CGM-Based Glycemic Metrics Support Estimating Nutritional Risk After Total Pancreatectomy: An Exploratory Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Nutritional Assessments
2.3. Evaluation of Glycemic Management and Glucose Fluctuation by CGM
2.4. Dietary Intake Surveys
2.5. Anthropometric Assessments
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Glycemic Comparison Between Malnutrition-Risk Progression (Group A) and Nutrition-Maintaining (Group B)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2021 [published correction appears in Diabetes Care. Diabetes Care 2021, 44, 2182, Erratum in Diabetes Care 2021, 44 (Suppl. S1), S15–S33. https://doi.org/10.2337/dc21-S002. [Google Scholar] [CrossRef]
- Nakamura, T.; Kikuchi, H.; Takebe, K.; Ishii, M.; Imamura, K.-I.; Yamada, N.; Kudoh, K.; Terada, A. Correlation between bile acid malabsorption and pancreatic exocrine dysfunction in patients with chronic pancreatitis. Pancreas 1994, 9, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J. Gastroenterol. 2013, 19, 7258–7266. [Google Scholar] [CrossRef] [PubMed]
- Dresler, C.M.; Fortner, J.G.; McDermott, K.; Bajorunas, D.R. Metabolic consequences of (regional) total pancreatectomy. Ann. Surg. 1991, 214, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Otsuki, M.; Igarashi, H.; Kihara, Y.; Kawabe, K.; Nakamura, T.; Fujimori, N.; Oono, T.; Takayanagi, R.; Shimosegawa, T. Epidemiological study of pancreatic diabetes in Japan in 2005: A nationwide study. Pancreas 2010, 39, 829–835. [Google Scholar] [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese clinical practice guideline for diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef]
- Thomas, M.G.; Avari, P.; Godsland, I.F.; Lett, A.M.; Reddy, M.; Oliver, N. Optimizing type 1 diabetes after multiple daily injections and capillary blood monitoring: Pump or sensor first? A meta-analysis using pooled differences in outcome measures. Diabetes Obes. Metab. 2021, 23, 2521–2528. [Google Scholar] [CrossRef]
- Lucidi, P.; Porcellati, F.; Bolli, G.B.; Fanelli, C.G. Prevention and management of severe hypoglycemia and hypoglycemia unawareness: Incorporating sensor technology. Curr. Diab Rep. 2018, 18, 83. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.F.; Javed, F.; Pratap, B.; Musat, D.; Nader, A.; Pulimi, S.; Alivar, C.L.; Herzog, E.; Kukin, M.L. Malnutrition as assessed by nutritional risk index is associated with worse outcome in patients admitted with acute decompensated heart failure: An ACAP-HF data analysis. Heart Int. 2011, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Kato, M.; Sugihara, S.; Hirai, M.; Yamada, K.; Yanagihara, K.; Yamamoto, K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ, J. 2013, 77, 705–711. [Google Scholar] [CrossRef]
- Shen, X.; Yang, L.; Gu, X.; Liu, Y.-Y.; Jiang, L. Geriatric Nutrition Risk Index as a Predictor of Cardiovascular and All-Cause Mortality in Older Americans with Diabetes. Diabetol. Metab. Syndr. 2023, 15, 89. [Google Scholar] [CrossRef]
- Sakamoto, A.; Funamizu, N.; Shine, M.; Uraoka, M.; Nagaoka, T.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Takada, Y. Geriatric Nutritional Risk Index Predicts Tolerability of S-1 as Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Pancreas 2023, 52, e196–e202. [Google Scholar]
- Higashi, T.; Murase, K.; Yokoi, R.; Kuno, M.; Fukada, M.; Tajima, J.Y.; Kiyama, S.; Tanaka, Y.; Okumura, N.; Matsuhashi, N. Association of Pre-Operative Geriatric Nutritional Risk Index with Complete Adjuvant Chemotherapy and Prognosis Post-Pancreatectomy. Anticancer. Res. 2024, 44, 427–434. [Google Scholar] [CrossRef]
- Liu, G.; Zou, C.; Jie, Y.; Wang, P.; Wang, X.; Fan, Y. Predictive Value of Geriatric Nutritional Risk Index in Patients with Lower Extremity Peripheral Artery Disease: A Meta-Analysis. Front. Nutr. 2022, 9, 903293. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Zhang, T.; Guo, J.; Liao, Q.; Song, S.; Duo, Y.; Gao, Y.; Yuan, T.; Zhao, W. Diabetes Management in Patients Undergoing Total Pancreatectomy: A Single Center Cohort Study. Front. Endocrinol. 2023, 14, 1097139. [Google Scholar] [CrossRef]
- Shi, H.J.; Jin, C.; Fu, D.L. Impact of postoperative glycemic control and nutritional status on clinical outcomes after total pancreatectomy. World J. Gastroenterol. 2017, 23, 265–274. [Google Scholar] [CrossRef]
- Hiromine, Y.; Noso, S.; Rakugi, H.; Sugimoto, K.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Osawa, H.; Tabara, Y.; et al. Poor glycemic control rather than types of diabetes is a risk factor for sarcopenia in diabetes mellitus: The MUSCLES-DM study. J. Diabetes Investig. 2022, 13, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Swiercz, A.; Pludowski, P.; Jaworski, M.; Szalecki, M. Skeletal status, body composition, and glycaemic control in adolescents with type 1 diabetes mellitus. J. Diabetes Res. 2018, 2018, 8121634. [Google Scholar] [CrossRef]
- Sugimoto, K.; Tabara, Y.; Ikegami, H.; Takata, Y.; Kamide, K.; Ikezoe, T.; Kiyoshige, E.; Makutani, Y.; Onuma, H.; Gondo, Y.; et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The Multicenter Study for Clarifying Evidence for Sarcopenia in Patients with Diabetes Mellitus. J. Diabetes Investig. 2019, 10, 1471–1479. [Google Scholar] [CrossRef]
- Tabara, Y.; Ikezoe, T.; Yamanaka, M.; Setoh, K.; Segawa, H.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Ichihashi, N.; Tsuboyama, T.; et al. Advanced glycation end product accumulation is associated with low skeletal muscle mass, weak muscle strength, and reduced bone density: The Nagahama Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1446–1453. [Google Scholar] [CrossRef]
- Tam, C.S.; Chaudhuri, R.; Hutchison, A.T.; Samocha-Bonet, D.; Heilbronn, L.K. Skeletal muscle extracellular matrix remodeling after short-term overfeeding in healthy humans. Metabolism. 2017, 67, 26–30. [Google Scholar] [CrossRef]
- Juel, C.T.B.; Dejgaard, T.F.; Hansen, C.P.; Storkholm, J.H.; Vilsbøll, T.; Lund, A.; Knop, F.K. Glycemic control and variability of diabetes secondary to total pancreatectomy assessed by continuous glucose monitoring. J. Clin. Endocrinol. Metab. 2021, 106, 168–173, Erratum in J. Clin. Endocrinol. Metab. 2021, 106, e2854. https://doi.org/10.1210/clinem/dgab285. [Google Scholar] [CrossRef] [PubMed]
- ISCHIA Study Group. Prevention of hypoglycemia by intermittent-scanning continuous glucose monitoring device combined with structured education in patients with type 1 diabetes mellitus: A randomized, crossover trial. Diabetes Res. Clin. Pract. 2023, 195, 110147. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 14) | Group A (n = 4) | Group B (n = 10) | p-Value | |
|---|---|---|---|---|
| Gender (male/female) | 4/10 | 1/3 | 2/8 | 1.000 |
| Age (years) | 70.0 (61.0–76.0) | 68.0 (57.8–73.0) | 70.5 (59.0–76.3) | 0.571 |
| Primary diagnosis (PDAC/IPMC/PanNET/others) | 6/5/2/1 | 1/2/0/1 | 5/3/2/0 | 0.171 |
| Year of surgery | 2017 (2014–2021) | 2017 (2015–2020) | 2017 (2014–2021) | 0.943 |
| Time since pancreatectomy (months) | 6.0 (1.0–29.0) | 1.0 (1.0–11.5) | 8.0 (3.3–42.5) | 0.198 |
| Adjunct antidiabetic therapies, n (%) | ||||
| None | 9 (64.3) | 0 | 0 | 0.777 |
| DPP-4 inhibitor | 2 (14.3) | 0 | 2 (20) | 0.225 |
| GLP-1 receptor agonist | 1 (7.1) | 1 (25) | 0 | 0.100 |
| Sulfonylurea | 1 (7.1) | 0 | 1 (10) | 0.402 |
| Glinide | 2 (14.3) | 1 (25) | 0 | 0.100 |
| Insulin dose | ||||
| Insulin regimen (MDI/pump) | 13/1 | 4/0 | 9/1 | - |
| Total insulin dose (U/day) | 26.0 (20.0–30.3) | 19.5 (15.1–28.0) | 27.5 (23.4–31.8) | 0.157 |
| Insulin Basal/Total (%) | 22.2 (14.2–28.8) | 17.9 (14.8–22.9) | 26.4 (13.9–30.1) | 0.289 |
| Digestive enzyme supplementation | ||||
| Pancrelipase (mg/day) | 1800 (1800–2250) | 1800 (1800–2137.5) | 1800 (1800–2250) | 1.000 |
| Pancrelipase (mg/kg IBW) | 40.0 (32.7–46.2) | 46.8 (33.9–54.1) | 38.4 (32.7–43.5) | 0.289 |
| Number (%) of other digestive enzyme concomitant users | 35.7 (5/14) | 25% (1/4) | 40% (4/10) | 0.728 |
| Adherence * | No documented non-adherence in medical records | - | - | - |
| Steatorrhea | No patients reported steatorrhea during the observation period | - | - | - |
| Metabolic control | ||||
| HbA1c (%) | 7.3 (6.6–7.4) | 8.5 (6.3–9.2) | 7.0 (6.7–7.3) | 0.177 |
| Average glucose (mg/dL) | 166.3 (152.7–194.1) | 213.2 (184.4–239.9) | 159.4 (147.2–168.7) | 0.013 |
| Evaluation of glycemic management | ||||
| CGM device (rtCGM/ isCGM) | 1/13 | 0/4 | 1/9 | 0.512 |
| Duration of CGM use | 10.0 (6.8–16.5) | 10.0 (7.5–13.3) | 11.0 (5.5–26.5) | 0.943 |
| GMI (%) | 7.3 (7.0–8.0) | 8.4 (7.7–9.1) | 7.1 (6.8–7.3) | 0.013 |
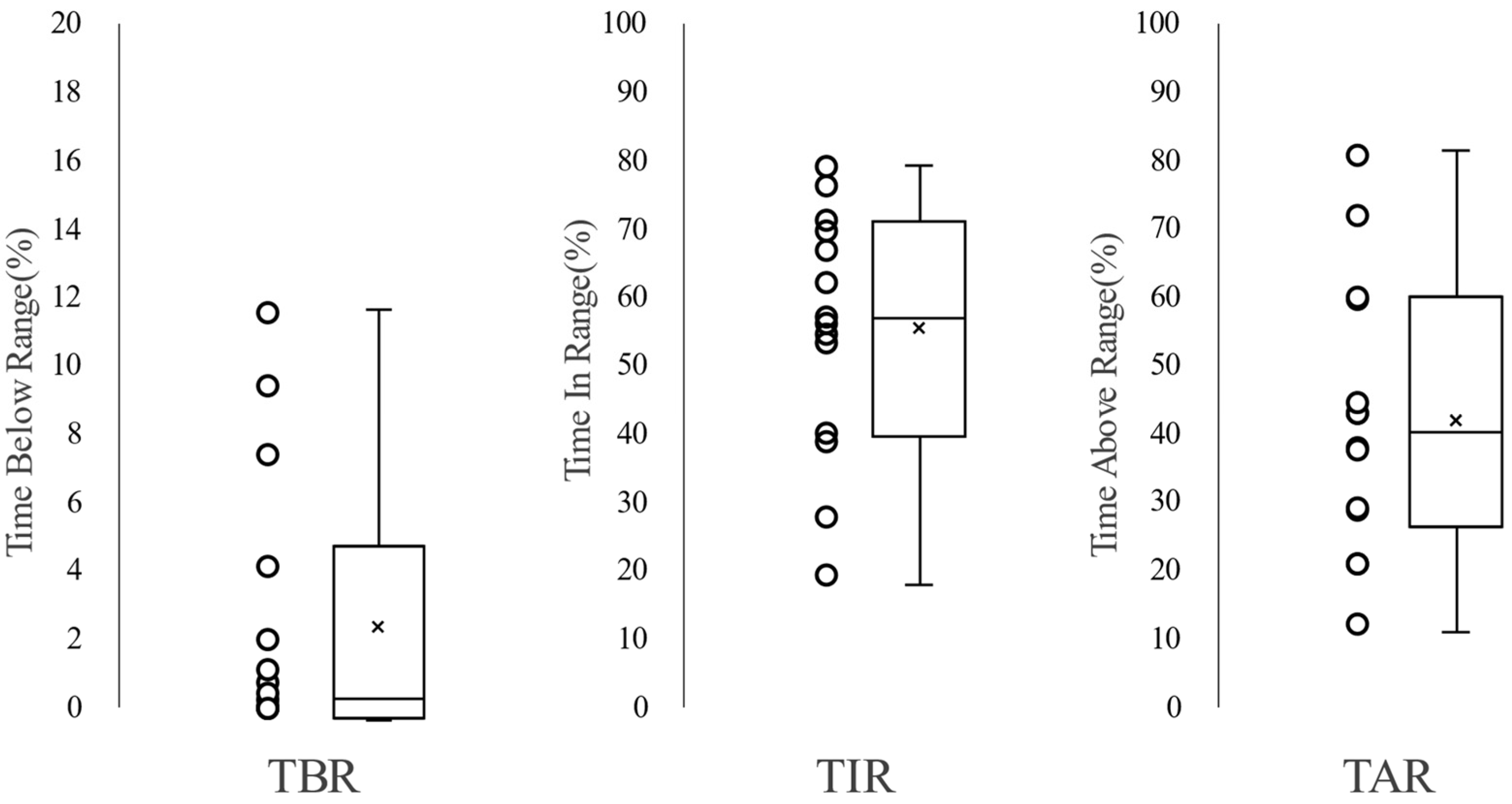
| Time In Range (%) | 63.4 (40.0–71.3) | 34.0 (21.4–58.0) | 65.6 (60.2–76.7) | 0.040 |
| Time Above Range (%) | 36.2 (21.7–59.7) | 65.7 (40.9–78.4) | 32.4 (20.7–37.8) | 0.048 |
| Time Below Range (%) | 0.41 (0.04–4.95) | 0.25 (0.01–1.62) | 0.74 (0.16–0.74) | 0.567 |
| Percentage of TIR target achieved (>50%) | 10/14 (71%) | 1/4 (25%) | 9/10 (90%) | 0.023 |
| Percentage of TBR target achieved (<1%) | 9/14 (64%) | 3/4 (75%) | 6/10 (60%) | 0.728 |
| Percentage of TAR target achieved (<50%) | 10/14 (71%) | 1/4 (25%) | 9/10 (90%) | 0.023 |
| Achievement of 3 targets | 5/14 (36%) | 0/4 (0%) | 5/10 (50%) | 0.112 |
| Achievement of 3 targets+HbA1c < 7.0 | 3/14 (21%) | 0/4 (0%) | 3/10 (30%) | 0.414 |
| Achievement of 3 targets+HbA1c < 7.5 | 5/14 (36%) | 0/4 (0%) | 5/10 (50%) | 0.112 |
| Dietary intake | ||||
| Energy (kcal/kg IBW) | 34.6 (29.2–39.6) | 33.8 (24.0–46.3) | 34.6 (29.2–39.6) | 1.000 |
| Carbohydrate (g/day) (12 cases) | 283.3 (250.3–315.3) | 316.0 (276.0–332.0) | 280.7 (214.1–300.2) | 0.267 |
| Protein (g/kg IBW) (12 cases) | 1.4 (1.0–1.7) | 1.39 (1.38–1.84) | 1.36 (0.92–1.86) | 0.579 |
| Fat (g/day) (12 cases) | 53.5 (47.3–67.6) | 56.0 (47.0–87.0) | 51.0 (40.5–65.2) | 0.853 |
| Anthropometric assessment | ||||
| Weight (kg) | 45.8 (40.0–56.5) | 39.8 (38.0–55.0) | 47.2 (43.5–56.5) | 0.288 |
| BMI (kg/m2) | 19.5 (16.9–21.1) | 17.1 (15.9–22.9) | 20.1 (18.0–22.9) | 0.229 |
| Body fat percentage (%) (10 cases) | 18.2 (13.3–23.5) | 11.5 (8.0–15.0) | 19.5 (16.1–27.5) | 0.090 |
| Lean body weight (kg) (10 cases) | 37.45 (31.5–50.5) | 33.3 (32.2–34.3) | 38.1 (35.2–47.7) | 0.188 |
| SMI (kg/m2) (8 cases) | 5.9 (5.4–6.8) | 5.2 (4.9–5.4) | 6.6 (5.7–7.1) | 0.094 |
| Nutritional assessment | ||||
| Alb (g/dL) | 3.9 (3.6–4.1) | 3.6 (3.2–3.9) | 4.0 (3.6–4.1) | 0.086 |
| T-Cho (mg/dL) | 173.5 (160.3–200.0) | 184.0 (113.0–210.8) | 168.5 (160.3–200.0) | 0.777 |
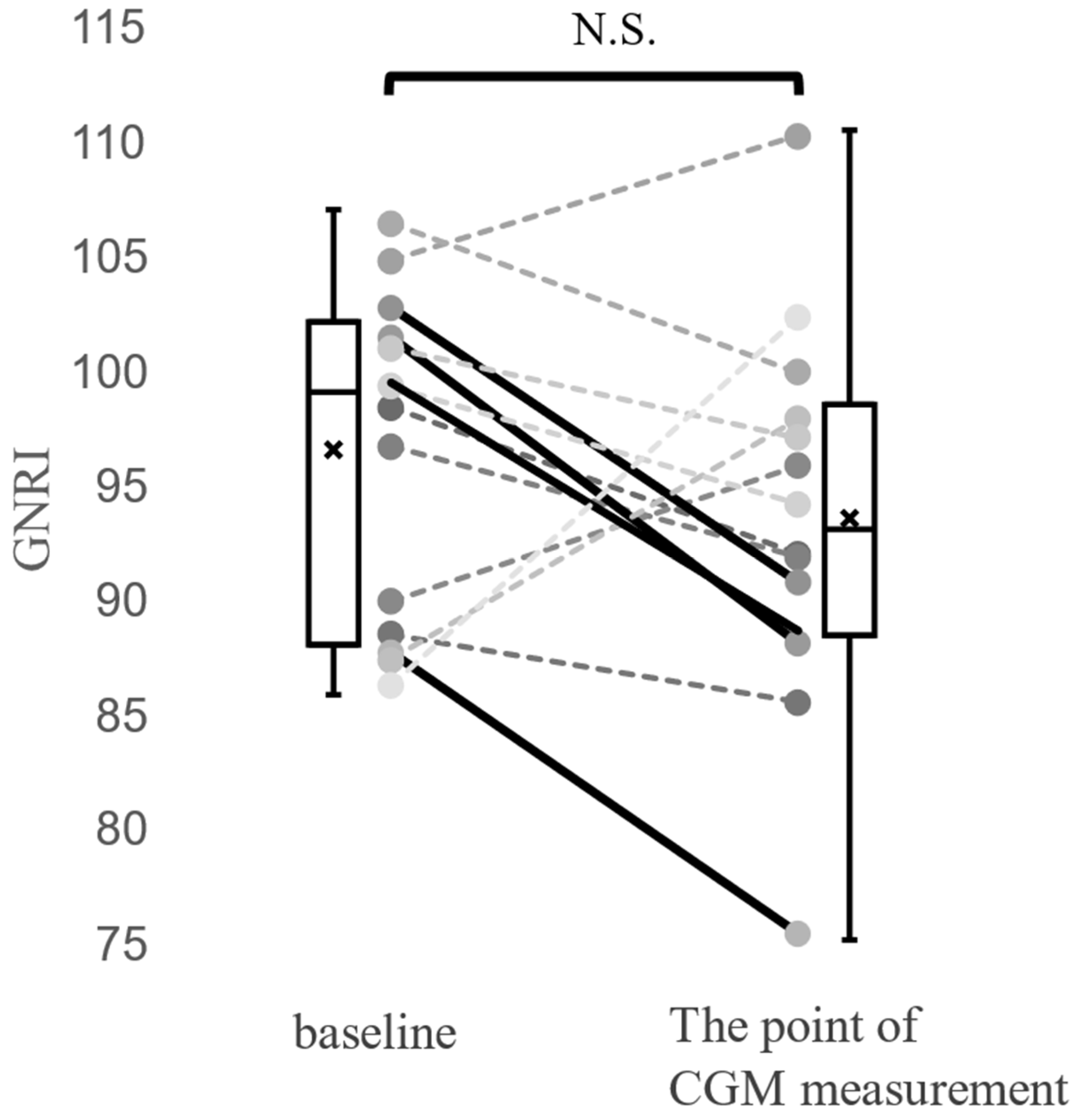
| GNRI | 92.5 (88.2–97.7) | 88.0 (78.5–90.1) | 96.5 (90.8–99.6) | 0.020 |
| Variable | OR | 95% CI (Lower–Upper) | p-Value |
|---|---|---|---|
| TIR (per 10% increase) | 0.34 | 0.15–0.76 | 0.008 |
| TAR (per 10% increase) | 2.88 | 1.28–11.68 | 0.006 |
| Mean glucose (per 10 mg/dL) | 2.22 | 1.22–8.43 | 0.003 |
| HbA1c (per 1% increase) | 2.64 | 0.68–19.3 | 0.165 |
| Time since pancreatectomy (per year) | 0.92 | 0.71–1.01 | 0.108 |
| Primary diagnosis (PDAC vs. non-PDAC) | 0.33 | 0.01–3.72 | 0.383 |
| Pancrelipase dose (per 10 mg/kg) | 1.89 | 0.51–8.69 | 0.334 |
| Variables Included | OR for TIR (per 10% Increase) | 95% CI (Lower–Upper) | p-Value (TIR) | OR for Covariate | 95% CI (Lower–Upper) | p-Value (Covariate) |
|---|---|---|---|---|---|---|
| TIR only | 0.34 | 0.15–0.76 | 0.008 | – | – | – |
| TIR + Age | 0.33 | 0.07–0.77 | 0.007 | 1.03 | 0.91–1.26 | 0.645 |
| TIR + Time since pancreatectomy | 0.35 | 0.07–0.82 | 0.011 | 0.88 | 0.56–1.02 | 0.161 |
| TIR + Primary diagnosis (PDAC vs. non-PDAC) | 0.25 | 0.012–0.75 | 0.006 | 0.11 | 0.0001–4.29 | 0.263 |
| TIR + Insulin regimen (pump/automated vs. MDI) | 0.35 | 0.085–0.81 | 0.01 | 0.00 (unstable) | <0.001–68.5 | 0.600 |
| TIR + Pancrelipase dose (per 10 mg/kg) | 0.07 | 8.9 × 10−6–0.55 | 0.001 | 51.4 | 1.25–2.7 × 107 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, R.; Yanagimachi, M.; Mitsuhashi, K.; Yamaichi, M.; Onodera, W.; Matsumoto, A.; Sato, E.; Tando, Y.; Fujita, Y. CGM-Based Glycemic Metrics Support Estimating Nutritional Risk After Total Pancreatectomy: An Exploratory Retrospective Study. J. Clin. Med. 2025, 14, 7124. https://doi.org/10.3390/jcm14197124
Nakamura R, Yanagimachi M, Mitsuhashi K, Yamaichi M, Onodera W, Matsumoto A, Sato E, Tando Y, Fujita Y. CGM-Based Glycemic Metrics Support Estimating Nutritional Risk After Total Pancreatectomy: An Exploratory Retrospective Study. Journal of Clinical Medicine. 2025; 14(19):7124. https://doi.org/10.3390/jcm14197124
Chicago/Turabian StyleNakamura, Ryoma, Miyuki Yanagimachi, Kento Mitsuhashi, Masato Yamaichi, Wataru Onodera, Atsufumi Matsumoto, Eri Sato, Yusuke Tando, and Yukihiro Fujita. 2025. "CGM-Based Glycemic Metrics Support Estimating Nutritional Risk After Total Pancreatectomy: An Exploratory Retrospective Study" Journal of Clinical Medicine 14, no. 19: 7124. https://doi.org/10.3390/jcm14197124
APA StyleNakamura, R., Yanagimachi, M., Mitsuhashi, K., Yamaichi, M., Onodera, W., Matsumoto, A., Sato, E., Tando, Y., & Fujita, Y. (2025). CGM-Based Glycemic Metrics Support Estimating Nutritional Risk After Total Pancreatectomy: An Exploratory Retrospective Study. Journal of Clinical Medicine, 14(19), 7124. https://doi.org/10.3390/jcm14197124






