A Real-World Comparison of the Safety Profile for Immune Checkpoint Inhibitors in Oncology Patients
Abstract
1. Introduction
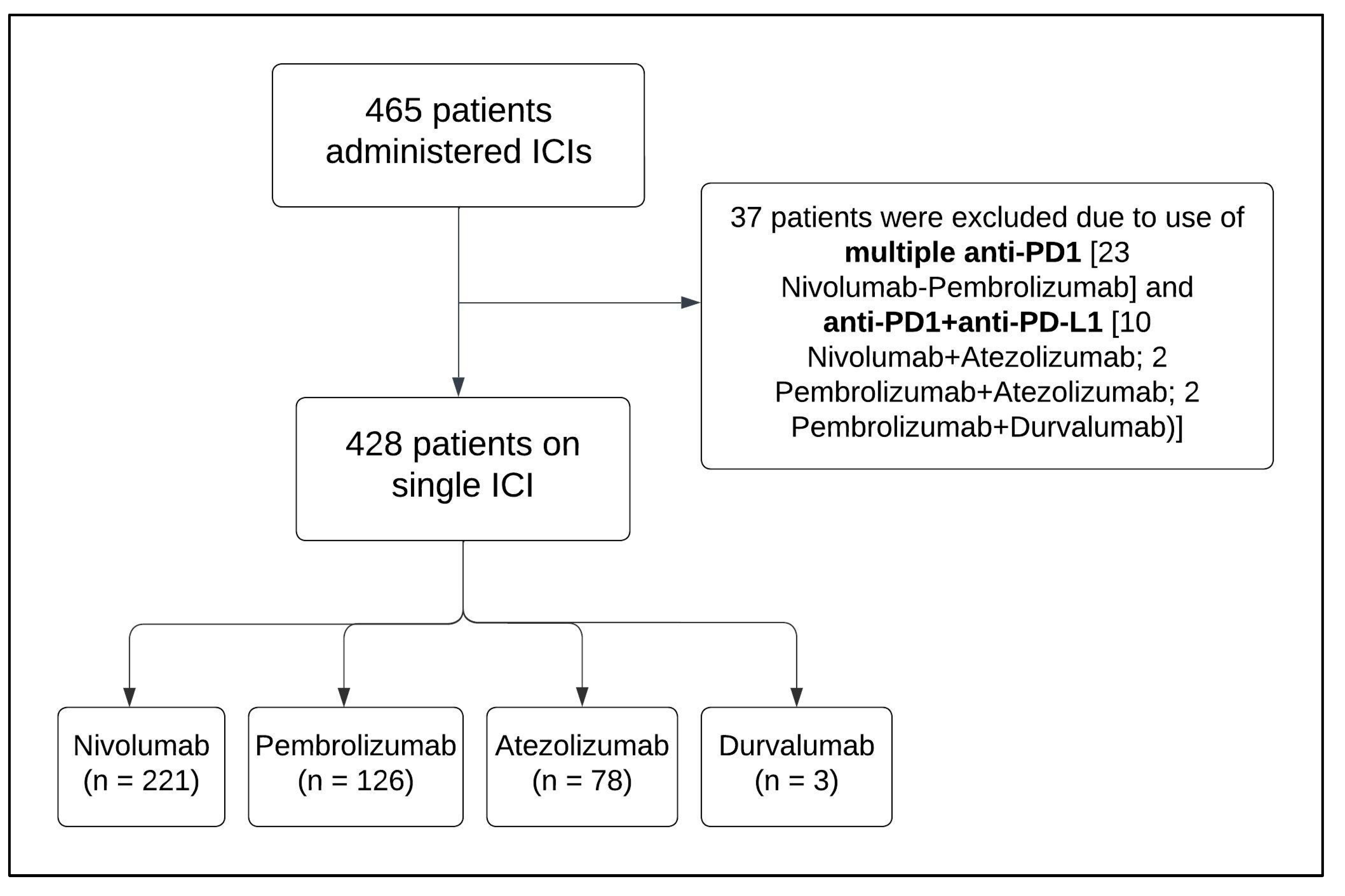
2. Methods
Statistical Analysis
3. Results
3.1. Patient Selection and Baseline Characteristics
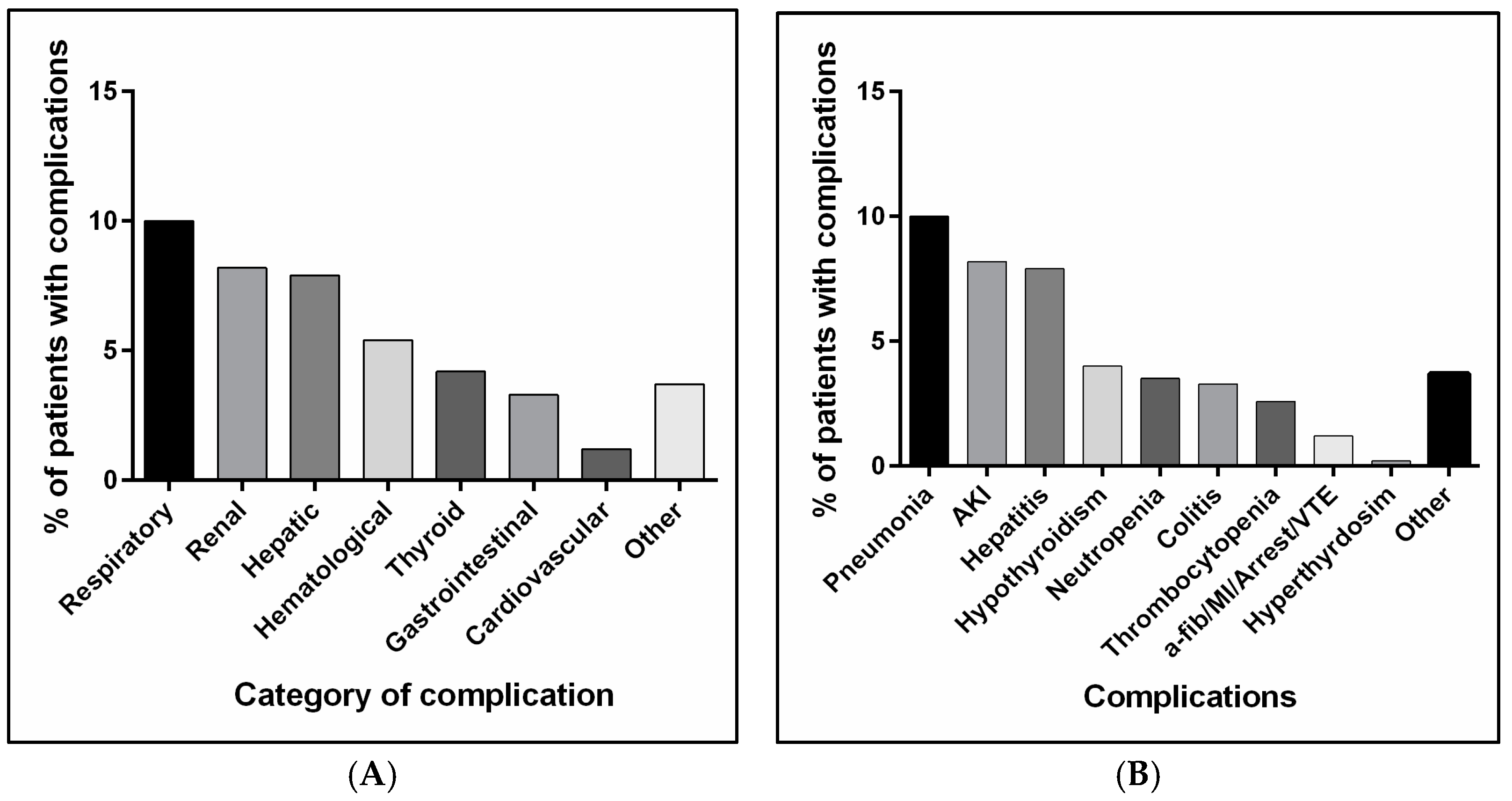
3.2. Complications and Side Effects Associated with Anti-PD1 or Anti-PD-L1 Administration
3.3. Onset of Complications/Side Effects Associated with Anti-PD1 or Anti-PD-L1
3.4. Assessment of Risk Factors Triggering Complications and Side Effect Occurrence with Anti-PD1 or Anti-PD-L1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Homet Moreno, B.; Ribas, A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br. J. Cancer 2015, 112, 1421–1427. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef]
- Hoshiko, S.; Makabe, O.; Nojiri, C.; Katsumata, K.; Satoh, E.; Nagaoka, K. Molecular cloning and characterization of the Streptomyces hygroscopicus alpha-amylase gene. J. Bacteriol. 1987, 169, 1029–1036. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Marlette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Wang, F.; Yang, S.; Palmer, N.; Fox, K.; Kohane, I.S.; Liao, K.P.; Yu, K.H.; Kou, S.C. Real-world data analyses unveiled the immune-related adverse effects of immune checkpoint inhibitors across cancer types. NPJ Precis. Oncol. 2021, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Kim, R.; Yu, T.; Gayle, J.A.; Wassel, C.L.; Dreyfus, J.; Phatak, H.; George, S. Real-World Clinical and Economic Outcomes in Selected Immune-Related Adverse Events Among Patients with Cancer Receiving Immune Checkpoint Inhibitors. Oncologist 2021, 26, e2002–e2012. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Corrigan-Curay, J.; Sacks, L.; Woodcock, J. Real-World Evidence and Real-World Data for Evaluating Drug Safety and Effectiveness. JAMA 2018, 320, 867–868. [Google Scholar] [CrossRef]
- Jarow, J.P.; LaVange, L.; Woodcock, J. Multidimensional Evidence Generation and FDA Regulatory Decision Making: Defining and Using “Real-World” Data. JAMA 2017, 318, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.C.; Liao, B.; Markovic, S.N.; Klein, C.J.; Naddaf, E.; Staff, N.P.; Liewluck, T.; Hammack, J.E.; Sandroni, P.; Finnes, H.; et al. Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol. 2017, 74, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Liaw, B.; Asada, M.; Niimura, T.; Zamami, Y.; Green-LaRoche, D.; Pai, L.; Levy, M.; Jeyapalan, S. Neuroimmunological adverse events associated with immune checkpoint inhibitor: A retrospective, pharmacovigilance study using FAERS database. J. Neurooncol 2021, 152, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hodi, F.S.; Giobbie-Hurder, A.; Ott, P.A.; Buchbinder, E.I.; Haq, R.; Tolaney, S.; Barroso-Sousa, R.; Zhang, K.; Donahue, H.; et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol. Res. 2017, 5, 1133–1140. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Ali, F.S.; Qiao, W.; Lum, P.; Raju, G.; Shuttlesworth, G.; Stroehlein, J.; et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J. Immunother. Cancer 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Lin, J.Y.; Hsu, M.Y.; Lin, P.C. Effectiveness and safety of immune checkpoint inhibitors: A retrospective study in Taiwan. PLoS ONE 2018, 13, e0202725. [Google Scholar] [CrossRef]
- Ide, T.; Araki, T.; Koizumi, T. Thromboembolism during immune checkpoint inhibitor therapy: Frequency and risk factors. Discov. Oncol. 2024, 15, 527. [Google Scholar] [CrossRef]
- Benkhadra, M.; Elazzazy, S.; Hamad, A.A.; Elkhatim, M.S.; Gulied, A.; Sahal, A.O.; Alasmar, A.; Jibril, F.I.; Afifi, H.M.; Nasser, S.; et al. Hematological Toxicity of Immune Checkpoint Inhibitors: Real-World Retrospective Outcomes from a Cohort Study in Qatar. Blood 2023, 142, 5378. [Google Scholar] [CrossRef]
- Hountondji, L.; Ferreira De Matos, C.; Lebosse, F.; Quantin, X.; Lesage, C.; Palassin, P.; Rivet, V.; Faure, S.; Pageaux, G.P.; Assenat, E.; et al. Clinical pattern of checkpoint inhibitor-induced liver injury in a multicentre cohort. JHEP Rep. 2023, 5, 100719. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, B.J.; Zhang, Y.H.; Qiu, X.Y. Hepatitis B virus reactivation risk associated with immune checkpoint inhibitors in tumor treatment: A retrospective study. Jpn. J. Clin. Oncol. 2024, 54, 1288–1297. [Google Scholar] [CrossRef]
- She, J.; Liu, H.; Wu, H.; Tuerhongjiang, G.; Zheng, T.; Bai, L. Cardiotoxicity Related to Immune Checkpoint Inhibitors: A Real-World Retrospective Analysis. Front. Cardiovasc. Med. 2022, 9, 838488. [Google Scholar] [CrossRef]
- Waheed, N.; Fradley, M.G.; DeRemer, D.L.; Mahmoud, A.; Shah, C.P.; Langaee, T.Y.; Lipori, G.P.; March, K.; Pepine, C.J.; Cooper-DeHoff, R.M.; et al. Newly diagnosed cardiovascular disease in patients treated with immune checkpoint inhibitors: A retrospective analysis of patients at an academic tertiary care center. Cardiooncology 2021, 7, 10. [Google Scholar] [CrossRef]
- Chauhan, A.; Burkeen, G.; Houranieh, J.; Arnold, S.; Anthony, L. Immune checkpoint-associated cardiotoxicity: Case report with systematic review of literature. Ann. Oncol. 2017, 28, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; de Lima Lopes, G.; Yusof, M.M.; Rubagumya, F.; Rutkowski, P.; Sengar, M. Barriers in access to oncology drugs—A global crisis. Nat. Rev. Clin. Oncol. 2023, 20, 7–15. [Google Scholar] [CrossRef]
- Leheny, S. ‘Adverse Event’, Not the Same as ‘Side Effect’. 2017. Available online: https://www.pharmacytimes.com/view/adverse-event-not-the-same-as-side-effect#:~:text=Adverse%20events%20are%20unintended%20pharmacologic,occurs%20due%20to%20drug%20therapy (accessed on 3 November 2024).
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Hu, S.; Tang, Z.; Harrison, J.P.; Hertel, N.; Penrod, J.R.; May, J.R.; Juarez-Garcia, A.; Holdgate, O. Economic Evaluation of Nivolumab Versus Docetaxel for the Treatment of Advanced Squamous and Non-squamous Non-small Cell Lung Cancer After Prior Chemotherapy in China. Pharmacoecon Open 2023, 7, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Winer, A.; Bodor, J.N.; Borghaei, H. Identifying and managing the adverse effects of immune checkpoint blockade. J. Thorac. Dis. 2018, 10, S480–S489. [Google Scholar] [CrossRef]
- Villadolid, J.; Amin, A. Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl. Lung Cancer Res. 2015, 4, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Chirila, R.M.; Dronca, R.S. Immune Checkpoint Inhibitor Toxicities. Mayo Clin. Proc. 2019, 94, 1321–1329. [Google Scholar] [CrossRef]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018, 124, 271–277. [Google Scholar] [CrossRef] [PubMed]
- El Majzoub, I.; Qdaisat, A.; Thein, K.Z.; Win, M.A.; Han, M.M.; Jacobson, K.; Chaftari, P.S.; Prejean, M.; Reyes-Gibby, C.; Yeung, S.J. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann. Emerg. Med. 2019, 73, 79–87. [Google Scholar] [CrossRef]
- Sengul Samanci, N.; Cikman, D.I.; Oruc, K.; Bedir, S.; Celik, E.; Degerli, E.; Derin, S.; Demirelli, F.H.; Ozguroglu, M. Immune-related adverse events associated with immune checkpoint inhibitors in patients with cancer. Tumori 2021, 107, 304–310. [Google Scholar] [CrossRef]
- Al Nuhait, M.; Bajnaid, E.; Al Otaibi, A.; Al Shammari, A.; Al Awlah, Y. Real-world safety experience with immune checkpoint inhibitors in Saudi Arabia. Sci. Prog. 2021, 104, 36850421997302. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, N.; Liang, X.; Zhang, S.; Fan, Q.; Yin, X.; Zhuang, Z.; Liu, Y.; Zhang, J.; et al. Immune checkpoint inhibitors-associated cardiotoxicity in immunotherapy trials on gastrointestinal cancer patients. Chin. Med. J. 2022, 135, 988–990. [Google Scholar] [CrossRef]
- Benz, S.; Sherman, K.A.; Dasanu, C.A.; Alvarez-Argote, J. Immune checkpoint inhibitor-related adverse events: Real-world experience from a single veterans’ affairs medical center. J. Oncol. Pharm. Pract. 2024, 30, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Gougis, P.; Jochum, F.; Abbar, B.; Dumas, E.; Bihan, K.; Lebrun-Vignes, B.; Moslehi, J.; Spano, J.P.; Laas, E.; Hotton, J.; et al. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective. EClinicalMedicine 2024, 70, 102536. [Google Scholar] [CrossRef]
- Albarran-Artahona, V.; Laguna, J.C.; Gorria, T.; Torres-Jimenez, J.; Pascal, M.; Mezquita, L. Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy. Diagnostics 2022, 12, 2091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, A.; Gridelli, C. “Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer”: Is there a substantial difference or not? J. Thorac. Dis. 2018, 10, S4065–S4068. [Google Scholar] [CrossRef]
- Rofi, E.; Del Re, M.; Arrigoni, E.; Rizzo, M.; Fontanelli, L.; Crucitta, S.; Gianfilippo, G.; Restante, G.; Fogli, S.; Porta, C.; et al. Clinical pharmacology of monoclonal antibodies targeting anti-PD-1 axis in urothelial cancers. Crit. Rev. Oncol. Hematol. 2019, 144, 102812. [Google Scholar] [CrossRef]
- Banna, G.L.; Cantale, O.; Bersanelli, M.; Del Re, M.; Friedlaender, A.; Cortellini, A.; Addeo, A. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol. Rev. 2020, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Gatti, M.; Gelsomino, F.; Ardizzoni, A.; Poluzzi, E.; De Ponti, F. Lessons to be Learnt from Real-World Studies on Immune-Related Adverse Events with Checkpoint Inhibitors: A Clinical Perspective from Pharmacovigilance. Target. Oncol. 2020, 15, 449–466. [Google Scholar] [CrossRef]
- Weber, J.S. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am. Soc. Clin. Oncol. Educ. Book. 2012, 32, 174–177. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Cheema, P.K.; Iafolla, M.A.J.; Abdel-Qadir, H.; Bellini, A.B.; Chatur, N.; Chandok, N.; Comondore, V.R.; Cunningham, M.; Halperin, I.; Hu, A.B.; et al. Managing Select Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitors. Curr. Oncol. 2024, 31, 6356–6383. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Wanchoo, R.; Karam, S.; Uppal, N.N.; Barta, V.S.; Deray, G.; Devoe, C.; Launay-Vacher, V.; Jhaveri, K.D.; Cancer; Kidney International Network Workgroup on Immune Checkpoint, I. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am. J. Nephrol. 2017, 45, 160–169. [Google Scholar] [CrossRef]
- Khan, O.F.; Monzon, J. Diagnosis, monitoring, and management of adverse events from immune checkpoint inhibitor therapy. Curr. Oncol. 2020, 27 (Suppl. S2), S43–S50. [Google Scholar] [CrossRef]

| Characteristics | All Patients (n = 428) | Nivolumab (n = 221) | Pembrolizumab (n = 126) | Atezolizumab (n = 78) | Durvalumab (n = 3) | p-Value |
|---|---|---|---|---|---|---|
| Age [median (IQR)] | 64 (20) | 64 (21) | 61 (19) | 64 (17.75) | 67 (5.5) | 0.147 |
| Male gender, n (%) | 278 (65.0) | 154 (69.7) | 73 (57.9) | 48 (61.5) | 3 (100) | 0.075 |
| Age group, n (%) | 0.005 | |||||
| <30 yrs | 11 (2.6) | 10 (4.5) | 1 (0.8) | 0 | 0 | |
| 30 to <60 yrs | 154 (36.0) | 74 (33.5) | 59 (46.8) | 21 (26.9) | 0 | |
| ≥60 yrs | 263 (61.4) | 137 (62.0) | 66 (52.4) | 57 (73.1) | 3 (100.0) | |
| Primary diagnosed cancer, n (%) * | 0.046 | |||||
| Solid | 406 (95.3) | 203 (92.7) | 122 (96.8) | 78 (100.0) | 3 (100.0) | |
| Hematological | 20 (4.7) | 16 (7.3) | 4 (3.2) | 0 | 0 | |
| Cancer stage right before ICI initiation, n (%) * | 0.003 | |||||
| Stage II | 40 (9.4) | 20 (9.1) | 8 (6.4) | 12 (15.4) | 0 | |
| Stage III | 65 (15.3) | 47 (21.5) | 9 (7.2) | 9 (11.5) | 0 | |
| Stage IV | 320 (75.3) | 152 (69.4) | 108 (86.4) | 57 (73.1) | 3 (100.0) | |
| Risk factors of heart diseases (HTN, DM, DLD, etc.) | 0.335 | |||||
| Yes | 232 (54.2) | 115 (52.0) | 72 (57.1) | 42 (53.8) | 3 (100.0) | |
| No | 196 (45.8) | 106 (48.0) | 54 (42.9) | 36 (46.2) | 0 | |
| Heart diseases | 0.002 | |||||
| Yes | 35 (8.2) | 15 (6.8) | 12 (9.5) | 6 (7.7) | 2 (66.7) | |
| No | 393 (91.8) | 206 (93.2) | 114 (90.5) | 72 (92.3) | 1 (33.3) | |
| Heart diseases | 35 (8.2) | 15 (6.8) | 12 (9.5) | 6 (7.7) | 2 (66.7) | |
| a-fib | 2 (5.7) | 1 (6.7) | 1 (8.3) | 0 | 0 | 0.882 |
| IHD | 21 (60) | 7 (46.7) | 8 (66.7) | 4 (66.7) | 2 (100.0) | 0.427 |
| CAD | 3 (8.6) | 2 (13.3) | 1 (8.3) | 0 | 0 | 0.757 |
| CABG | 4 (11.4) | 2 (13.3) | 1 (8.3) | 1 (16.7) | 0 | 0.899 |
| CHF | 5 (14.3) | 3 (20.0) | 1 (8.3) | 1 (16.7) | 0 | 0.775 |
| Other comorbidities | 0.091 | |||||
| Yes | 136 (31.8) | 75 (33.9) | 31 (24.6) | 30 (38.5) | 0 | |
| No | 292 (68.2) | 146 (66.1) | 95 (75.4) | 48 (61.5) | 3 (100.0) | |
| History of hepatitis, n (%) | 0.009 | |||||
| Yes | 36 (8.4) | 24 (10.9) | 2 (1.6) | 10 (12.8) | 0 | |
| No | 392 (91.6) | 197 (89.1) | 124 (98.4) | 68 (87.2) | 3 (100.0) | |
| Previous cancer therapy, n (%) | 0.565 | |||||
| Yes | 316 (73.8) | 165 (74.7) | 94 (74.6) | 54 (69.2) | 3 (100.0) | |
| No | 112 (26.2) | 56 (25.3) | 32 (25.4) | 24 (30.8) | 0 | |
| BMI, kg/m2 [median (IQR)] | 24.45 (7.8) | 24.03 (7.9) | 26.06 (9.6) | 24.48 (5.8) | 24.11 (0.24) | 0.027 |
| BMI, n (%) | 0.014 | |||||
| Underweight | 59 (13.8) | 40 (18.1) | 11 (8.7) | 8 (10.3) | 0 | |
| Healthy weight | 169 (39.5) | 85 (38.5) | 48 (38.1) | 33 (42.3) | 3 (100.0) | |
| Overweight | 111 (25.9) | 60 (27.1) | 28 (22.2) | 23 (29.5) | 2 (40) | |
| Obesity | 89 (20.8) | 36 (16.3) | 39 (31.0) | 14 (17.9) | 0 | |
| Labs [median (IQR)] | ||||||
| LDL (mmol/L) * | 2.93 (1.0) | 3.04 (1.56) | 2.81 (1.27) | 3.04 (1.08) | 2.77 (0.49) | 0.714 |
| HDL (mmol/L) * | 1.0 (0) | 1.04 (0.36) | 0.92 (0.31) | 0.99 (0.23) | 0.9 (0.26) | 0.299 |
| HbA1C% * | 6.55 (3.0) | 6.45 (3.13) | 6.6 (2.7) | 6.6 (2.5) | 7.85 (0.85) | 0.892 |
| ALT (U/L) * | 20.5 (20.8) | 23.0 (20.75) | 19.0 (16) | 22.0 (24) | 10 (3.5) | 0.017 |
| AST (U/L) * | 21 (16.0) | 22.0 (16.75) | 18.0 (8) | 23.0 (26) | 16.0 (2) | <0.001 |
| Bilirubin (Umol/L) * | 8.9 (6.5) | 9.5 (6.9) | 7.77 (5.53) | 10.0 (7.2) | 7.1 (1.05) | 0.003 |
| Scr (Umol/L) * | 70 (23) | 70 (23) | 69 (25) | 71 (17) | 74 (2) | 0.857 |
| CrCl (mL/min) * | 78 (43.5) | 74.5 (41.25) | 85 (47) | 78 (34) | 70 (19.5) | 0.148 |
| Troponin (ng/mL) | 5.4 (11) | 4.65 (8.27) | 9.55 (12.9) | 6.73 (10.58) | 7.85 (0) | 0.341 |
| Complication | All Patients (n = 428) | Nivolumab (n = 221) | Pembrolizumab (n = 126) | Atezolizumab (n = 78) | Durvalumab (n = 3) | p-Value |
|---|---|---|---|---|---|---|
| Respiratory, n (%) | 0.161 | |||||
| Pneumonia | ||||||
| Yes | 43 (10.0) | 23 (10.4) | 8 (6.3) | 11 (14.1) | 1 (33.3) | |
| No | 385 (90.0) | 198 (89.6) | 118 (93.7) | 67 (85.9) | 2 (66.7) | |
| Renal, n (%) | 0.426 | |||||
| Acute kidney injury | ||||||
| Yes | 35 (8.2) | 21 (9.5) | 11 (8.7) | 3 (3.8) | 0 | |
| No | 393 (91.8) | 200 (90.5) | 115 (91.3) | 75 (96.2) | 3 (100.0) | |
| Hepatic, n (%) | <0.001 | |||||
| Hepatitis | ||||||
| Yes | 34 (7.9) | 17 (7.7) | 3 (2.4) | 14 (17.9) | 0 | |
| No | 394 (92.1) | 204 (92.3) | 123 (97.6) | 64 (82.1) | 3 (100.0) | |
| Hematological, n (%) | 0.256 | |||||
| Yes | 23 (5.4) | 9 (4.1) | 11 (8.7) | 3 (3.8) | 0 | |
| No | 405 (94.6) | 212 (95.9) | 115 (91.3) | 75 (96.2) | 3 (100.0) | |
| Hematological, n (%) | 0.940 | |||||
| Neutropenia | ||||||
| Yes | 15 (3.5) | 8 (3.6) | 5 (4.0) | 2 (2.6) | 0 | |
| No | 413 (96.5) | 213 (96.4) | 121 (96.0) | 76 (97.4) | 3 (100.0) | |
| Thrombocytopenia | 0.072 | |||||
| Yes | 11 (2.6) | 2 (0.9) | 7 (5.6) | 2 (2.6) | 0 | |
| No | 417 (97.4) | 219 (99.1) | 119 (94.4) | 76 (97.4) | 3 (100.0) | |
| Thyroid, n (%) | 0.692 | |||||
| Yes | 18 (4.2) | 7 (3.2) | 7 (5.6) | 4 (5.1) | 0 | |
| No | 410 (95.8) | 214 (96.8) | 119 (94.4) | 74 (94.4) | 3 (100.0) | |
| Thyroid, n (%) | 0.722 | |||||
| Hypothyroidism | ||||||
| Yes | 17 (4.0) | 7 (3.2) | 7 (5.6) | 3 (3.8) | 0 | |
| No | 411 (96) | 214 (96.8) | 119 (94.4) | 75 (96.2) | 3 (100.0) | |
| Hyperthyroidism | 0.212 | |||||
| Yes | 1 (0.2) | 0 | 0 | 1 (1.3) | 0 | |
| No | 427 (99.8) | 221 (100.0) | 126 (100.0) | 77 (98.7) | 3 (100.0) | |
| Gastrointestinal complications, n (%) | 0.008 | |||||
| Colitis | ||||||
| Yes | 14 (3.3) | 10 (4.5) | 2 (1.6) | 1 (1.3) | 1 (33.3) | |
| No | 414 (96.7) | 211 (95.5) | 124 (98.4) | 77 (98.7) | 2 (66.7) | |
| Cardiovascular, n (%) | <0.001 | |||||
| Yes | 5 (1.2) | 1 (0.5) | 0 | 3 (3.8) | 1 (33.3) | |
| No | 423 (498.8) | 220 (99.5) | 126 (100.0) | 75 (96.2) | 2 (66.7) | |
| Others, n (%) | 0.039 | |||||
| Yes | 16 (3.7) | 4 (1.8) | 5 (4.0) | 7 (9.0) | 0 | |
| No | 412 (96.3) | 217 (98.2) | 121 (96.0) | 71 (91.0) | 3 (100.0) |
| Side Effect | All Patients (n = 428) | Nivolumab (n = 221) | Pembrolizumab (n = 126) | Atezolizumab (n = 78) | Durvalumab (n = 3) | p-Value |
|---|---|---|---|---|---|---|
| Musculoskeletal, n (%) | 0.663 | |||||
| Fatigue/arthralgia | ||||||
| Yes | 169 (39.5) | 83 (37.6) | 51 (40.5) | 33 (42.3) | 2 (66.7) | |
| No | 259 (60.5) | 138 (62.4) | 75 (59.5) | 45 (57.7) | 1 (33.3) | |
| Musculoskeletal, n (%) | 0.625 | |||||
| Fatigue | ||||||
| Yes | 164 (38.3) | 80 (36.2) | 51 (40.5) | 31 (39.7) | 2 (66.7) | |
| No | 264 (61.7) | 141 (63.8) | 75 (59.5) | 47 (60.3) | 1 (33.3) | |
| Musculoskeletal, n (%) | 0.007 | |||||
| Arthralgia | ||||||
| Yes | 19 (4.4) | 17 (7.7) | 0 | 2 (2.6) | 0 | |
| No | 409 (95.6) | 204 (92.3) | 126 (100.0) | 76 (97.4) | 3 (100.0) | |
| Gastrointestinal, n (%) | 0.144 | |||||
| Nausea/vomiting/diarrhea | ||||||
| Yes | 153 (35.7) | 68 (30.8) | 54 (42.9) | 30 (38.5) | 1 (33.3) | |
| No | 275 (64.3) | 153 (69.2) | 72 (57.1) | 48 (61.5) | 3 (66.7) | |
| Gastrointestinal, n (%) | 0.273 | |||||
| Nausea and vomiting | ||||||
| Yes | 128 (29.9) | 59 (26.7) | 42 (33.3) | 27 (34.6) | 0 | |
| No | 300 (70.1) | 162 (73.3) | 84 (66.7) | 51 (65.4) | 3 (100.0) | |
| Gastrointestinal, n (%) | 0.324 | |||||
| Diarrhea | ||||||
| Yes | 53 (12.4) | 22 (10.0) | 19 (15.1) | 11 (14.1) | 1 (33.3) | |
| No | 375 (87.6) | 199 (90.0) | 107 (84.9) | 67 (85.9) | 2 (66.7) | |
| Dermatological (pruritis–dermatitis–eczema), n (%) | 0.298 | |||||
| Yes | 32 (7.5) | 18 (8.1) | 9 (7.1) | 4 (5.1) | 1 (33.3) | |
| No | 396 (92.5) | 203 (91.1) | 117 (92.9) | 74 (94.9) | 2 (66.7) |
| Characteristics | All Patients (n = 428) | Nivolumab (n = 221) | Pembrolizumab (n = 126) | Atezolizumab (n = 78) | Durvalumab (n = 3) | p-Value |
|---|---|---|---|---|---|---|
| Total complications | 0.162 | |||||
| 0 | 276 (64.5) | 145 (65.6) | 84 (66.7) | 46 (59) | 1 (33.3) | |
| 1 | 122 (28.5) | 62 (28.1) | 38 (30.2) | 21 (26.9) | 1 (33.3) | |
| 2 | 25 (5.8) | 12 (5.4) | 3 (2.4) | 9 (11.5) | 1 (33.3) | |
| 3 | 4 (0.9) | 2 (0.9) | 1 (0.8) | 1 (1.3) | 0 (0.0) | |
| 4 | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (1.3) | 0 (0.0) | |
| Total side effects | 0.173 | |||||
| 0 | 176 (41.1) | 100 (45.2) | 47 (37.3) | 29 (37.2) | 0 (0.0) | |
| 1 | 140 (32.7) | 68 (30.8) | 41 (32.5) | 29 (37.2) | 2 (66.7) | |
| 2 | 82 (19.2) | 33 (14.9) | 34 (27.0) | 14 (17.9) | 1 (33.3) | |
| 3 | 28 (6.5) | 18 (8.1) | 4 (3.2) | 6 (7.7) | 0 (0.0) | |
| 4 | 2 (0.5) | 2 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Complication/Side Effect | Average Week Elapsed Until Complication [Median (IQR)] | Weeks Elapsed Since Treatment Initiation Until Adverse Effect Occurrence [Median (IQR)] | p-Value | |||
|---|---|---|---|---|---|---|
| Nivolumab (n = 221) | Pembrolizumab (n = 126) | Atezolizumab (n = 78) | Durvalumab (n = 3) | |||
| Hepatic (hepatitis) | 6.0 (14.0) | 6.0 (12.5) | 10.0 (31.5) | 2.0 (3.5) | NA | 0.084 |
| Cardiovascular | 7.5 (14.0) | NA | NA | 5.0 (8.5) | 10.0 (0.0) | 0.655 |
| Hematological | 8.0 (26.0) | 24.0 (31.0) | 6.0 (9.5) | 30.0 (6.5) | NA | 0.145 |
| Neutropenia | 22.0 (30.0) | 25.0 (35.25) | 7.0 (18.0) | 34.5 (4.5) | NA | 0.174 |
| Thrombocytopenia | 7.0 (24.0) | 4.0 (3.0) | 6.0 (11.0) | 32.5 (6.5) | NA | 0.222 |
| Respiratory (pneumonia) | 8.0 (27.0) | 10.0 (35.0) | 11.0 (38.0) | 4.0 (5.0) | 8.0 (0.0) | 0.182 |
| Renal (AKI) | 10.0 (15.0) | 10.0 (9.0) | 2.0 (3.0) | 26.0 (10.5) | NA | 0.062 |
| Gastrointestinal complications (colitis) | 10.0 (22.0) | 8.5 (18.5) | 6.0 (38.0) | 16.0 (0.0) | 27.0 (0.0) | 0.631 |
| Thyroid | 10.5 (22.0) | 8.0 (11.5) | 23.0 (7.0) | 6.0 (14.5) | NA | 0.218 |
| Hypothyroidism | 12 (19) | 8.0 (11.5) | 23.0 (7.0) | 9.0 (20) | NA | 0.385 |
| Skin reaction | 12.0 (21.25) | 11.5 (19.0) | 10 (42) | 18.5 (65) | NA | 0.414 |
| Complication/Side Effect | Patients’ Factors | Coef (B) | S.E | Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Hepatic complication | Gender | Male | Ref | ||||
| Female | 0.996 | 0.473 | 2.71 | 1.07–6.85 | 0.035 | ||
| History of hepatitis | No | Ref | |||||
| Yes | 2.41 | 0.597 | 11.14 | 3.46–35.88 | <0.001 | ||
| Respiratory complication | Previous cancer therapy | No | Ref | ||||
| Yes | 1.125 | 0.515 | 3.08 | 1.12–8.85 | 0.029 | ||
| Hematological complication | Type of malignancy | Solid | Ref | ||||
| Liquid | 2.84 | 0.736 | 17.18 | 4.06–72.71 | <0.001 | ||
| Neutropenia | Type of malignancy | Solid | |||||
| Liquid | 1.79 | 0.816 | 6.01 | 1.21–29.75 | 0.028 | ||
| Thrombocytopenia | Type of malignancy | Solid | |||||
| Liquid | 3.28 | 1.08 | 26.5 | 3.22–218.38 | 0.002 | ||
| Musculoskeletal | Gender | Male | |||||
| Female | 0.419 | 0.21 | 1.52 | 1.01–2.29 | 0.046 | ||
| Arthralgia | Previous cancer therapy | No | Ref | ||||
| Yes | −1.07 | 0.531 | 0.344 | 0.121–0.974 | 0.045 | ||
| Fatigue | Gender | Male | Ref | ||||
| Female | 0.503 | 0.212 | 1.65 | 1.09–2.51 | 0.018 | ||
| Nausea and vomiting | Gender | Male | Ref | ||||
| Female | 0.731 | 0.222 | 2.08 | 1.34–3.21 | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwhaibi, A.; Alenazi, M.A.; Alghadeer, S.; Mansy, W.; Alsaif, R.A.; Abualreesh, N.E.; Alanazi, R.J.; Alroumi, A.; Alanazi, S.A. A Real-World Comparison of the Safety Profile for Immune Checkpoint Inhibitors in Oncology Patients. J. Clin. Med. 2025, 14, 388. https://doi.org/10.3390/jcm14020388
Alwhaibi A, Alenazi MA, Alghadeer S, Mansy W, Alsaif RA, Abualreesh NE, Alanazi RJ, Alroumi A, Alanazi SA. A Real-World Comparison of the Safety Profile for Immune Checkpoint Inhibitors in Oncology Patients. Journal of Clinical Medicine. 2025; 14(2):388. https://doi.org/10.3390/jcm14020388
Chicago/Turabian StyleAlwhaibi, Abdulrahman, Miteb A. Alenazi, Sultan Alghadeer, Wael Mansy, Reem A. Alsaif, Nawaf E. Abualreesh, Rakan J. Alanazi, Abdullah Alroumi, and Saleh A. Alanazi. 2025. "A Real-World Comparison of the Safety Profile for Immune Checkpoint Inhibitors in Oncology Patients" Journal of Clinical Medicine 14, no. 2: 388. https://doi.org/10.3390/jcm14020388
APA StyleAlwhaibi, A., Alenazi, M. A., Alghadeer, S., Mansy, W., Alsaif, R. A., Abualreesh, N. E., Alanazi, R. J., Alroumi, A., & Alanazi, S. A. (2025). A Real-World Comparison of the Safety Profile for Immune Checkpoint Inhibitors in Oncology Patients. Journal of Clinical Medicine, 14(2), 388. https://doi.org/10.3390/jcm14020388






