Beyond Racial Categorization in Sports Cardiology: A Systematic Review of Cardiac Adaptations in Athletes
Abstract
1. Introduction
2. Materials and Methods
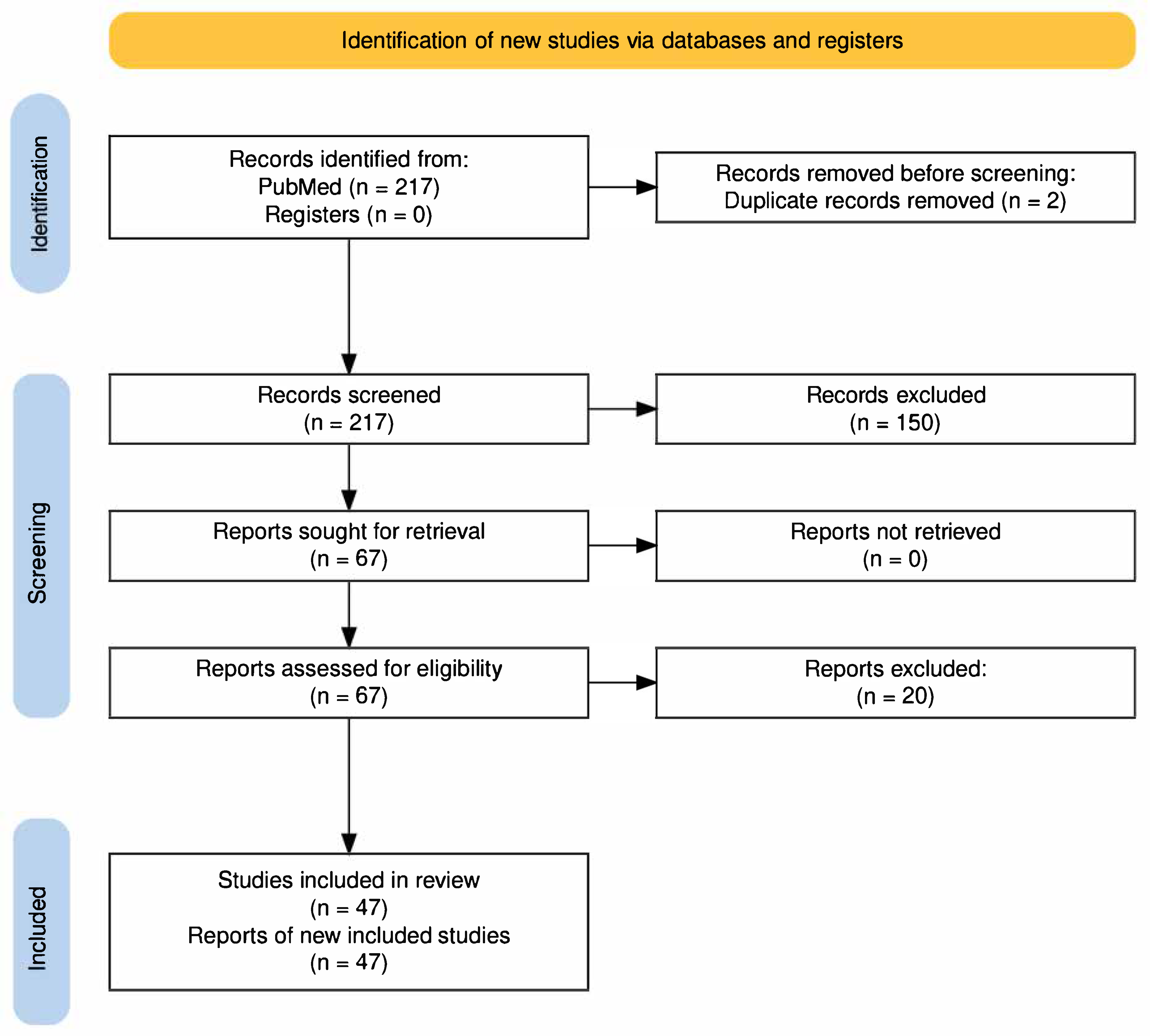
2.1. Search Strategy and Study Selection
2.2. Data Synthesis
2.3. Assessment of Study Quality and Evidence Certainty
3. Results
| Study (Author & Number) | Year | Design | Population N | (Black) | Sport(s) | Geographic Origin | Population Key | Comparator(s)/Purpose |
|---|---|---|---|---|---|---|---|---|
| ECG Studies | ||||||||
| Sokunbi et al. [1] | 2021 | Cross-sectional observational comparative study | 360 | 360 | Multiple | Nigeria | Adolescent athletes and controls | Prevalence/distribution of ECG patterns; athletes vs. controls; training vs. non-training findings |
| Sheikh et al. [2] | 2014 | Retrospective cross-sectional diagnostic accuracy | 5505 | 1208 | Mixed | UK | Black/White athletes, HCM | ESC vs. Seattle vs. refined ECG criteria |
| Conway et al. [3] | 2022 | Retrospective cross-sectional diagnostic accuracy | 1686 | 123 | Mixed | USA | NCAA Division I athletes | Seattle vs. Refined vs. International ECG criteria |
| Lander et al. [4] | 2024 | Cross-sectional observational | 173 | 129 | Basketball | USA | Elite female professional basketball athletes (WNBA) | Reference ECG range/prevalence of findings |
| Malhotra et al. [5] | 2020 | Retrospective cross-sectional diagnostic accuracy | 11,168 | 1005 | Soccer | UK | Adolescent soccer players (white/black) | ESC vs. Seattle vs. refined vs. international ECG criteria |
| McClean et al. [6] | 2019 | Retrospective cross-sectional diagnostic accuracy | 1304 | 428 | Mixed | UK/Qatar | Arab/black male pediatric athletes (11–18 years) | 2010 ESC vs. Seattle vs. international ECG criteria |
| Zorzi et al. [7] | 2022 | Cross-sectional observational comparative study | 2229 | 1115 | Multiple | Italy | Young competitive athletes | Prevalence/clinical significance of isolated low QRS voltage |
| Miragoli et al. [8] | 2019 | Retrospective cross-sectional observational | 414 | 69 | Mixed | Italy | Non-professional adolescent athletes | Prevalence/correlates of early repolarization pattern |
| Junttila et al. [9] | 2011 | Cross-sectional observational study | 503 | 151 | Multiple | USA | Young collegiate athletes | Prevalence/characteristics of inferolateral early repolarization; association with LVH, gender |
| Papadakis et al. [10] | 2011 | Prospective longitudinal observational cohort | 2894 | 904 | Mixed | UK | Black/White athletes, controls, HCM patients | ECG patterns/incidence of HCM by group |
| Ferrari et al. [11] | 2024 | Multicentre retrospective cross-sectional observational | 6125 | 1625 | Soccer | Brazil | Male Brazilian football players | Prevalence/correlates of abnormal ECG/imaging findings |
| Pambo et al. [12] | 2021 | Cross-sectional observational | 159 | 159 | Soccer | Ghana | Male competitive athletes | Cardiac findings by geographic/ethnic subgroup |
| Pambo et al. [13] | 2021 | Cross-sectional observational | 75 | 75 | Soccer | Ghana | Female competitive athletes | Cardiac findings by geographic/ethnic subgroup |
| Riding et al. [14] | 2019 | Cross-sectional observational comparative | 1698 | 1019 | Mixed | Multi-regional | Adolescent athletes and healthy controls | Prevalence and significance of T-wave inversions |
| Muramoto et al. [15] | 2014 | Retrospective cross-sectional observational comparative | 1114 | 71 | Multiple | USA | Varsity athletes | Prevalence, pattern, and prognostic significance of J-wave/early repolarization by group, race, and sex |
| Papadakis et al. [16] | 2009 | Cross-sectional observational comparative | 2110 | 65 | Mixed | UK | Healthy athletes, HCM patients, ARVC patients | ECG repolarization markers for distinguishing physiological vs. pathological anterior T-wave inversion |
| Calore et al. [17] | 2016 | Cross-sectional observational comparative | 233 | 53 | Mixed | Italy | Pre-adolescent athletes undergoing ECG screening | Prevalence and natural history of T-wave inversion subtypes |
| D’Ascenzi et al. [18] | 2019 | Prospective longitudinal observational cohort | 2227 | 0 | Mixed | Italy | Healthy athletes vs. ARVC patients, matched for age, sex, ethnicity | ECG markers for distinguishing athlete’s heart from ARVC |
| McClean et al. [6] | 2019 | Cross-sectional comparative diagnostic accuracy | 732 | 314 | Mixed | UK/Qatar | Arab and black male pediatric athletes (11–18 years) | Diagnostic accuracy: international vs. refined ECG recommendations for ATWI |
| Brosnan et al. [19] | 2018 | Matched cross-sectional comparative observational | 200 | 3 | Mixed | Multi-national | Mixed-race, Black, and White adolescent male soccer players | Cardiac electrical/structural adaptation by race/ethnicity |
| Jacob et al. [20] | 2015 | Prospective cross-sectional observational study | 1755 | 352 | Multiple | USA | Collegiate athletes | Prevalence and significance of isolated T wave inversion |
| Malhotra et al. [21] | 2021 | Cross-sectional observational comparative | 3000 | 1000 | Soccer | UK | Elite male American football players (NFL Combine) | ECG abnormalities by race and player position |
| Wilson et al. [22] | 2012 | Cross-sectional observational comparative study | 1220 | 300 | Multiple | Qatar/West Asia | National-level male athletes | ECG abnormalities |
| Bryde et al. [23] | 2025 | Cross-sectional observational comparative study | 706 | 85 | Soccer | USA | MLS professional athletes | ECG findings by ethnicity; targeted echo follow-up for abnormalities |
| Magalski et al. [24] | 2008 | Cross-sectional observational comparative | 1959 | 1321 | American Football | USA | Competitive collegiate athletes | Incremental value of ECG and echocardiography for preparticipation screening |
| Magalski et al. [25] | 2011 | Prospective cross-sectional observational | 964 | 188 | Mixed | USA | Professional male athletes (various sports, ethnicities) | ECG findings by sport and ethnicity |
| Raman & Vyselaar [26] | 2022 | Cross-sectional observational comparative | 753 | 285 | Mixed | Canada | Male African American basketball players/youth athletes | Cardiac screening findings by athlete group |
| Crouse et al. [27] | 2009 | Cross-sectional observational comparative study | 77 | 54 | American football | USA | NCAA Division I football athletes | Prevalence and types of ECG abnormalities by race |
| Grace et al. [28] | 2015 | Cross-sectional observational comparative study | 45 | 45 | Boxing, Body building | South Africa | University students of Zulu descent | ECG patterns: endurance vs. resistance vs. controls; prevalence of LVH & repolarization changes |
| Rambarat et al. [29] | 2020 | Multicenter retrospective cohort study | 329 | 64 | NCAA Division I (multiple) | USA | Collegiate female athletes | Preparticipation cardiac screening: sport, race differences in ECG/echo parameters |
| Corsi et al. [30] | 2025 | Retrospective cross-sectional observational comparative | 8303 | 200 | Basketball | USA | American collegiate football players | Cardiac remodeling: pre- vs. post-training echocardiography |
| Echocardiographic Studies | ||||||||
| Hamburger et al. [31] | 2023 | Prospective longitudinal observational cohort | 85 | 52 | American Football | USA | Collegiate athletes | Utility of echocardiography as primary screening |
| Engel et al. [32] | 2016 | Cross-sectional observational study | 526 | 406 | Basketball | USA | NBA players | Cardiac structure and function by race/anthropometry |
| Basavarajaiah et al. [33] | 2008 | Cross-sectional observational comparative | 900 | 450 | Mixed | UK | Black athletes (by region), comparator non-Black | Cardiac electrical/structural patterns by geographic/ethnic origin |
| Gjerdalen et al. [34] | 2014 | Cross-sectional observational comparative study | 553 | 49 | Soccer | Norway/Scandinavia | Male professional football players | Cardiac chamber remodeling by ethnicity; BSA-indexed LV/RV measurements |
| Pelà et al. [35] | 2015 | Cross-sectional observational comparative | 138 | 41 | Soccer | Italy | Amateur footballers (West-African Black/Italian White) | LV structural remodeling by ethnicity |
| Tso et al. [36] | 2022 | Prospective longitudinal observational cohort | 249 | 124 | American Football | USA | Collegiate football athletes (Black/White) | Association of race and position with acquired concentric LVH over time |
| Moneghetti et al. [37] | 2019 | Cross-sectional observational comparative study | 230 | 98 | American football | USA | NCAA Division I ASF players | Race differences in LV remodeling (mass-to-volume, sphericity, strain, etc.) |
| Di Paolo et al. [38] | 2012 | Cross-sectional observational comparative | 216 | 154 | Mixed | Sub-Saharan Africa | Adolescent African/Italian soccer players | ECG and echocardiographic findings by ethnicity and country |
| Crouse et al. [39] | 2016 | Cross-sectional observational comparative | 80 | 36 | American Football | USA | Collegiate ASF athletes | Echo/BP characteristics vs. reference and by ethnicity |
| Dzudie et al. [40] | 2007 | Cross-sectional observational comparative | 21 | 21 | Handball | Cameroon | Elite handball players/controls | Cardiac structure/function by athletic status |
| Kervio et al. [41] | 2013 | Cross-sectional observational comparative | 282 | 96 | Soccer | Multi-national | Japanese, African-Caribbean, Caucasian soccer players | ECG/echo characteristics by ethnicity |
| Cho et al. [42] | 2019 | Cross-sectional observational comparative study | 1185 | 140 | Multiple | International (South Korea) | University athletes (Universiade) | Incidence and predictors of abnormal LV geometry by race, sport type, and training time |
| Edenfield et al. [43] | 2019 | Retrospective cross-sectional observational comparative | 375 | 218 | American Football | USA | Collegiate football players | ARD by position, race, BSA; generation of BSA-specific ARD norms |
| Augustine et al. [44] | 2024 | Cross-sectional observational comparative | 1087 | 163 | Soccer | UK | Adolescent academy footballers (White/Black/Mixed-race) | RV dimension and ECG features by ethnicity; prevalence/overlap with ARVC criteria |
| Zaidi et al. [45] | 2013 | Cross-sectional observational comparative | 675 | 297 | Mixed | UK | Black/White athletes, sedentary controls | RV structure/function and ECG findings by ethnicity and activity |
| ECG + Electrocardiogram Studies | ||||||||
| Waase et al. [46] | 2018 | Coress-sectional observational comparative study | 519 | 409 | Basketball | USA | NBA athletes | Generate normative ECG data for elite professional athletes/Assess athlete ECG interpretation criteria |
| Sheikh et al. [47] | 2013 | Cross-sectional observational comparative study | 329 | 329 | Multiple | UK | Adolescent Black athletes | LVH and ECG repolarization changes |
| Schmied et al. [48] | 2009 | Cross-sectional observational screening study | 155 | 155 | Soccer | Algeria | U-17 football players | Precompetition cardiac screening: prevalence & ethnic variation in ECG/echo findings; risk factors for SCD |
| Uberoi et al. [49] | 2013 | Cross-sectional observational comparative study | 85 | Not specified | American football | USA | NCAA football players | Cardiac dimensions and ECG/echo remodeling by lineup position and race |
| Haddad et al. [50] | 2013 | Cross-sectional observational comparative study | 112 | 38 | American football | USA | NCAA Division I football players | Race differences in ventricular mass/volume ratio, function, and ECG |
| Demola et al. [51] | 2019 | Cross-sectional observational comparative study | 90 | 30 | Multiple | Italy | Early adolescent athletes | Ethnicity-related differences in hemodynamic and ECG adaptation to exercise; relation to LV remodeling |
| Di Gioia et al. [52] | 2024 | Prospective longitudinal observational cohort study (cross-sectional & comparative analyses) | 1492 | 57 | Multiple sports (including endurance) | Italy | Olympic elite athletes | Prevalence, morphology, and prognosis of LVTs; comparisons by sex, race/ethnicity, and sport type |
| Reviews | ||||||||
| Davis et al. [53] | 2022 | Systematic Review | 51 studies; 65,629 individuals | Variable | Mixed | Multi-national | Athletes | Ethnic differences in athlete ECGs, focus on T-wave inversion and race/ancestry impact on ECG interpretation |
| McClean et al. [54] | 2018 | Meta-analysis | 43 studies; 16,396 individuals | Variable | Mixed | Multi-national | Pediatric Athletes | Impact of age, race, and sex on electrical and structural cardiac remodeling in pediatric athletes |
| Pambo & Scharhag [55] | 2021 | Systematic Review | 16 studies; 5632 individuals | Variable | Mixed | Multi-national | Black African and Afro-Caribbean athletes | ECG/ECHO findings in Black athletes; prevalence and characteristics of repolarization and hypertrophy patterns |
| Christou et al. [56] | 2020 | Systematic Review | 58 studies; 7221 individuals | Variable | Multiple | Multi-national | Athletes | Impact of demographic, anthropometric, and athletic factors on left atrial size in athletes |
3.1. Study Populations and Participant Characteristics
3.2. Electrocardiogram Findings in Black Athletes
The Black Athlete Repolarization Variant
| ECG Finding | IC Classification | In Black Athletes | In Athletes of Other Ethnicities |
|---|---|---|---|
| Sinus bradycardia | Normal | Mixed findings in prevalence vs. White athletes [5,10,21] | Mixed findings in White athletes vs. Black athletes; more prevalent in mixed-race athletes than both Black and White athletes [5,10,21] |
| Incomplete RBBB | Normal | Increased prevalence vs. White athletes [10] | Less prevalent in White athletes [10] |
| Complete RBBB | Borderline | Decreased prevalence vs. White athletes [10] | More prevalent in White athletes [10] |
| Voltage criteria for LVH or RVH | Normal | LVH: less prevalent in soccer players; more prevalent in football players and athletes of African-American/Caribbean, Middle African, and West African descent [5,14,21,24,25,26]; RVH: increased prevalence vs. White athletes, more pronounced in Middle Africans [5,10,14,21] | LVH: more prevalent in White and mixed-race soccer players; less prevalent in White football players [5,21,24,25,26]; RVH: less prevalent in White athletes, comparable in mixed-race athletes [5,10,21] |
| Voltage criteria for LAE or RAE | Borderline | Increased prevalence of left atrial enlargement (LAE) and right atrial enlargement (RAE) vs. White athletes [5,10,21,22] | Less prevalent in White athletes; comparable in mixed-race athletes vs. Black athletes [5,21] |
| Right axis deviation | Borderline | Decreased prevalence vs. White athletes [10] | More prevalent in White athletes [10] |
| 1º AV block | Normal | Increased prevalence vs. White athletes [5,10] | Less prevalent in White athletes [5] |
| ER/STE | Normal | Black athlete repolarization variant: convex STE followed by TWI in V1-V4, more prevalent in Middle Africans [4,10,14,57,59,61]; Increased prevalence of ER vs. White athletes [8,15,26]; Increased prevalence of STE, including ascending convex and ascending concave, vs. White athletes [10,21,49]; Increased prevalence of nonspecific ST changes vs. athletes of other ethnicities [26,30] | Decreased prevalence of ER in White athletes [8,26]; Decreased prevalence of all STE in White and mixed-race athletes; comparable ascending concave STE in Black and mixed-race athletes [10,21]; Decreased prevalence of nonspecific ST changes in non-Black athletes [26,30] |
| TWI | Abnormal (except Black athlete repolarization variant and juvenile T-wave pattern) | Black athlete repolarization variant: benign TWI following convex STE in V1-V4, more pronounced in Middle and West Africans [2,4,10,11,14,49,59,61,64]; Increased prevalence of abnormal TWI in inferior/lateral leads vs. White and/or mixed-race athletes; most pronounced in Middle and West Africans [5,10,11,14,21,22,24,30] | Less prevalent in non-Black athletes, including those identifying as White and mixed-race [5,10,11,21,30] |
| ST segment depression | Abnormal | Increased prevalence vs. non-Black athletes [10,30] | Less prevalent in non-Black athletes [10,30] |
3.3. T-Wave Inversion Patterns
3.4. Impact of Geographic Origin, Sport Type, and Sex
3.5. Echocardiographic Findings in Black Athletes
| Parameter | Normal Values in Adult Males | In Black Athletes | In Athletes of Other Ethnicities | Key Difference |
|---|---|---|---|---|
| LV Wall Thickness (LVWT) | <11 mm. LVH is considered mild if it measures 11–13 mm, moderate if it measures 14–15 mm, and severe if it measures >15 mm [68]. | Frequently >12 mm; up to 16–18 mm; LVH more common [12,33,38,40,54]. | Rarely >12 mm; lower prevalence of LVH [12,33,38,40,54]. | LVH was up to 17.1 times more common in Black athletes [54]. |
| Relative Wall Thickness (RWT) | 0.42. Values greater than 0.42 usually reflect a concentric pattern, whereas values less than 0.42 usually predict an eccentric pattern of remodeling [69]. | Higher; suggesting concentric remodeling [12,14,35,37,51]. | Lower; suggesting a more eccentric pattern [12,14,35,37]. | RWT ≥ 0.44 in 43% of Black athletes vs. 7% of White [35]. |
| LV Mass | 72–210 g. (40–110 g/m2 if indexed for BSA) [69]. | Increased, with average LV mass at 286 g [33]. LV Mass Index increased with results at 101.4 g/m2 in Black Athletes vs. 92.4 g/m2 in Caucasian Athletes [38]. As high as 117 g/m2 in Black athletes [35]. | Values can be at upper limits of normal, or increased, but generally lower than those of Black athletes [33,35,38]. | Up to 13% greater in Black athletes [33]. Some studies found no difference [37]. |
| LV Cavity Size (LVEDD) | 42–58 mm [70]. | Slightly smaller or similar; no values > 60 mm [35,41,43]. | Higher proportion > 60 mm in some groups (e.g., Japanese) [35,41,43]. | Some studies found no differences [33]. |
| Left Atrial Diameter (LAD) | 30–40 mm [70]. | Larger average values: 35.4 mm [54]; 35.5 mm [38]. | Smaller average values: 30.5 mm [54]; 32.3 mm [38]. | Up to 13.4% greater in Black athletes [54]. Some studies found no difference [33]. |
| Posterior Wall Thickness (PWTd) | 6–12 mm [69]. | Higher average values: 9.7 mm [54]; 10.0 mm [35]. | Lower average values: 8.5 mm [54]; 8.1 mm [35]. | Up to 12.4% increase in Black athletes [54]. |
3.6. Left Ventricular Wall Thickness and Hypertrophy
3.7. Patterns of Ventricular Remodeling
3.8. Functional Parameters and Chamber Geometry
3.9. Right Ventricular Adaptations
3.10. Clinical Thresholds and Regional Variations
4. Discussion
4.1. Systematic Analysis of Electrocardiographic and Echocardiographic Findings
4.2. Electrocardiographic Adaptations: Evidence Synthesis
4.3. Echocardiographic Remodeling: Quantitative Assessment
4.4. Perpetuation of “White as Normal” Paradigm
4.5. Consequences of Diagnostic Disparities
4.6. Lessons from Medical History
4.7. Role of Thoracic Morphology
4.8. Moving Beyond Race-Based Categorization
- Geographic and ancestral origins: Continental ancestry and specific regional backgrounds may provide more precise information than broad racial categories.
- Anthropometric and training factors: BSA, training intensity, sport type, and duration of athletic participation demonstrate strong associations with cardiac adaptations that may be more relevant than racial classification.
- Social determinants of health: Socioeconomic status, access to healthcare, environmental exposures, and nutritional factors may influence cardiovascular development and should be considered in comprehensive assessment.
- Individual clinical factors: Family history, symptoms, functional capacity, and comprehensive cardiac evaluation remain the cornerstone of appropriate clinical decision-making.
4.9. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | atrioventricular |
| ARVC | arrhythmogenic right ventricular cardiomyopathy |
| BSA | body surface area |
| C-LVH | concentric left ventricular hypertrophy |
| ECG | electrocardiogram |
| ER | early repolarization |
| ESC | European Society of Cardiology |
| HCM | hypertrophic cardiomyopathy |
| IC | International Criteria |
| LAD | left atrial diameter |
| LAE | left atrial enlargement |
| LAVI | left atrial volume index |
| LV | left ventricular |
| LVEDD | left ventricular end-diastolic diameter |
| LVESD | left ventricular end-systolic diameter |
| LVEF | left ventricular ejection fraction |
| LVH | left ventricular hypertrophy |
| LVWT | left ventricular wall thickness |
| NCAA | National Collegiate Athletic Association |
| PWTd | posterior wall thickness |
| RAE | right atrial enlargement |
| RBBB | right bundle branch block |
| RV | right ventricular |
| RVH | right ventricular hypertrophy |
| RVID | right ventricular internal diameter |
| RWT | relative wall thickness |
| SCD | sudden cardiac death |
| STE | ST segment elevation |
| TWI | T-wave inversion |
References
- Sokunbi, O.J.; Okoromah, C.A.; Ekure, E.N.; Olawale, O.A.; Eke, W.S. Electrocardiographic pattern of apparently healthy African adolescent athletes in Nigeria. BMC Pediatr. 2021, 21, 97. [Google Scholar] [CrossRef]
- Sheikh, N.; Papadakis, M.; Ghani, S.; Zaidi, A.; Gati, S.; Adami, P.E.; Carré, F.; Schnell, F.; Wilson, M.; Avila, P. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation 2014, 129, 1637–1649. [Google Scholar] [CrossRef]
- Conway, J.J.; Krystofiak, J.; Quirolgico, K.; Como, B.; Altobelli, A.; Putukian, M. Evaluation of a Preparticipation cardiovascular screening program among 1686 national collegiate athletic Association division I athletes: Comparison of the Seattle, refined, and international electrocardiogram screening criteria. Clin. J. Sport Med. 2022, 32, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lander, B.S.; Duffy, E.Y.; Hennessey, J.A.; Tolani, S.; Patel, N.; Bohnen, M.S.; Hsu, J.J.; Danielian, A.; Shah, A.B.; Goolsby, M. Electrocardiographic Findings in Female Professional Basketball Athletes. JAMA Cardiol. 2024, 9, 475–479. [Google Scholar] [CrossRef]
- Malhotra, A.; Dhutia, H.; Yeo, T.-J.; Finocchiaro, G.; Gati, S.; Bulleros, P.; Fanton, Z.; Papatheodorou, E.; Miles, C.; Keteepe-Arachi, T. Accuracy of the 2017 international recommendations for clinicians who interpret adolescent athletes’ ECGs: A cohort study of 11 168 British white and black soccer players. Br. J. Sports Med. 2020, 54, 739–745. [Google Scholar] [CrossRef]
- McClean, G.; Riding, N.R.; Pieles, G.; Watt, V.; Adamuz, C.; Sharma, S.; George, K.P.; Oxborough, D.; Wilson, M.G. Diagnostic accuracy and Bayesian analysis of new international ECG recommendations in paediatric athletes. Heart 2019, 105, 152–159. [Google Scholar] [CrossRef]
- Zorzi, A.; Bettella, N.; Tatangelo, M.; Del Monte, A.; Vessella, T.; Poscolieri, B.; Crescenzi, C.; Pegorin, D.; D’Ascenzi, F.; Pescatore, V. Prevalence and clinical significance of isolated low QRS voltages in young athletes. EP Eur. 2022, 24, 1484–1495. [Google Scholar] [CrossRef]
- Miragoli, M.; Goldoni, M.; Demola, P.; Paterlini, A.; Li Calzi, M.; Gioia, M.I.; Visioli, F.; Rossi, S.; Pelà, G. Left ventricular geometry correlates with early repolarization pattern in adolescent athletes. Scand. J. Med. Sci. Sports 2019, 29, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.J.; Sager, S.J.; Freiser, M.; McGonagle, S.; Castellanos, A.; Myerburg, R.J. Inferolateral early repolarization in athletes. J. Interv. Card. Electrophysiol. 2011, 31, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Carre, F.; Kervio, G.; Rawlins, J.; Panoulas, V.F.; Chandra, N.; Basavarajaiah, S.; Carby, L.; Fonseca, T.; Sharma, S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur. Heart J. 2011, 32, 2304–2313. [Google Scholar] [CrossRef]
- Ferrari, F.; da Silveira, A.D.; Ziegelmann, P.K.; Aleixo, H.; Dilda, G.D.; Emed, L.G.; Magalhães, F.C.; Cardoso, F.B.; da Silva, H.C.; Guerra, F.E. Imaging associations enhance the understanding of ECG abnormalities in male Brazilian football players: Findings from the B-Pro Foot ECG study. Br. J. Sports Med. 2024, 58, 598–605. [Google Scholar] [CrossRef]
- Pambo, P.; Adu-Adadey, M.; Agbodzakey, H.; Scharhag, J. Electrocardiographic and echocardiographic findings in elite Ghanaian male soccer players. Clin. J. Sport Med. 2021, 31, e373–e379. [Google Scholar] [CrossRef]
- Pambo, P.; Adu-Adadey, M.; Ankrah, P.T.; Agbodzakey, H.; Scharhag, J. Electrocardiographic and echocardiographic findings in Ghanaian female soccer players. Clin. J. Sport Med. 2021, 31, e367–e372. [Google Scholar] [CrossRef]
- Riding, N.R.; Sharma, S.; McClean, G.; Adamuz, C.; Watt, V.; Wilson, M.G. Impact of geographical origin upon the electrical and structural manifestations of the black athlete’s heart. Eur. Heart J. 2019, 40, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, D.; Yong, C.M.; Singh, N.; Aggarwal, S.; Perez, M.; Ashley, E.; Hadley, D.; Froelicher, V. Patterns and prognosis of all components of the J-wave pattern in multiethnic athletes and ambulatory patients. Am. Heart J. 2014, 167, 259–266. [Google Scholar] [CrossRef]
- Papadakis, M.; Basavarajaiah, S.; Rawlins, J.; Edwards, C.; Makan, J.; Firoozi, S.; Carby, L.; Sharma, S. Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. Eur. Heart J. 2009, 30, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Calore, C.; Zorzi, A.; Sheikh, N.; Nese, A.; Facci, M.; Malhotra, A.; Zaidi, A.; Schiavon, M.; Pelliccia, A.; Sharma, S. Electrocardiographic anterior T-wave inversion in athletes of different ethnicities: Differential diagnosis between athlete’s heart and cardiomyopathy. Eur. Heart J. 2016, 37, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Anselmi, F.; Berti, B.; Capitani, E.; Chiti, C.; Franchini, A.; Graziano, F.; Nistri, S.; Focardi, M.; Capitani, M. Prevalence and significance of T-wave inversion in children practicing sport: A prospective, 4-year follow-up study. Int. J. Cardiol. 2019, 279, 100–104. [Google Scholar] [CrossRef]
- Brosnan, M.J.; Te Riele, A.S.; Bosman, L.P.; Hoorntje, E.T.; van den Berg, M.P.; Hauer, R.N.; Flannery, M.D.; Kalman, J.M.; Prior, D.L.; Tichnell, C. Electrocardiographic features differentiating arrhythmogenic right ventricular cardiomyopathy from an athlete’s heart. JACC Clin. Electrophysiol. 2018, 4, 1613–1625. [Google Scholar] [CrossRef]
- Jacob, D.; Main, M.L.; Gupta, S.; Gosch, K.; McCoy, M.; Magalski, A. Prevalence and significance of isolated T wave inversion in 1755 consecutive American collegiate athletes. J. Electrocardiol. 2015, 48, 407–414. [Google Scholar] [CrossRef]
- Malhotra, A.; Oxborough, D.; Rao, P.; Finocchiaro, G.; Dhutia, H.; Prasad, V.; Miller, C.; Keavney, B.; Papadakis, M.; Sharma, S. Defining the normal spectrum of electrocardiographic and left ventricular adaptations in mixed-race male adolescent soccer players. Circulation 2021, 143, 94–96. [Google Scholar] [CrossRef]
- Wilson, M.G.; Chatard, J.; Carré, F.; Hamilton, B.; Whyte, G.; Sharma, S.; Chalabi, H. Prevalence of electrocardiographic abnormalities in West-Asian and African male athletes. Br. J. Sports Med. 2012, 46, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Bryde, R.E.; Soutar, M.F.; Bavishi, A.A.; Kim, J.H.; Chung, E.; Gallucci, J.A.; Street, J.; Silvers, H.; Putukian, M.; Martinez, M.W. Electrocardiographic Findings Among Major League Soccer Athletes: Normative Data From a Diverse Population. JACC Adv. 2025, 4, 101886. [Google Scholar] [CrossRef]
- Magalski, A.; Maron, B.J.; Main, M.L.; McCoy, M.; Florez, A.; Reid, K.J.; Epps, H.W.; Bates, J.; Browne, J.E. Relation of race to electrocardiographic patterns in elite American football players. J. Am. Coll. Cardiol. 2008, 51, 2250–2255. [Google Scholar] [CrossRef]
- Magalski, A.; McCoy, M.; Zabel, M.; Magee, L.M.; Goeke, J.; Main, M.L.; Bunten, L.; Reid, K.J.; Ramza, B.M. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am. J. Med. 2011, 124, 511–518. [Google Scholar] [CrossRef]
- Raman, K.S.; Vyselaar, J.R. Electrocardiographic Findings in Professional Male Athletes. Clin. J. Sport Med. 2022, 32, e513–e520. [Google Scholar] [CrossRef]
- Crouse, S.F.; Meade, T.; Hansen, B.E.; Green, J.S.; Martin, S.E. Electrocardiograms of collegiate football athletes. Clin. Cardiol. Int. Index. Peer-Rev. J. Adv. Treat. Cardiovasc. Dis. 2009, 32, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.; Duvenage, E.; Jordaan, J.P. Electrocardiographic patterns in African University strength and endurance athletes of Zulu descent. J. Sports Med. Phys. Fit. 2015, 55, 1383–1389. [Google Scholar]
- Rambarat, C.A.; Reifsteck, F.; Clugston, J.R.; Handberg, E.M.; Martinez, M.W.; Hamburger, R.; Street, J.M.; Asken, B.; Taha, Y.; Kelling, M. Preparticipation cardiac evaluation findings in a cohort of collegiate female athletes. Am. J. Cardiol. 2021, 140, 134–139. [Google Scholar] [CrossRef]
- Corsi, D.; Saraiya, A.; Doyle, M.; Shah, V.; O’Malley, B.; Qiu, G.; Lanstaff, R.; Masood, I.; Osler, B.; Hajduczok, A.G. Cardiac screening findings and referral patterns in male African-American basketball players: Analysis of the HeartBytes Registry. Am. J. Cardiol. 2025, 243, 73–80. [Google Scholar] [CrossRef]
- Hamburger, R.F.; Taha, Y.; Ruzieh, M.; Clugston, J.R.; Handberg, E.M.; Reifsteck, F.; Martinez, M.W.; Pepine, C.J.; Edenfield, K.M. Longitudinal cardiac remodeling in collegiate American football players as assessed by echocardiography during their collegiate career. Clin. Cardiol. 2023, 46, 1090–1096. [Google Scholar] [CrossRef]
- Engel, D.J.; Schwartz, A.; Homma, S. Athletic cardiac remodeling in US professional basketball players. JAMA Cardiol. 2016, 1, 80–87. [Google Scholar] [CrossRef]
- Basavarajaiah, S.; Boraita, A.; Whyte, G.; Wilson, M.; Carby, L.; Shah, A.; Sharma, S. Ethnic differences in left ventricular remodeling in highly-trained athletes relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2256–2262. [Google Scholar] [CrossRef]
- Gjerdalen, G.; Hisdal, J.; Solberg, E.; Andersen, T.; Radunovic, Z.; Steine, K. The S candinavian athlete’s heart; echocardiographic characteristics of male professional football players. Scand. J. Med. Sci. Sports 2014, 24, e372–e380. [Google Scholar] [CrossRef] [PubMed]
- Pelà, G.; Li Calzi, M.; Crocamo, A.; Pattoneri, P.; Goldoni, M.; Anedda, A.; Musiari, L.; Biggi, A.; Bonetti, A.; Montanari, A. Ethnicity-related variations of left ventricular remodeling in adolescent amateur football players. Scand. J. Med. Sci. Sports 2015, 25, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Tso, J.V.; Turner, C.G.; Liu, C.; Galante, A.; Gilson, C.R.; Clark, C.; Taylor, H.A.; Quyyumi, A.A.; Baggish, A.L.; Kim, J.H. Association between race and maladaptive concentric left ventricular hypertrophy in American-style football athletes. Br. J. Sports Med. 2022, 56, 151–157. [Google Scholar] [CrossRef]
- Moneghetti, K.J.; Singh, T.; Hedman, K.; Christle, J.W.; Kooreman, Z.; Kobayashi, Y.; Bouajila, S.; Amsallem, M.; Wheeler, M.; La Gerche, A. Echocardiographic assessment of left ventricular remodeling in American style footballers. Int. J. Sports Med. 2020, 41, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, F.M.; Schmied, C.; Zerguini, Y.A.; Junge, A.; Quattrini, F.; Culasso, F.; Dvorak, J.; Pelliccia, A. The athlete’s heart in adolescent Africans: An electrocardiographic and echocardiographic study. J. Am. Coll. Cardiol. 2012, 59, 1029–1036. [Google Scholar] [CrossRef]
- Crouse, S.F.; White, S.; Erwin, J.P.; Meade, T.H.; Martin, S.E.; Oliver, J.M.; Joubert, D.P.; Lambert, B.S.; Bramhall, J.P.; Gill, K. Echocardiographic and blood pressure characteristics of first-year collegiate American-style football players. Am. J. Cardiol. 2016, 117, 131–134. [Google Scholar] [CrossRef]
- Dzudie, A.; Menanga, A.; Hamadou, B.; Kengne, A.P.; Atchou, G.; Kingue, S. Ultrasonographic study of left ventricular function at rest in a group of highly trained black African handball players. Eur. J. Echocardiogr. 2007, 8, 122–127. [Google Scholar] [CrossRef]
- Kervio, G.; Pelliccia, A.; Nagashima, J.; Wilson, M.G.; Gauthier, J.; Murayama, M.; Uzan, L.; Ville, N.; Carré, F. Alterations in echocardiographic and electrocardiographic features in Japanese professional soccer players: Comparison to African-Caucasian ethnicities. Eur. J. Prev. Cardiol. 2013, 20, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, K.H.; Rink, L.; Hornsby, K.; Park, H.; Park, J.-H.; Yoon, H.J.; Ahn, Y.; Jeong, M.H.; Cho, J.G. University athletes and changes in cardiac geometry: Insight from the 2015 Gwangju Summer Universiade. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 407–416. [Google Scholar] [CrossRef]
- Edenfield, K.M.; Reifsteck, F.; Carek, S.; Harmon, K.G.; Asken, B.M.; Dillon, M.C.; Street, J.; Clugston, J.R. Echocardiographic measurements of left ventricular end-diastolic diameter and interventricular septal diameter in collegiate football athletes at preparticipation evaluation referenced to body surface area. BMJ Open Sport Exerc. Med. 2019, 5, e000488. [Google Scholar] [CrossRef]
- Augustine, D.; Willis, J.; Sivalokanathan, S.; Wild, C.; Sharma, A.; Zaidi, A.; Pearce, K.; Stuart, G.; Papadakis, M.; Sharma, S. Right ventricular assessment of the adolescent footballer’s heart. Echo Res. Pract. 2024, 11, 7. [Google Scholar] [CrossRef]
- Zaidi, A.; Ghani, S.; Sharma, R.; Oxborough, D.; Panoulas, V.F.; Sheikh, N.; Gati, S.; Papadakis, M.; Sharma, S. Physiological right ventricular adaptation in elite athletes of African and Afro-Caribbean origin. Circulation 2013, 127, 1783–1792. [Google Scholar] [CrossRef]
- Waase, M.P.; Mutharasan, R.K.; Whang, W.; DiTullio, M.R.; DiFiori, J.P.; Callahan, L.; Mancell, J.; Phelan, D.; Schwartz, A.; Homma, S. Electrocardiographic findings in national basketball association athletes. JAMA Cardiol. 2018, 3, 69–74. [Google Scholar] [CrossRef]
- Sheikh, N.; Papadakis, M.; Carre, F.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Zaidi, A.; Gati, S.; Rawlins, J.; Wilson, M.G. Cardiac adaptation to exercise in adolescent athletes of African ethnicity: An emergent elite athletic population. Br. J. Sports Med. 2013, 47, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Schmied, C.; Zerguini, Y.; Junge, A.; Tscholl, P.; Pelliccia, A.; Mayosi, B.; Dvorak, J. Cardiac findings in the precompetition medical assessment of football players participating in the 2009 African Under-17 Championships in Algeria. Br. J. Sports Med. 2009, 43, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Sadik, J.; Lipinski, M.J.; Van Le, V.; Froelicher, V. Association between cardiac dimensions and athlete lineup position: Analysis using echocardiography in NCAA football team players. Physician Sportsmed. 2013, 41, 58–66. [Google Scholar] [CrossRef]
- Haddad, F.; Peter, S.; Hulme, O.; Liang, D.; Schnittger, I.; Puryear, J.; Gomari, F.A.; Finocchiaro, G.; Myers, J.; Froelicher, V. Race differences in ventricular remodeling and function among college football players. Am. J. Cardiol. 2013, 112, 128–134. [Google Scholar] [CrossRef]
- Demola, P.; Crocamo, A.; Ceriello, L.; Botti, A.; Cremonini, I.; Pattoneri, P.; Corradi, D.; Visioli, F.; Goldoni, M.; Pelà, G. Hemodynamic and ECG responses to stress test in early adolescent athletes explain ethnicity-related cardiac differences. Int. J. Cardiol. 2019, 289, 125–130. [Google Scholar] [CrossRef]
- Di Gioia, G.; Crispino, S.P.; Monosilio, S.; Maestrini, V.; Nenna, A.; Spinelli, A.; Lemme, E.; Squeo, M.R.; Pelliccia, A. Left ventricular trabeculation: Arrhythmogenic and clinical significance in elite athletes. J. Am. Soc. Echocardiogr. 2024, 37, 577–586. [Google Scholar] [CrossRef]
- Davis, A.J.; Semsarian, C.; Orchard, J.W.; La Gerche, A.; Orchard, J.J. The impact of ethnicity on athlete ECG interpretation: A systematic review. J. Cardiovasc. Dev. Dis. 2022, 9, 183. [Google Scholar] [CrossRef]
- McClean, G.; Riding, N.R.; Ardern, C.L.; Farooq, A.; Pieles, G.E.; Watt, V.; Adamuz, C.; George, K.P.; Oxborough, D.; Wilson, M.G. Electrical and structural adaptations of the paediatric athlete’s heart: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 230. [Google Scholar] [CrossRef]
- Pambo, P.; Scharhag, J. Electrocardiographic and echocardiographic findings in black athletes: A general review. Clin. J. Sport Med. 2021, 31, 321–329. [Google Scholar] [CrossRef]
- Christou, G.A.; O′Driscoll, J.M. The impact of demographic, anthropometric and athletic characteristics on left atrial size in athletes. Clin. Cardiol. 2020, 43, 834–842. [Google Scholar] [CrossRef]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Patton, K.K.; Ellinor, P.T.; Ezekowitz, M.; Kowey, P.; Lubitz, S.A.; Perez, M.; Piccini, J.; Turakhia, M.; Wang, P.; Viskin, S. Electrocardiographic early repolarization: A scientific statement from the American Heart Association. Circulation 2016, 133, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso, C.; Biffi, A.; Buja, G.; Delise, P.; Gussac, I. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur. Heart J. 2010, 31, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Drezner, J.A.; Ackerman, M.J.; Anderson, J.; Ashley, E.; Asplund, C.A.; Baggish, A.L.; Börjesson, M.; Cannon, B.C.; Corrado, D.; DiFiori, J.P. Electrocardiographic interpretation in athletes: The ‘Seattle criteria’. Br. J. Sports Med. 2013, 47, 122–124. [Google Scholar] [CrossRef]
- Drezner, J.A.; Fischbach, P.; Froelicher, V.; Marek, J.; Pelliccia, A.; Prutkin, J.M.; Schmied, C.M.; Sharma, S.; Wilson, M.G.; Ackerman, M.J. Normal electrocardiographic findings: Recognising physiological adaptations in athletes. Br. J. Sports Med. 2013, 47, 125–136. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef]
- Ilodibia, T.F.; Odia, J.O. Evaluation of the Seattle and International Criteria in elite Nigerian athletes. J. Electrocardiol. 2021, 68, 14–23. [Google Scholar] [CrossRef]
- Lyon, A.; Bueno-Orovio, A.; Zacur, E.; Ariga, R.; Grau, V.; Neubauer, S.; Watkins, H.; Rodriguez, B.; Minchole, A. Electrocardiogram phenotypes in hypertrophic cardiomyopathy caused by distinct mechanisms: Apico-basal repolarization gradients vs. Purkinje-myocardial coupling abnormalities. EP Eur. 2018, 20 (Suppl. S3), iii102–iii112. [Google Scholar] [CrossRef]
- McClean, G.; Riding, N.R.; Pieles, G.; Sharma, S.; Watt, V.; Adamuz, C.; Johnson, A.; Tramullas, A.; George, K.P.; Oxborough, D. Prevalence and significance of T-wave inversion in Arab and Black paediatric athletes: Should anterior T-wave inversion interpretation be governed by biological or chronological age? Eur. J. Prev. Cardiol. 2019, 26, 641–652. [Google Scholar] [CrossRef]
- Baggish, A.L. Cardiac data from the Women’s National Basketball Association—Caring for women requires studying women. JAMA Cardiol. 2020, 5, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.; Papadakis, M.; Sharma, S. Cardiac adaptation in athletes of black ethnicity: Differentiating pathology from physiology. Heart 2012, 98, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Grajewski, K.G.; Stojanovska, J.; Ibrahim, E.-S.H.; Sayyouh, M.; Attili, A. Left ventricular hypertrophy: Evaluation with cardiac MRI. Curr. Probl. Diagn. Radiol. 2020, 49, 460–475. [Google Scholar] [CrossRef]
- Harkness, A.; Ring, L.; Augustine, D.X.; Oxborough, D.; Robinson, S.; Sharma, V. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G1–G18. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.N.; Shen, S.; Chukwurah, M.I.; Churchill, T.W.; Stewart, K.M.; Chung, E.H.; Weiner, R.B.; Li, H.; Guseh, J.S. Athlete’s Heart Revisited: Historical, Clinical, and Molecular Perspectives. Circ. Res. 2025, 137, 231–254. [Google Scholar] [CrossRef]
- Papadakis, M.; Wilson, M.G.; Ghani, S.; Kervio, G.; Carre, F.; Sharma, S. Impact of ethnicity upon cardiovascular adaptation in competitive athletes: Relevance to preparticipation screening. Br. J. Sports Med. 2012, 46 (Suppl. S1), i22–i28. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, J.; Carré, F.; Kervio, G.; Papadakis, M.; Chandra, N.; Edwards, C.; Whyte, G.; Sharma, S. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation 2010, 121, 1078–1085. [Google Scholar] [CrossRef]
- Petek, B.J.; Drezner, J.A.; Churchill, T.W. The International Criteria for Electrocardiogram Interpretation in Athletes Common Pitfalls and Future Directions. Card. Electrophysiol. Clin. 2024, 16, 35. [Google Scholar] [CrossRef]
- Kim, J.H.; Baggish, A.L.; Levine, B.D.; Ackerman, M.J.; Day, S.M.; Dineen, E.H.; Guseh Ii, J.S.; La Gerche, A.; Lampert, R.; Martinez, M.W.; et al. Clinical Considerations for Competitive Sports Participation for Athletes with Cardiovascular Abnormalities: A Scientific Statement From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2025, 85, 1059–1108. [Google Scholar] [CrossRef] [PubMed]
- Petek, B.J.; Churchill, T.W.; Moulson, N.; Kliethermes, S.A.; Baggish, A.L.; Drezner, J.A.; Patel, M.R.; Ackerman, M.J.; Kucera, K.L.; Siebert, D.M. Sudden cardiac death in National Collegiate Athletic Association athletes: A 20-year study. Circulation 2024, 149, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.A.; Eisenstein, L.G.; Jones, D.S. Hidden in plain sight—Reconsidering the use of race correction in clinical algorithms. New Engl. J. Med. 2020, 383, 874–882. [Google Scholar] [CrossRef]
- Bragg-Gresham, J.; Zhang, X.; Le, D.; Heung, M.; Shahinian, V.; Morgenstern, H.; Saran, R. Prevalence of chronic kidney disease among black individuals in the US after removal of the black race coefficient from a glomerular filtration rate estimating equation. JAMA Netw. Open 2021, 4, e2035636. [Google Scholar] [CrossRef]
- Hasley, H.L.; Iarajuli, T.; Nguyen, J.; Thiemann, D.; Malik, M.; Roth, J.; Raver, M.; Stifelman, M.; Munver, R.; Ahmed, M. Race-modified estimated glomerular filtration rate underestimates chronic kidney disease prevalence in Black patients undergoing partial and radical nephrectomy: Implications for surgical planning. Urol. Ann. 2024, 16, 221–226. [Google Scholar] [CrossRef]
- Khan, S.S.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Blaha, M.J.; Carson, A.P.; Chang, A.R.; Ciemins, E. Development and validation of the American Heart Association’s PREVENT equations. Circulation 2024, 149, 430–449. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Lombardo, M.; Grasso, E.; Gensini, G.F.; Ambrosio, G. The influence of pectus excavatum on cardiac kinetics and function in otherwise healthy individuals: A systematic review. Int. J. Cardiol. 2023, 381, 135–144. [Google Scholar] [CrossRef]
- Zuidema, W.P.; Oosterhuis, J.W.A.; Zijp, G.W.; van Baren, R.; de Lange-de Klerk, E.S.M.; van der Heide, S.M.; van der Steeg, A.F.W.; van Heurn, L.W.E. Sports activity in adolescents in the Netherlands with a pectus excavatum; the impact of surgery. J. Pediatr. Surg. 2019, 54, 1671–1674. [Google Scholar] [CrossRef] [PubMed]
- Hyde, N.; Prutkin, J.M.; Drezner, J.A. Electrocardiogram interpretation in NCAA athletes: Comparison of the ‘Seattle’and ‘International’criteria. J. Electrocardiol. 2019, 56, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsi, D.; Hernandez, R.; Bao, J.Y.; Garrova, S.; Shipon, D. Beyond Racial Categorization in Sports Cardiology: A Systematic Review of Cardiac Adaptations in Athletes. J. Clin. Med. 2025, 14, 7107. https://doi.org/10.3390/jcm14197107
Corsi D, Hernandez R, Bao JY, Garrova S, Shipon D. Beyond Racial Categorization in Sports Cardiology: A Systematic Review of Cardiac Adaptations in Athletes. Journal of Clinical Medicine. 2025; 14(19):7107. https://doi.org/10.3390/jcm14197107
Chicago/Turabian StyleCorsi, Douglas, Rafael Hernandez, Jasmine Yimeng Bao, Stephen Garrova, and David Shipon. 2025. "Beyond Racial Categorization in Sports Cardiology: A Systematic Review of Cardiac Adaptations in Athletes" Journal of Clinical Medicine 14, no. 19: 7107. https://doi.org/10.3390/jcm14197107
APA StyleCorsi, D., Hernandez, R., Bao, J. Y., Garrova, S., & Shipon, D. (2025). Beyond Racial Categorization in Sports Cardiology: A Systematic Review of Cardiac Adaptations in Athletes. Journal of Clinical Medicine, 14(19), 7107. https://doi.org/10.3390/jcm14197107






