Motor Assessment Timed Test (MATT): A New Timed Test to Assess Functional Mobility in Parkinson’s Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Sample Size
2.3. Participants
2.4. Measures
2.4.1. Demographic Measures
2.4.2. Disease Severity
2.4.3. Clinical Motor Assessment
2.4.4. Fall History and Fear of Falling (FoF)
2.4.5. Cognitive Impairment
2.4.6. Executive Function
2.4.7. Physical Function and Motor Complications Assessment
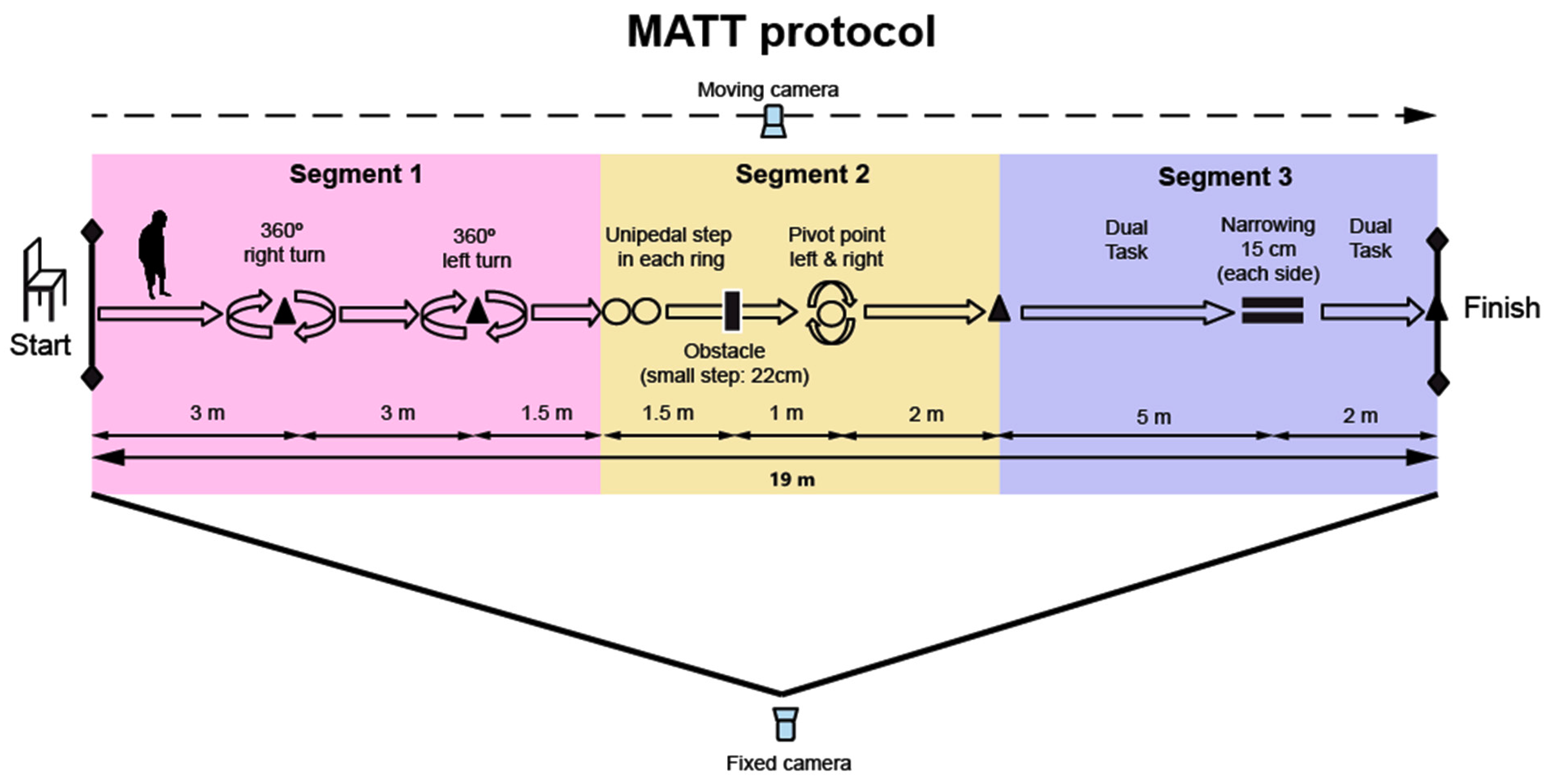
2.4.8. MATT (Motor Assessment Timed Test) Design
- Segment 1: Gait and dynamic turn (360° in both directions).
- Segment 2: Balance control (two tandem steps and stepping over an obstacle) and static turns in a narrow space (360° in both directions).
- Segment 3: Dual task and walking through a narrow passage.
2.5. Procedures
2.5.1. Clinical Evaluation
2.5.2. Questionnaire and Motor Scales Administration
2.5.3. Motor Examination (Balance and Timed Test Administration)
2.5.4. MATT Protocol
- Initial Position: Participants are instructed to sit in a chair with armrests at the starting point, as shown on the left side of Figure 1.
- Start of the Test: The procedure begins when participants stand up from the chair following the “GO” command. The time is then recorded in seconds from this moment.
- Walking and Right Turn: Participants walk 3 m to the first cone and perform a 360° right turn around the cone.
- Left Turn: Participants continue walking straight and perform a 360° left turn upon reaching the second cone (3 m from the first).
- Placing Feet on the Hoops: After passing the second cone, participants walk 1.5 m to the hoops, where they place their right foot on the first hoop and their left foot on the second hoop.
- Overcoming the Obstacle: Participants then overcome an obstacle (22 cm high) without jumping.
- Entering the Hoop and Performing Turns in a Restricted Space: After stepping over the obstacle, they enter a hoop 1 m ahead and perform two 360° turns inside the hoop (one clockwise and one counterclockwise).
- Walking to the Next Cone: After exiting the hoop, participants walk 2 m to the next cone.
- Counting Backwards and Crossing the Narrow Passage: Upon reaching the cone, participants begin counting aloud backwards from 100 while continuing to walk through a narrow passage (2 m long), marked by bars and cones, simulating a confined space (15 cm wide on each side of the body, at hip level).
- End of the Protocol: Timing stops when participants tap the last cone, located 2 m from the midpoint of the narrow corridor.
2.5.5. MATT Assessment
2.6. Clinimetric Properties
2.6.1. Internal Consistency
2.6.2. Intra-Rater (Test–Retest) Reliability
2.6.3. Inter-Rater Reliability
2.6.4. Intra-Session Reliability
2.6.5. Development and Content Validity
2.6.6. Content Validity
2.6.7. Clinical Applicability
2.7. Statistics
3. Results
3.1. Reliability
3.1.1. Internal Consistency
3.1.2. Intra-Rater Reliability
3.1.3. Inter-Rater Reliability
3.1.4. Intra-Session Reliability
3.1.5. Concurrent Validity
Correlation Analysis
- -
- Total Time: Very high correlations were found with the TUG (ρ = 0.86, p < 0.001) and BBS (ρ = −0.83, p < 0.001). Additionally, high correlations were found with the disease stage “H&Y scale” (ρ = 0.66, p < 0.001), the motor examination “MDS-UPDRS III” (ρ = 0.60, p < 0.001), the history of falls “6-M retrospective falls” (ρ = 0.65, p < 0.001), and the questionnaire of freezing of gait “FOG-Q” (ρ = 0.50, p < 0.001).
- -
- Segment 1: Very high correlations were found with gait speed (10-MWT: r = −0.88, p < 0.001) and also with functional mobility (TUG: ρ = 0.89, p < 0.001). A high correlation was found between Segment 1 and the Tinetti Gait Section (ρ = −0.64, p < 0.001).
- -
- Segment 2: Very high correlation was found with the specific balance scale (BBS: ρ = −0.84, p < 0.001). Moreover, high correlations were found with the Tinneti Balance section (ρ = −0.68, p < 0.001), the Tinneti total score (ρ = −0.74, p < 0.001), and the ABC scale: ρ = −0.69, p < 0.001). Nevertheless, a moderate correlation was found between Segment 2 and the FRT (r = −0.40, p < 0.001) and between this segment and the stabilometry variables, both open (TE COP: ρ = 0.33, p = 0.015; MS COP: ρ = 0.34, p = 0.011) and closed eye conditions (TE COP: ρ = 0.41, p = 0.002; MS COP: ρ = 0.41, p = 0.002).
- -
- Segment 3: Very high correlation was found with the Cognitive TUG (ρ = 0.84, p < 0.001). Moreover, Segment 3 showed high correlations with the executive function (TMT “A”: ρ = 0.53, p < 0.001; TMT “B”: ρ = 0.58; p < 0.001; TMT “B-A”: ρ = 0.55; p < 0.001).
3.1.6. Learning Effect
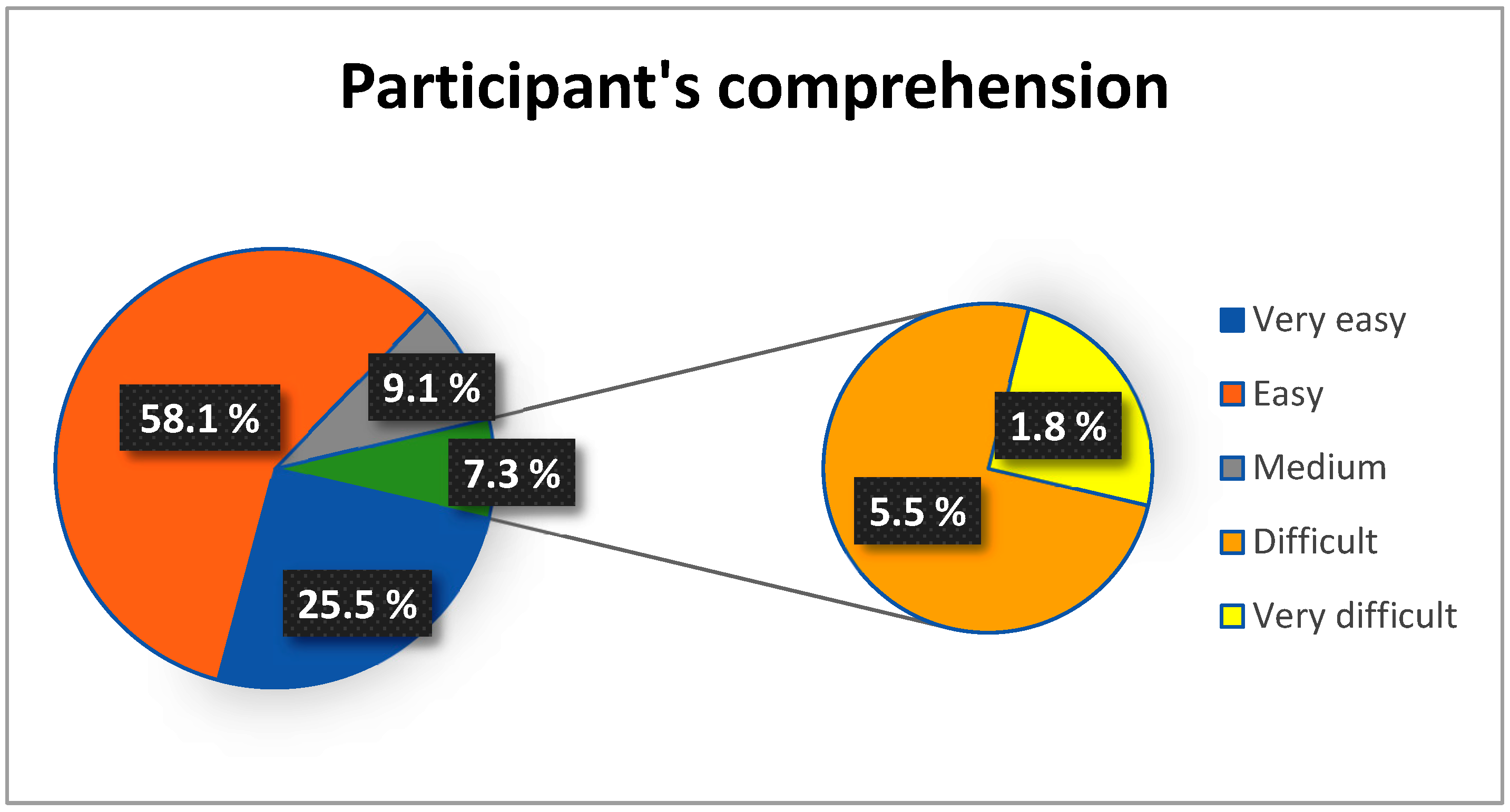
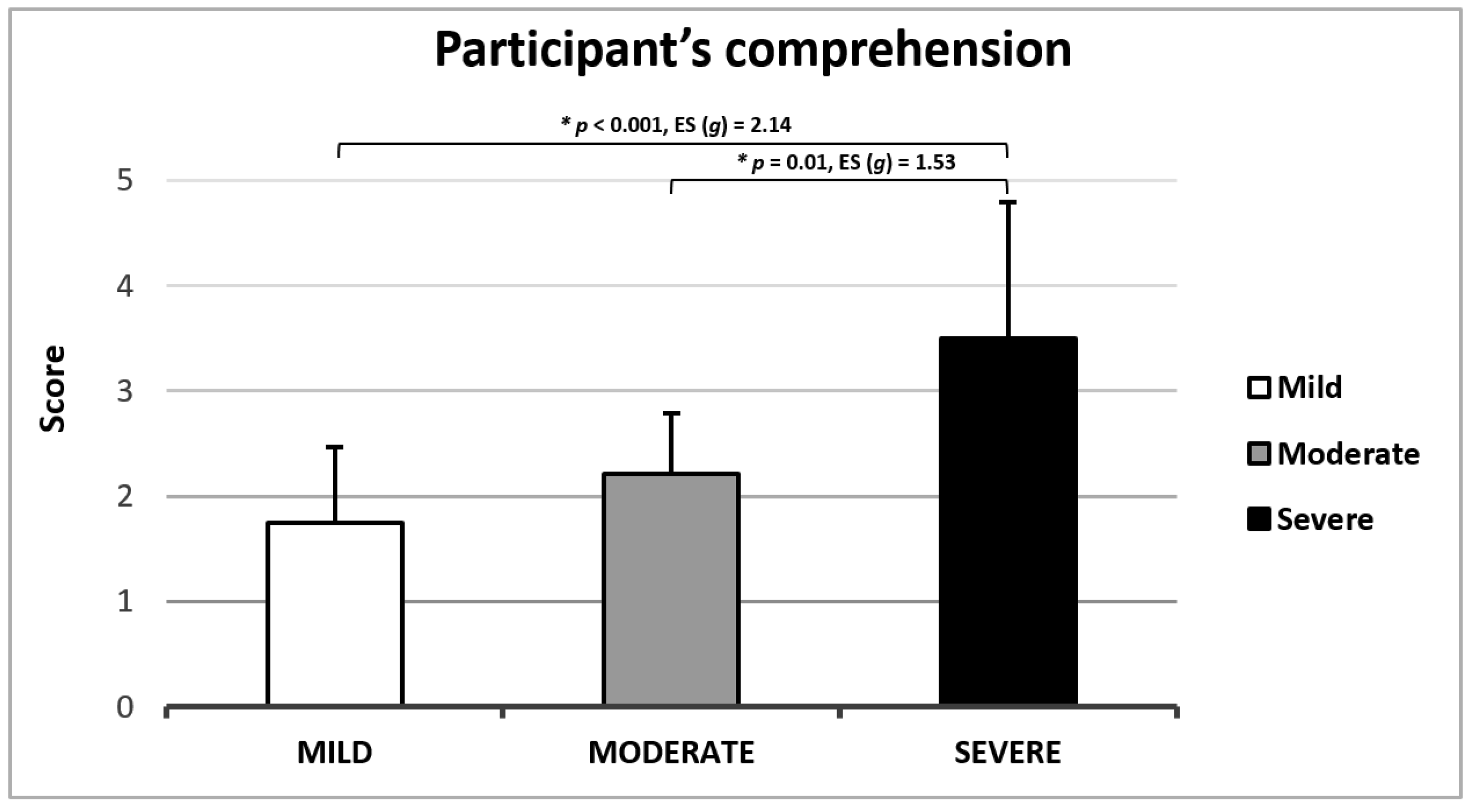
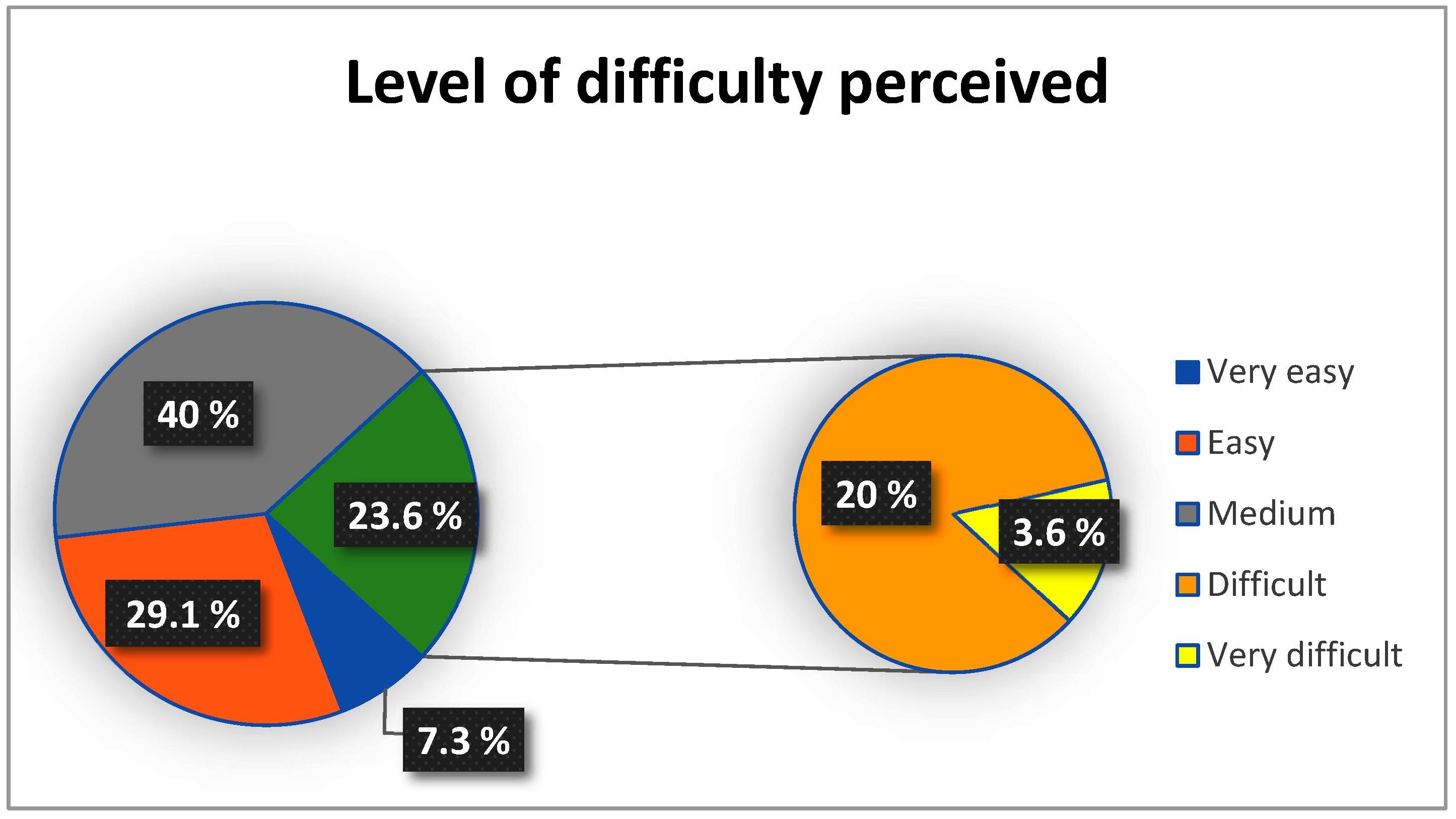
3.1.7. Clinical Applicability
4. Discussion
4.1. Functional Mobility
4.2. Gait and Dynamic Balance
5. Limitations
6. Conclusions
7. Application Development

Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahn, S.; Jankovic, J.; Hallett, M. Gait Disorders: Pathophysiology and Clinical Syndromes. In Principles and Practice of Movement Disorders; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 1–35. [Google Scholar]
- Raccagni, C.; Nonnekes, J.; Bloem, B.R.; Peball, M.; Boehme, C.; Seppi, K.; Wenning, G.K. Gait and Postural Disorders in Parkinsonism: A Clinical Approach. J. Neurol. 2020, 267, 3169–3176. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Giladi, N.; Hausdorff, J.M.; Balash, Y. Episodic and Continuous Gait Disturbances in Parkinson’s Disease. In Scientific Basis for the Treatment of Parkinson’s Disease; Galvez-Jimenez, N., Ed.; Taylor & Francis: London, UK, 2005; pp. 321–332. [Google Scholar]
- Hausdorff, J.M. Gait Dynamics in Parkinson’s Disease: Common and Distinct Behavior among Stride Length, Gait Variability, and Fractal-like Scaling. Chaos 2009, 19, 026113. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Negre-Pages, L.; Damier, P.; Delval, A.; Derkinderen, P.; Destée, A.; Meissner, W.G.; Schelosky, L.; Tison, F.; Rascol, O. Prevalence, Determinants, and Effect on Quality of Life of Freezing of Gait in Parkinson Disease. JAMA Neurol. 2014, 71, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of Gait: Moving Forward on a Mysterious Clinical Phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Giladi, N.; Shabtai, H.; Simon, E.S.; Biran, S.; Tal, J.; Korczyn, A.D. Construction of Freezing of Gait Questionnaire for Patients with Parkinsonism. Park. Relat. Disord. 2000, 6, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; Haaxma, C.A.; Hagen, Y.J.; Munneke, M.; Bloem, B.R. Freezer or Non-Freezer: Clinical Assessment of Freezing of Gait. Park. Relat. Disord. 2012, 18, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Spildooren, J.; Vercruysse, S.; Desloovere, K.; Vandenberghe, W.; Kerckhofs, E.; Nieuwboer, A. Freezing of Gait in Parkinson’s Disease: The Impact of Dual-tasking and Turning. Mov. Disord. 2010, 25, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and Freezing of Gait in Parkinson’s Disease: A Review of Two Interconnected, Episodic Phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Klebe, S.; Zechlin, C.; Baecker, C.; Friege, L.; Deuschl, G. Falls in Frequent Neurological Diseases: Prevalence, Risk Factors and Aetiology. J. Neurol. 2004, 251, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Grimbergen, Y.A.M.; Cramer, M.; Willemsen, M.; Zwinderman, A.H. Prospective Assessment of Falls in Parkinson’s Disease. J. Neurol. 2001, 248, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Bouça-Machado, R.; Maetzler, W.; Ferreira, J.J. What Is Functional Mobility Applied to Parkinson’s Disease? J. Park. Dis. 2018, 8, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Menz, H.B.; McGinley, J.L.; Huxham, F.E.; Murphy, A.T.; Iansek, R.; Danoudis, M.; Soh, S.E.; Kelly, D.; Watts, J.J. Falls and Mobility in Parkinson’s Disease: Protocol for a Randomised Controlled Clinical Trial. BMC Neurol. 2011, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yan, Y.; Kong, X.; Su, S. Risk Factors for Falls in Parkinson’s Disease: A Cross-Sectional Observational and Mendelian Randomization Study. Front. Aging Neurosci. 2024, 16, 1420885. [Google Scholar] [CrossRef] [PubMed]
- Bhidayasiri, R.; Martinez-Martin, P. Clinical Assessments in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 132, 129–182. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Marinus, J.; Almeida, Q.; Dibble, L.; Nieuwboer, A.; Post, B.; Ruzicka, E.; Goetz, C.; Stebbins, G.; Martinez-Martin, P.; et al. Measurement Instruments to Assess Posture, Gait, and Balance in Parkinson’s Disease: Critique and Recommendations. Mov. Disord. 2016, 31, 1342–1355. [Google Scholar] [CrossRef]
- Yang, K.; Xiong, W.X.; Liu, F.T.; Sun, Y.M.; Luo, S.; Ding, Z.T.; Wu, J.J.; Wang, J. Objective and Quantitative Assessment of Motor Function in Parkinson’s Disease-from the Perspective of Practical Applications. Ann. Transl. Med. 2016, 4, 90. [Google Scholar] [CrossRef]
- AlMahadin, G.; Lotfi, A.; Zysk, E.; Siena, F.L.; Mc Carthy, M.; Breedon, P. Parkinson’s Disease: Current Assessment Methods and Wearable Devices for Evaluation of Movement Disorder Motor Symptoms-a Patient and Healthcare Professional Perspective. BMC Neurol. 2020, 20, 419. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Cavallo, F. How Wearable Sensors Can Support Parkinson’s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef]
- Thomas, M.; Jankovic, J.; Suteerawattananon, M.; Wankadia, S.; Caroline, K.S.; Vuong, K.D.; Protas, E. Clinical Gait and Balance Scale (GABS): Validation and Utilization. J. Neurol. Sci. 2004, 217, 89–99. [Google Scholar] [CrossRef]
- Macleod, A.D.; Counsell, C.E. Parkinsonism and Related Disorders Timed Tests of Motor Function in Parkinson’s Disease. Park. Relat. Disord. 2010, 16, 442–446. [Google Scholar] [CrossRef]
- Evans, A.H.; Farrell, M.J.; Gibson, S.J.; Helme, R.D.; Lim, S. Dyskinetic Patients Show Rebound Worsening of Affect after an Acute L-Dopa Challenge. Park. Relat. Disord. 2012, 18, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J.; National Parkinson Foundation Quality Improvement Initiative Investigators. Using the Timed Up & Go Test in a Clinical Setting to Predict Falling in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Clarke, E.; Elver, L.; Gowman, E.; Mortimer, E.; Byrd, E. The Reliability and Validity of the L-Test in People with Parkinson’s Disease. Physiotherapy 2019, 105, 84–89. [Google Scholar] [CrossRef]
- Bouça-Machado, R.; Duarte, G.S.; Patriarca, M.; Castro Caldas, A.; Alarcão, J.; Fernandes, R.M.; Mestre, T.A.; Matias, R.; Ferreira, J.J. Measurement Instruments to Assess Functional Mobility in Parkinson’s Disease: A Systematic Review. Mov. Disord. Clin. Pract. 2020, 7, 129–139. [Google Scholar] [CrossRef]
- Wall, J.C.; Bell, C.; Campbell, S.; Davis, J. The Timed Get-up-and-Go Test Revisited: Measurement of the Component Tasks. J. Rehabil. Res. Dev. 2000, 37, 109–114. [Google Scholar]
- Botolfsen, P.; Helbostad, J.L.; Moe-Nilssen, R.; Wall, J.C. Reliability and Concurrent Validity of the Expanded Timed Up-and-Go Test in Older People with Impaired Mobility. Physiother. Res. Int. 2008, 13, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.D.O.; Voos, M.C.; Barbosa, A.F.; Chen, J.; Francato, D.C.V.; Milosevic, M.; Popovic, M.; Barbosa, E.R. Relationship Between Posturography, Clinical Balance and Executive Function in Parkinson’s Disease. J. Mot. Behav. 2018, 51, 212–221. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression and Mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O.; Kurtis, M.M.; Martinez-Castrillo, J.C.; et al. Expanded and Independent Validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). J. Neurol. 2013, 260, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Araya, A.X.; Valenzuela, E.; Padilla, O.; Iriarte, E.; Caro, C. Preocupación a Caer: Validación de Un Instrumento de Medición En Personas Mayores Chilenas Que Viven En La Comunidad. Rev. Esp. Geriatr. Gerontol. 2017, 52, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Rabin, L.A.; Barr, W.B.; Burton, L.A. Assessment Practices of Clinical Neuropsychologists in the United States and Canada: A Survey of INS, NAN, and APA Division 40 Members. Arch. Clin. Neuropsychol. 2005, 20, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Arriaga, A.; Rodríguez-Violante, M. Validación de La Versión En Español Del Cuestionario de Congelamiento de La Marcha (FOG-Q) En Enfermedad de Parkinson. Arch. Neurocienc. 2011, 16, 173–178. [Google Scholar]
- Powell, L.E.; Myers, A.M. The Activities-Specific Balance Confidence (ABC) Scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 28–34. [Google Scholar] [CrossRef]
- Montilla-Ibáñez, A.; Martínez-Amat, A.; Lomas-Vega, R.; Cruz-Díaz, D.; la Torre-Cruz, M.J.D.; Casuso-Pérez, R.; Hita-Contreras, F. The Activities-Specific Balance Confidence Scale: Reliability and Validity in Spanish Patients with Vestibular Disorders. Disabil. Rehabil. 2017, 39, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kegelmeyer, D.A.; Kloos, A.D.; Thomas, K.M.; Kostyk, S.K. Reliability and Validity of the Tinetti Mobility Test for Individuals with Parkinson Disease. Phys. Ther. 2007, 87, 1369–1378. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guevara, C.R.; Lugo, L.H. Validez y Confiabilidad de La Escala de Tinetti Para Población Colombiana. Rev. Colomb. Reumatol. 2012, 19, 218–233. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring Balance in the Elderly: Validation of an Instrument. Can. J. Public Health 1992, 83, 7–11. [Google Scholar]
- Qutubuddin, A.A.; Pegg, P.O.; Cifu, D.X.; Brown, R.; McNamee, S.; Carne, W. Validating the Berg Balance Scale for Patients with Parkinson’s Disease: A Key to Rehabilitation Evaluation. Arch. Phys. Med. Rehabil. 2005, 86, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Freixes, O.; Passuni, D.A.; Buffetti, E.; Elizalde, M.; Lastiri, F. Berg Balance Scale: Inter-Rater and Intra-Rater Reliability of the Spanish Version with Incomplete Spinal Cord Injured Subjects. Spinal Cord Ser. Cases 2020, 6, 28. [Google Scholar] [CrossRef]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional Reach: A New Clinical Measure of Balance. J. Gerontol. 1990, 45, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bataller-Cervero, A.V.; Cimarras-Otal, C.; Roche-Seruendo, L.E.; Alcázar-Crevillén, A.; Villalba-Ruete, J.A.; Berzosa, C. Static Balance Modification during the Workday in Assembly Chain Workers with and without Current Low Back Pain. Int. J. Environ. Res. Public. Health 2020, 17, 7385. [Google Scholar] [CrossRef] [PubMed]
- Krewer, C.; Bergmann, J.; Gräfrath, P.C.; Jahn, K. Influence of Foot Position on Static and Dynamic Standing Balance in Healthy Young Adults. Hear. Balance Commun. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- Kamieniarz, A.; Michalska, J.; Brachman, A.; Pawłowski, M.; Słomka, K.J.; Juras, G. A Posturographic Procedure Assessing Balance Disorders in Parkinson’s Disease: A Systematic Review. Clin. Interv. Aging 2018, 13, 2301–2316. [Google Scholar] [CrossRef]
- Lang, J.T.; Kassan, T.O.; Devaney, L.L.; Colon-semenza, C.; Joseph, M.F. Test-Retest Reliability and Minimal Detectable Change for the 10-Meter Walk Test in Older Adults with Parkinson’s Disease. J. Geriatr. Phys. Ther. 2015, 39, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Onder, H.; Ozyurek, O. The Impact of Distinct Cognitive Dual-Tasks on Gait in Parkinson’s Disease and the Associations with the Clinical Features of Parkinson’s Disease. Neurol. Sci. 2020, 42, 2775–2783. [Google Scholar] [CrossRef]
- Bouça-Machado, R.; Pona-Ferreira, F.; Gonçalves, N.; Leitão, M.; Cacho, R.; Castro-Caldas, A.; Ferreira, J.J.; CNS Multidisciplinary Team. Outcome Measures for Evaluating the Effect of a Multidisciplinary Intervention on Axial Symptoms of Parkinson’s Disease. Front. Neurol. 2020, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Parashos, S.A.; Elm, J.; Boyd, J.T.; Chou, K.L.; Dai, L.; Mari, Z.; Morgan, J.C.; Sudarsky, L.; Wielinski, C.L. Validation of an Ambulatory Capacity Measure in Parkinson Disease: A Construct Derived from the Unified Parkinson’s Disease Rating Scale. J. Park. Dis. 2015, 5, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Dagan, Y.; Gurevich, T.; Giladi, N.; Hausdorff, J.M. Effects of Cognitive Function on Gait and Dual Tasking Abilities in Patients with Parkinson’s Disease Suffering from Motor Response Fluctuations. Exp. Brain Res. 2011, 208, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Plummer, P.; Eskes, G. Measuring Treatment Effects on Dual-Task Performance: A Framework for Research and Clinical Practice. Front. Hum. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Raffegeau, T.E.; Krehbiel, L.M.; Kang, N.; Thijs, F.J.; Altmann, L.J.; Cauraugh, J.H.; Hass, C.J. A Meta-Analysis: Parkinson’s Disease and Dual-Task Walking. Park. Relat. Disord. 2019, 62, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.C.; Healy, D.G.; Galvin, R.; French, H.P. Dual Tasking with the Timed “up & Go” Test Improves Detection of Risk of Falls in People with Parkinson Disease. Phys. Ther. 2015, 95, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Helbostad, J.L.; Sturnieks, D.L.; Menant, J.; Delbaere, K.; Lord, S.R.; Pijnappels, M. Consequences of Lower Extremity and Trunk Muscle Fatigue on Balance and Functional Tasks in Older People: A Systematic Literature Review. BMC Geriatr. 2010, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T. Effects of General and Local Fatigue on Postural Control: A Review. Neurosci. Biobehav. Rev. 2012, 36, 162–176. [Google Scholar] [CrossRef]
- Haga, N.; van der Heijden-Maessen, H.C.; van Hoorn, J.F.; Boonstra, A.M.; Hadders-Algra, M. Test-Retest and Inter- and Intrareliability of the Quality of the Upper-Extremity Skills Test in Preschool-Age Children with Cerebral Palsy. Arch. Phys. Med. Rehabil. 2007, 88, 1686–1689. [Google Scholar] [CrossRef]
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of Measurements Obtained with the Timed “Up & Go” Test in People with Parkinson Disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar] [CrossRef]
- Hopkins, W.G. Measures of Reliability in Sports Medicine and Science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Lynn, M.R. Determination and Quantification of Content Validity. Nurs. Res. 1986, 35, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Frades-Payo, B. Specific Patient-Reported Outcome Measures for Parkinson’s Disease: Analysis and Applications. Expert. Rev. Pharmacoecon. Outcomes Res. 2008, 8, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, P.; López-Pina, J.A. Revisión Sistemática de Las Propiedades Psicométricas de Las Escalas de Valoración de La Enfermedad de Parkinson: Riesgo de Caídas, Congelaciones y Otras Alteraciones En La Marcha y El Control Postural. Fisioterapia 2014, 36, 288–297. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shechtman, O. The Coefficient of Variation as an Index of Measurement Reliability. In Methods of Clinical Epidemiology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–49. ISBN 9783642371318. [Google Scholar]
- Smidt, N.; Van der Windt, D.A.; Assendelft, W.J.; Mourits, A.J.; Devill, W.L.; De Winter, A.F.; Bouter, L.M. Interobserver Reproducibility of the Assessment of Severity of Complaints, Grip Strength, and Pressure Pain Threshold in Patients with Lateral Epicondylitis. Arch. Phys. Med. Rehabil. 2002, 83, 1145–1150. [Google Scholar] [CrossRef]
- Francq, B.G.; Govaerts, B. How to Regress and Predict in a Bland-Altman Plot? Review and Contribution Based on Tolerance Intervals and Correlated-Errors-in-Variables Models. Stat. Med. 2016, 35, 2328–2358. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Matinolli, M.; Korpelainen, J.T.; Korpelainen, R.; Sotaniemi, K.A.; Matinolli, V.M.; Myllylä, V.V. Mobility and Balance in Parkinson’s Disease: A Population-Based Study. Eur. J. Neurol. 2009, 16, 105–111. [Google Scholar] [CrossRef]
- Soke, F.; Guclu-Gunduz, A.; Ozkan, T.; Ozkul, C.; Gulsen, C.; Kocer, B. Reliability and Validity of the Timed 360° Turn Test in People with Parkinson’s Disease. Eur. Geriatr. Med. 2020, 11, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Smulders, K.; Cohen, R.G.; Horak, F.B.; Giladi, N.; Nutt, J.G. The Clinical Significance of Freezing While Turning in Parkinson’s Disease. Neuroscience 2017, 343, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Prime, M.; McKay, J.L.; Bay, A.; Hart, A.; Kim, C.; Abraham, A.; Hackney, M.E. Association between Parkinson’s Disease Subtypes and Tests of Physical Function: The 360-Degree Turn Test Is Most Predictive. bioRxiv 2018, 342733. [Google Scholar]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s Disease: Challenges and Opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- King, L.A.; Mancini, M.; Priest, K.; Salarian, A.; Rodrigues-De-Paula, F.; Horak, F. Do Clinical Scales of Balance Reflect Turning Abnormalities in People with Parkinson’s Disease? J. Neurol. Phys. Ther. 2012, 36, 25–31. [Google Scholar] [CrossRef]
- Johnson, L.; James, I.; Rodrigues, J.; Stell, R.; Thickbroom, G.; Mastaglia, F. Clinical and Posturographic Correlates of Falling in Parkinson’s Disease. Mov. Disord. 2013, 28, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Christofoletti, G.; de Andrade, L.P.; Beinotti, F.; Borges, G. Cognition and Dual-Task Performance in Older Adults with Parkinson’s and Alzheimer’s Disease. Int. J. Gen. Med. 2014, 7, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.C.A.; Ansai, J.H.; Andrade, L.P.; Takahashi, A.C.M. The Relationship between Dual-Task and Cognitive Performance among Elderly Participants Who Exercise Regularly. Braz. J. Phys. Ther. 2015, 19, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kelly, V.E.; Johnson, C.O.; Mcgough, E.L.; Horak, F.B.; Chung, K.A.; Espay, A.J.; Devoto, J.; Factor, S.A.; Cholerton, B.; Edwards, K.L.; et al. Association of Cognitive Domains with Postural Instability/Gait Disturbance in Parkinson’s Disease. Park. Relat. Disord. 2016, 21, 692–697. [Google Scholar] [CrossRef]
- Walton, C.C.; O’Callaghan, C.; Hall, J.M.; Gilat, M.; Mowszowski, L.; Naismith, S.L.; Burrell, J.R.; Shine, J.M.; Lewis, S.J.G. Antisaccade Errors Reveal Cognitive Control Deficits in Parkinson’s Disease with Freezing of Gait. J. Neurol. 2015, 262, 2745–2754. [Google Scholar] [CrossRef]
- Krzysztoń, K.; Stolarski, J.; Kochanowski, J. Evaluation of Balance Disorders in Parkinson’s Disease Using Simple Diagnostic Tests-Not So Simple to Choose. Front. Neurol. 2018, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.L.; Jønsson, L.R.; Kristensen, M.T. Introducing a Third Timed Up & Go Test Trial Improves Performances of Hospitalized and Community-Dwelling Older Individuals. J. Geriatr. Phys. Ther. 2017, 40, 121–126. [Google Scholar] [CrossRef] [PubMed]
| Characteristics (Mean ± SD) | Participants (n = 57; 38 Men and 19 Women) |
|---|---|
| Age (years) | 68.67 ± 8.49 |
| BMI (kg/m2) | 26.47 ± 3.52 |
| Education (years) | 10.84 ± 3.68 |
| Disease duration (years) | 6.16 ± 4.05 |
| 6-M retrospective falls (N) | 2.51 ± 2.81 |
| MMSE (0–30) | 26.71 ± 2.46 |
| H&Y (1–5) | 2.21 ± 0.90 |
| MDS-UPDRS-III (0–132) | 32.95 ± 17.75 |
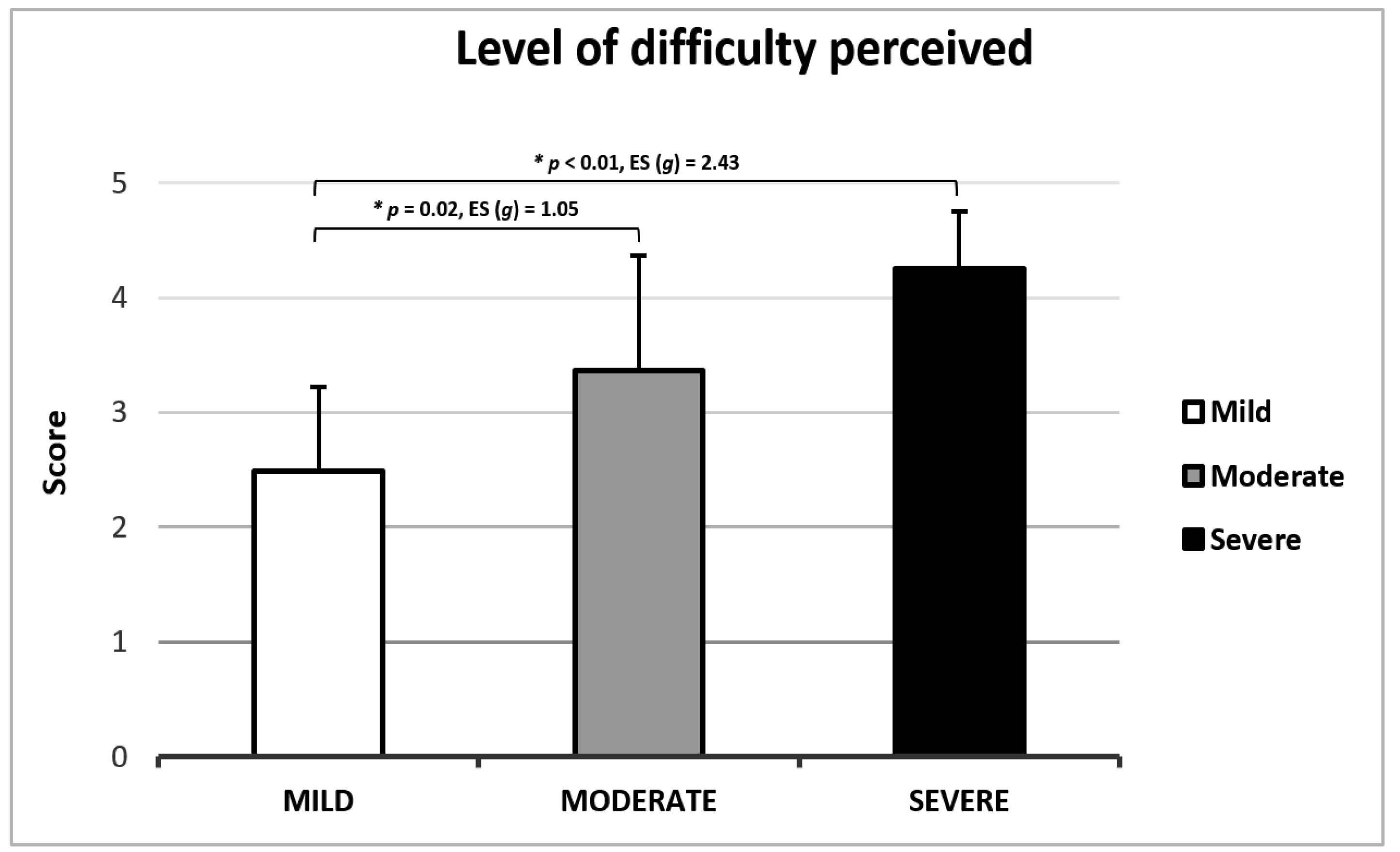
| MATT Score | ||||
|---|---|---|---|---|
| Total Sample (n = 57) | Mild (n = 37) | Moderate (n = 15) | Severe (n = 5) | |
| Total time (s) | 55.11 ± 38.53 | 40.48 ± 11.68 † | 74.57 ± 53.45 | 122.32 ± 47.64 # |
| Segment 1 (s) | 21.41 ± 16.07 | 16.18 ± 5.00 † | 30.07 ± 26.62 | 39.56 ± 12.19 # |
| Segment 2 (s) | 22.23 ± 18.57 | 15.31 ± 4.65 † | 29.93 ± 19.92 | 59.28 ± 39.08 # |
| Segment 3 (s) | 11.46 ± 6.69 | 8.99 ± 2.55 | 14.57 ± 9.36 | 23.48 ± 5.99 # |
| Segment 1 | Segment 2 | Segment 3 | Total Time | |
|---|---|---|---|---|
| Segment 1 | - | 0.93 ** | 0.83 ** | 0.96 ** |
| Segment 2 | - | 0.83 ** | 0.98 ** | |
| Segment 3 | - | 0.90 ** | ||
| Total time | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastia-Amat, S.; Tortosa-Martínez, J.; Pueo, B. Motor Assessment Timed Test (MATT): A New Timed Test to Assess Functional Mobility in Parkinson’s Disease Patients. J. Clin. Med. 2025, 14, 361. https://doi.org/10.3390/jcm14020361
Sebastia-Amat S, Tortosa-Martínez J, Pueo B. Motor Assessment Timed Test (MATT): A New Timed Test to Assess Functional Mobility in Parkinson’s Disease Patients. Journal of Clinical Medicine. 2025; 14(2):361. https://doi.org/10.3390/jcm14020361
Chicago/Turabian StyleSebastia-Amat, Sergio, Juan Tortosa-Martínez, and Basilio Pueo. 2025. "Motor Assessment Timed Test (MATT): A New Timed Test to Assess Functional Mobility in Parkinson’s Disease Patients" Journal of Clinical Medicine 14, no. 2: 361. https://doi.org/10.3390/jcm14020361
APA StyleSebastia-Amat, S., Tortosa-Martínez, J., & Pueo, B. (2025). Motor Assessment Timed Test (MATT): A New Timed Test to Assess Functional Mobility in Parkinson’s Disease Patients. Journal of Clinical Medicine, 14(2), 361. https://doi.org/10.3390/jcm14020361







