Macrophomina phaseolina: A Phytopathogen Associated with Human Ocular Infections—A Case Report of Endophthalmitis and Systematic Review of Human Infections
Abstract
1. Introduction
2. Case Presentation
3. Review of the Literature
3.1. Methods
3.1.1. Search Strategy
3.1.2. Eligibility Criteria
3.1.3. Data Items
3.1.4. Data Synthesis
3.2. Results
3.2.1. Study Selection Process and Study Characteristics
3.2.2. Clinical Profile of Patients with M. phaseolina Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Yoon, S.J.; Park, Y.J.; Kim, S.Y.; Ryu, C.M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- Enyiukwu, D.N.; Ononuju, C.C.; Maranzu, J.O. Plant Pathogenic Fungi—Novel Agents of Human Diseases: Implications for public Health. Greener J. Epidemiol. Public Health 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S.; Brar, S.K.; Vallad, G.E.; Chand, R.; Chauhan, V.B. Emerging phytopathogenMacrophomina phaseolina: Biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 2012, 38, 136–151. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Arora, P.; Dilbaghi, N.; Chaudhury, A. Opportunistic invasive fungal pathogen Macrophomina phaseolina prognosis from immunocompromised humans to potential mitogenic RBL with an exceptional and novel antitumor and cytotoxic effect. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Sigler, L.; Gibas, C.F.C.; Fong, I.W. Disseminated fungal infection in a renal transplant recipient involving Macrophomina phaseolina and Scytalidium dimidiatum: Case report and review of taxonomic changes among medically important members of the Botryosphaeriaceae. Med. Mycol. 2008, 46, 285–292. [Google Scholar] [CrossRef]
- Bagyalakshmi, R.; Therese, K.L.; Prasanna, S.; Madhavan, H.N. Newer emerging pathogens of ocular non-sporulating molds (NSM) identified by polymerase chain reaction (PCR)-Based DNA sequencing technique targeting internal transcribed spacer (ITS) region. Curr. Eye Res. 2008, 33, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Wickes, B.L.; Romanelli, A.M.; Debelenko, L.; Rubnitz, J.E.; Sutton, D.A.; Thompson, E.H.; Fothergill, A.W.; Rinaldi, M.G.; Hayden, R.T.; et al. Cutaneous Infection Caused by Macrophomina phaseolina in a Child with Acute Myeloid Leukemia. J. Clin. Microbiol. 2009, 47, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Premamalini, T.; Ambujavalli, B.T.; Vijayakumar, R.; Rajyoganandh, S.V.; Kalpana, S.; Kindo, A.J. Fungal keratitis caused by Macrophomina phaseolina—A case report. Med. Mycol. Case Rep. 2012, 1, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A.; Kapila, R. Macrophomina phaseolina: An overlooked cutaneous infection, seed rot disease in humans. Int. J. Dermatol. 2020, 59, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, L.K.; Sheba, E.; Jakati, S.; Jayasudha, R.; Padakandla, S.R.; Bagga, B.; Sharma, S. Elucidating the clinical, microbiological and molecular diagnostic aspects of Macrophomina phaseolina keratitis. Med. Mycol. 2022, 60, 10. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Payne, A.R. Liquid Culture Production of Fungal Microsclerotia. In Microbial-Based Biopesticides; Methods in Molecular Biology; Humana: New York, NY, USA, 2016; Volume 1477, pp. 71–83. [Google Scholar] [CrossRef]
- Toumasis, P.; Tsantes, A.G.; Tsiogka, A.; Samonis, G.; Vrioni, G. From Clinical Suspicion to Diagnosis: A Review of Diagnostic Approaches and Challenges in Fungal Keratitis. J. Clin. Med. 2024, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular mechanisms governing antifungal drug resistance. NPJ Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue Penetration of Antifungal Agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Novack, G.D.; Robin, A.L. Ocular Pharmacology. J. Clin. Pharmacol. 2024, 64, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Bisen, A.C.; Sanap, S.N.; Agrawal, S.; Biswas, A.; Mishra, A.; Verma, S.K.; Singh, V.; Bhatta, R.S. Etiopathology, Epidemiology, Diagnosis, and Treatment of Fungal Keratitis. ACS Infect. Dis. 2024, 10, 2356–2380. [Google Scholar] [CrossRef] [PubMed]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Kailasam, V.; Sai Veda Koduganti, S.; Dasgupta, O.; Garg, P.; Nirmal, J. Ocular delivery of Amphotericin B: Current challenges and future perspectives. Expert Opin. Drug Deliv. 2024, 21, 1793–1805. [Google Scholar] [CrossRef]
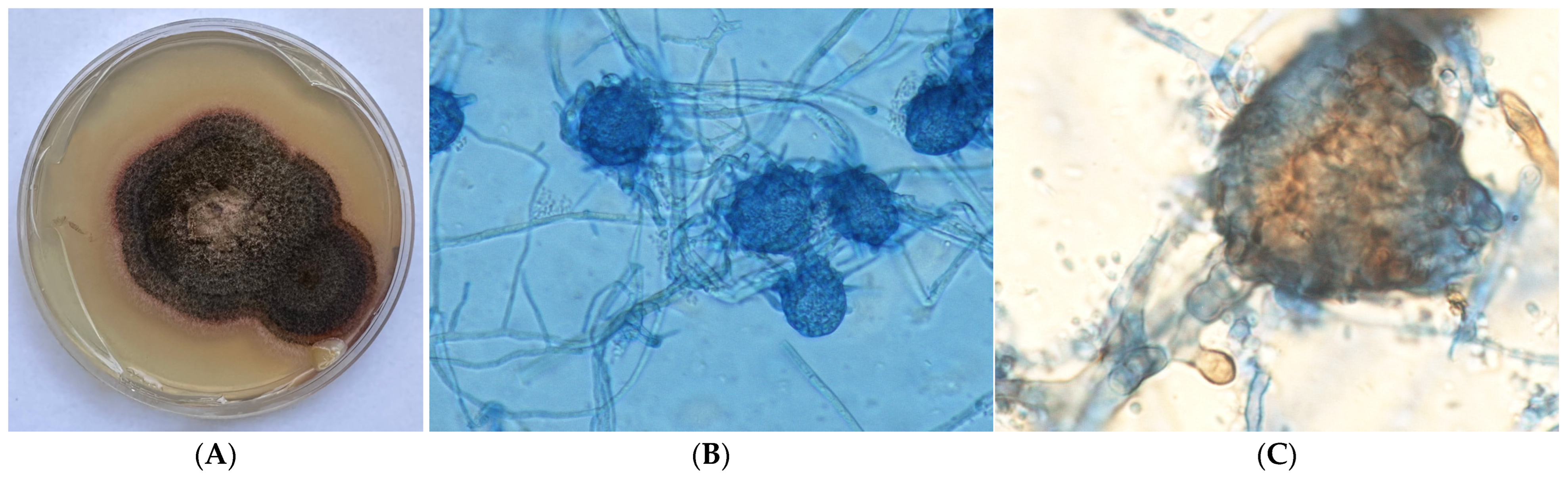
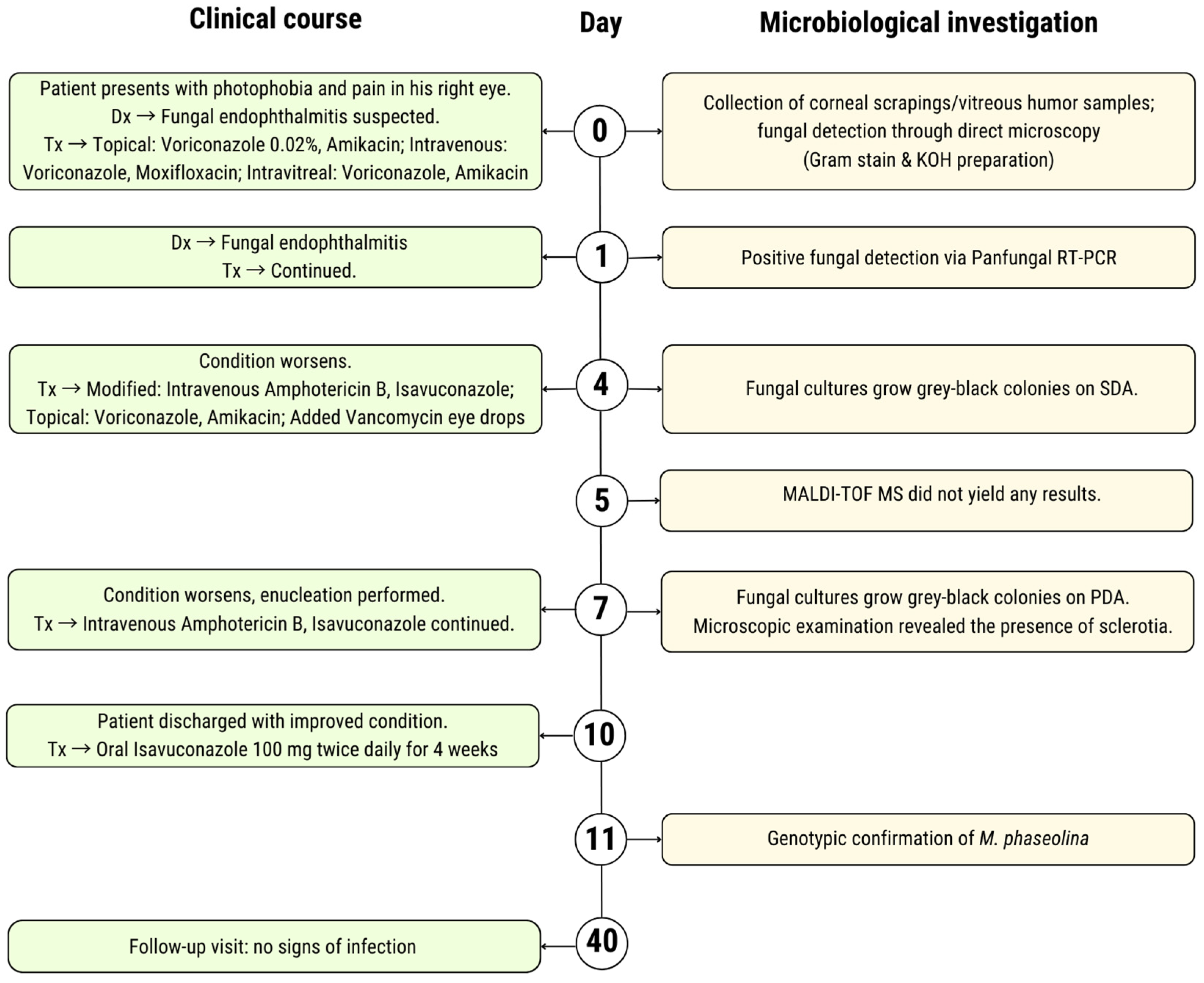
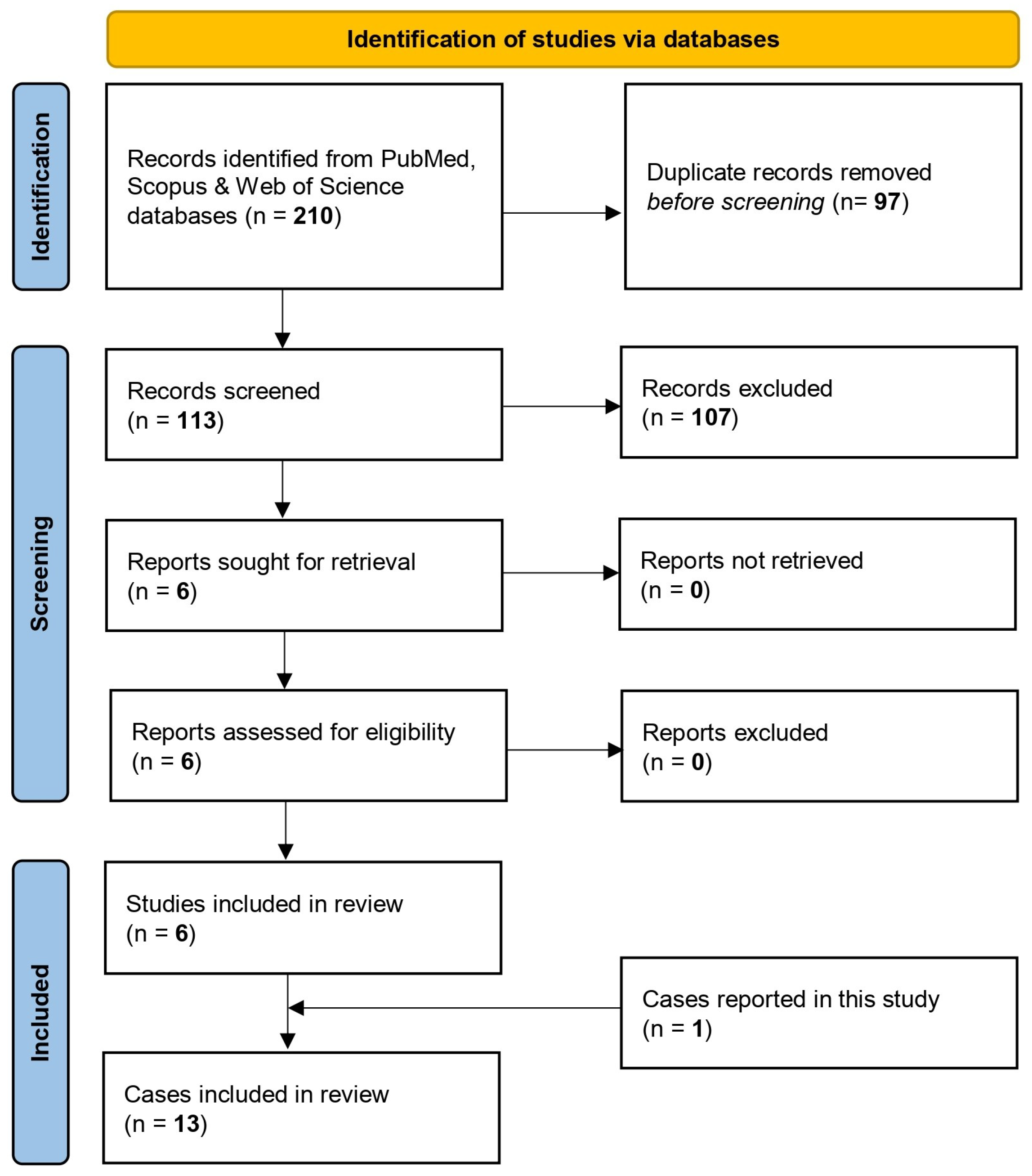
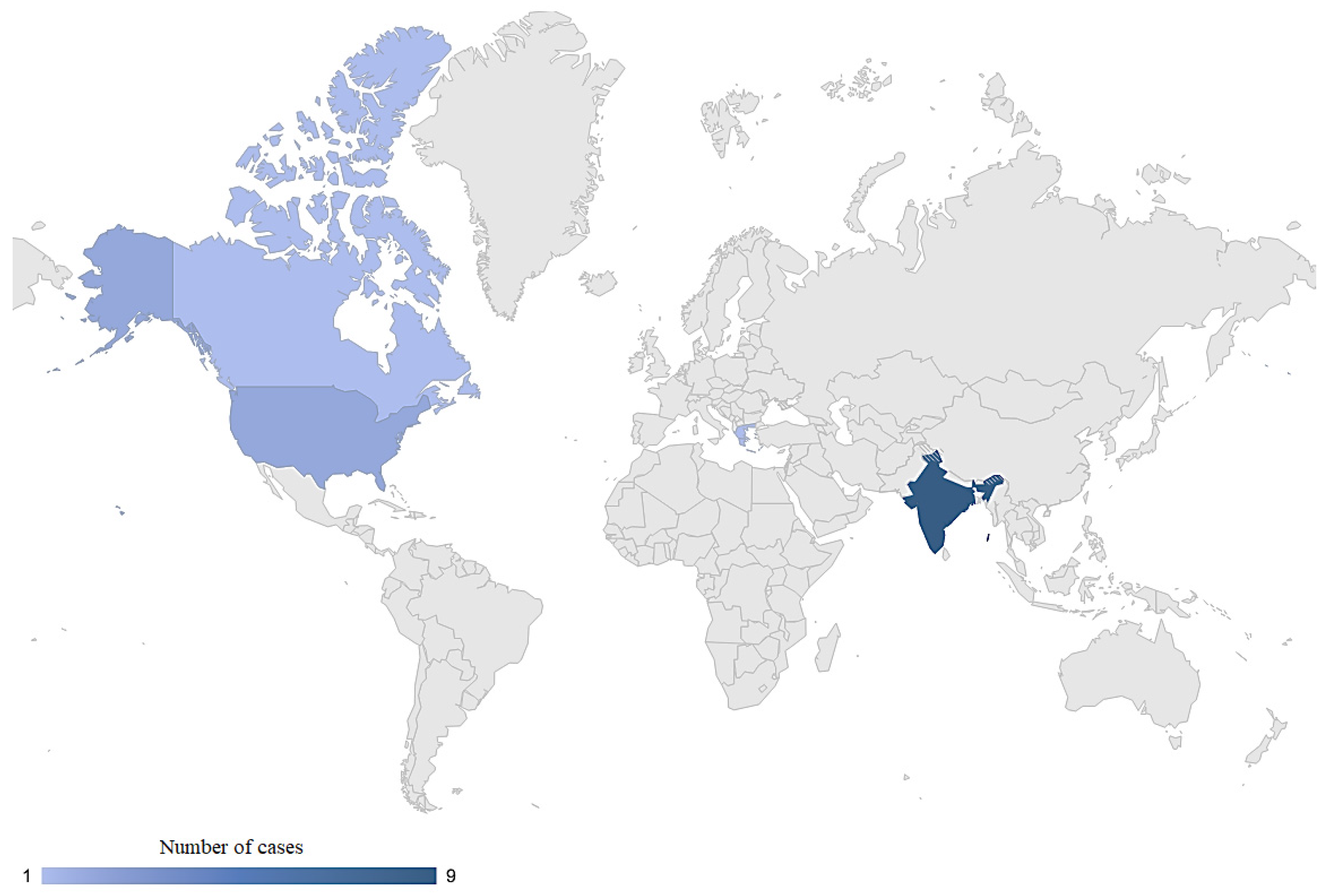
| Case | Article (Year) | Country | Age (y) | Gender | Site(s) of Infection | Predisposing Factor | Comorbidities | Microbiological Method to Confirm M. phaseolina Infection | Antifungal Susceptibility—MIC | Antifungal Treatment | Outcome Regarding M. phaseolina Infection | REF. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tan et al. (2008) | Canada | 31 | M | Skin and joint (Left great toe) | Unknown | Hypertension End-stage renal disease (Renal transplantation two months prior to the infection. Under immunosuppression regimen.) | DNA sequencing | AΜΒ = 0.06 μg/mL VRZ = 0.015 μg/mL KCZ = 0.03 μg/mL CAS = 0.015 μg/mL ITZ = 0.015 μg/mL FLU = 0.12 μg/mL 5-FC = 4 μg/mL | Oral VRZ 200 mg twice daily | Favorable (Symptoms improved) However, unfavorable overall outcome (Death of invasive infection by the related pathogen, Scytalidium dimidiatum) | [7] |
| 2 | Bagyalakshmi et al. (2008) | India | N/A | N/A | Eye (Keratitis) | Unknown | N/A | DNA sequencing | N/A | AMB eye drops | Favorable (Ocular lesion resolved completely) | [8] |
| 3 | // | // | N/A | N/A | Eye (Keratitis) | Trauma | N/A | DNA sequencing | N/A | AMB eye drops | Favorable (Ocular lesion resolved completely) | // |
| 4 | Srinivasan et al. (2009) | USA | 6 | F | “Skin (Right medial malleolus)” | Unknown | Acute myeloid leukemia | DNA sequencing | AΜΒ = 0.25 μg/mL VRZ = 0.5 μg/mL PZC = 1 μg/mL TER = 0.06 μg/mL CAS = 1 μg/mL | VRZ (route of admission not mentioned) → no improvement → change to PZC (route of admission not mentioned) | Favorable (Dramatic improvement with PZC; complete healing with central scarring within 3 weeks) | [9] |
| 5 | Premamalini et al. (2012) | India | 70 | F | Eye (Keratitis) | Trauma | Anemia Chronic kidney disease | DNA sequencing | N/A | Initially: Natamycin eye drops hourly, oral KCZ 200 mg twice daily After identification: Natamycin eye drops two hourly, oral VRZ 200 mg twice daily | Favorable (Ocular lesion and symptoms resolved completely) | [10] |
| 6 | Schwartz et al. (2020) | USA | 42 | M | Skin (soft tissue mass on the right dorsal foot) | Unknown | Diabetes mellitus | N/A | N/A | Oral VRZ 300 mg twice daily for 1 day and then 200 mg twice daily for 2 weeks | Favorable (The mass resolved completely) | [11] |
| 7 | Ahirwar et al. (2022) | India | 46 | M | Eye (Keratitis) | Foreign body | No | PCR assay of MpCal gene | Ν/A | NAM eye drops, VRZ eye drops, oral KCZ 200 mg | Favorable (Ocular lesion resolved completely) | [12] |
| 8 | // | // | 58 | M | Eye (Keratitis) | Unknown | No | PCR assay of MpCal gene | N/A | NAM eye drops, KCZ 200 mg | Unfavorable (Therapeutic penetrating keratoplasty) | // |
| 9 | // | // | 46 | F | Eye (Keratitis) | Unknown | No | PCR assay of MpCal gene | AMB = 2 μg/mL VRZ = 0.1 μg/mL PZC = 0.1 μg/mL KCZ = 16 μg/mL CAS = 0.03 μg/mL NAT = 2 μg/mL | NAM eye drops, KCZ 200 mg | Unfavorable (Therapeutic penetrating keratoplasty) | // |
| 10 | // | // | 52 | F | Eye (Keratitis) | Foreign body | No | PCR assay of MpCal gene | AMB = 8 μg/mL VRZ < 0.01 μg/mL PZC < 0.01 μg/mL KCZ = 1 μg/mL CAS = 0.03 μg/mL NAT = 4 μg/mL | NAM eye drops, KCZ 200 mg | Unfavorable (Therapeutic penetrating keratoplasty) | // |
| 11 | // | // | 65 | M | Eye (Keratitis) | Trauma | No | PCR assay of MpCal gene | AMB = 4 μg/mL VRZ = 0.03 μg/mL PZC = 2 μg/mL KCZ = 16 μg/mL CAS = 4 μg/mL NAT = 2 μg/mL | NAM eye drops, KCZ 200 mg | Unfavorable (Therapeutic penetrating keratoplasty) | // |
| 12 | // | // | 56 | F | Eye (Keratitis) | Foreign body | No | PCR assay of MpCal gene | AMB = 4 μg/mL VRZ = 2 μg/mL PZC = 2 μg/mL KCZ = 2 μg/mL CAS = 8 μg/mL NAT = 2 μg/mL | NAM eye drops, KCZ 200 mg | Unfavorable (Therapeutic penetrating keratoplasty) | // |
| 13 | Toumasis et al. (2024) | Greece | 78 | M | Eye (Endophthalmitis) | Foreign body | Heterozygous beta-thalassemia Coronary artery disease Hypertension Dyslipidemia | DNA sequencing | AΜΒ = 0.047 μg/mL VRZ = 0.032 μg/mL ISAV = 0.064 μg/mL ITZ > 32 μg/mL PZC = 1 μg/mL | VRZ eye drops, IVT and IV for 4 days → no improvement → change to AMB/ISAV IVT and IV for 3 days → no improvement → enucleation After enucleation: IV AMB/ISAV for 3 days After discharge: oral ISAV for 4 weeks | Unfavorable (Enucleation of the affected eye) | [*] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toumasis, P.; Vrioni, G.; Gardeli, I.; Michelaki, A.; Exindari, M.; Orfanidou, M. Macrophomina phaseolina: A Phytopathogen Associated with Human Ocular Infections—A Case Report of Endophthalmitis and Systematic Review of Human Infections. J. Clin. Med. 2025, 14, 430. https://doi.org/10.3390/jcm14020430
Toumasis P, Vrioni G, Gardeli I, Michelaki A, Exindari M, Orfanidou M. Macrophomina phaseolina: A Phytopathogen Associated with Human Ocular Infections—A Case Report of Endophthalmitis and Systematic Review of Human Infections. Journal of Clinical Medicine. 2025; 14(2):430. https://doi.org/10.3390/jcm14020430
Chicago/Turabian StyleToumasis, Panagiotis, Georgia Vrioni, Ioanna Gardeli, Aikaterini Michelaki, Maria Exindari, and Maria Orfanidou. 2025. "Macrophomina phaseolina: A Phytopathogen Associated with Human Ocular Infections—A Case Report of Endophthalmitis and Systematic Review of Human Infections" Journal of Clinical Medicine 14, no. 2: 430. https://doi.org/10.3390/jcm14020430
APA StyleToumasis, P., Vrioni, G., Gardeli, I., Michelaki, A., Exindari, M., & Orfanidou, M. (2025). Macrophomina phaseolina: A Phytopathogen Associated with Human Ocular Infections—A Case Report of Endophthalmitis and Systematic Review of Human Infections. Journal of Clinical Medicine, 14(2), 430. https://doi.org/10.3390/jcm14020430






