Benign and Malignant Parotid Gland Tumors: Insights from a Five-Year Northeast Romanian Population
Abstract
1. Introduction
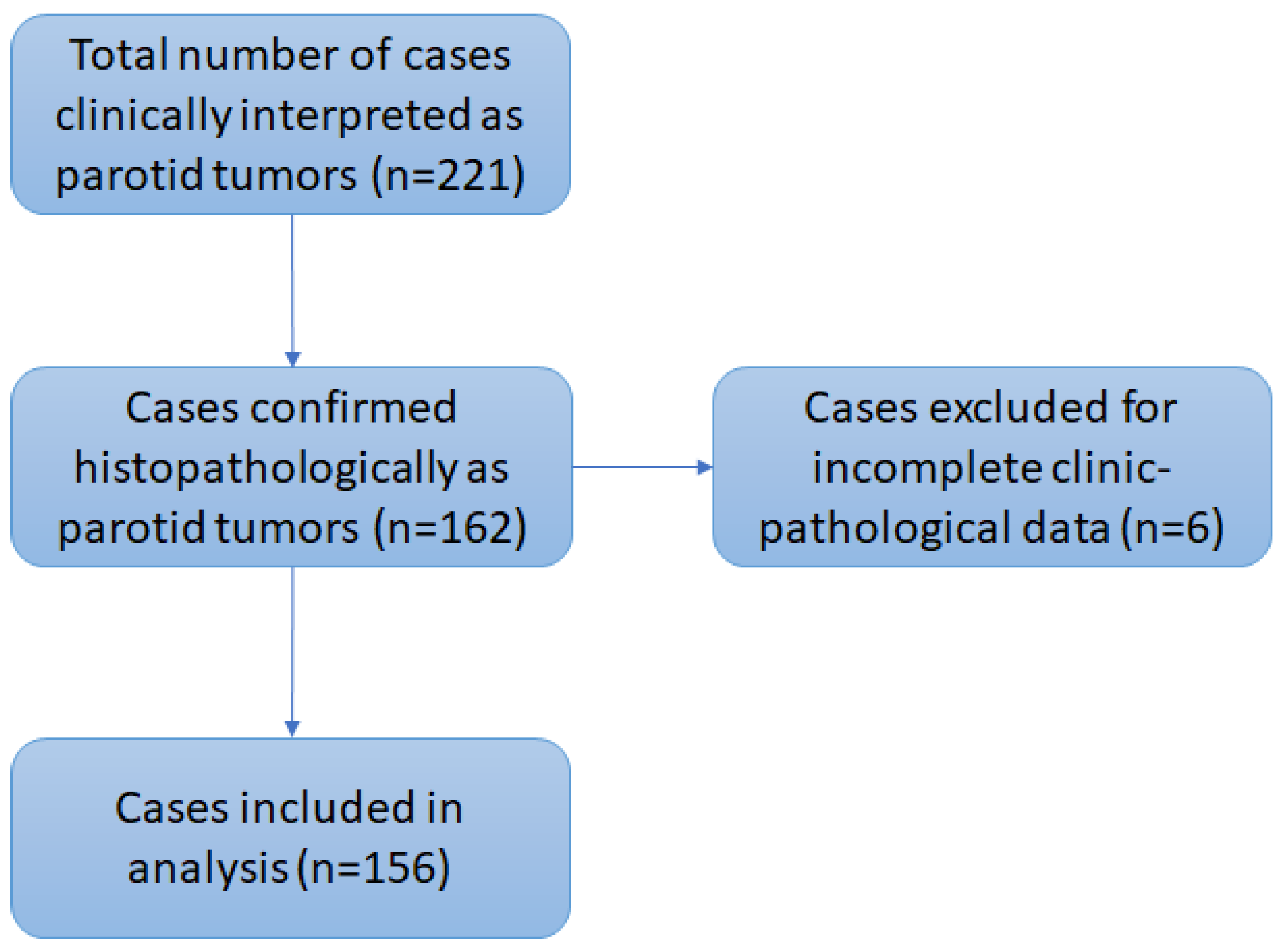
2. Materials and Methods
2.1. Study Population
2.2. Data Description
2.3. Statistical Analysis
3. Results
3.1. Study Sample Overview
3.2. Demographics
3.3. Tumor Distribution (Benign vs. Malignant)
3.4. Clinicopathological Characteristics of Benign Tumors
3.5. Clinicopathological Characteristics of Malignant Tumors
3.6. Surgical Management
3.7. Postoperative Outcomes
3.8. Incidental Findings or Unusual Associations
3.9. Diagnostic Accuracy (Sensitivity/Specificity)
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | acinic cell carcinoma |
| ADC | adenocarcinoma |
| AdCC | adenoid cystic carcinoma |
| AJCC | American Joint Committee on Cancer |
| CA ex PA | carcinoma ex pleomorphic adenoma |
| G | histological grade |
| Ly | lymphoma |
| MTS | metastasis |
| n | number of cases |
| NOS | not otherwise specified |
| p | probability |
| pTNM | pathological tumor node metastasis |
| SCC | squamous cell carcinoma |
| SMAS | superficial musculoaponeurotic system |
| WHO | World Health Organization |
References
- Cristofaro, M.G.; Allegra, E.; Giudice, A.; Colangeli, W.; Caruso, D.; Barca, I.; Giudice, M. Pleomorphic Adenoma of the Parotid: Extracapsular Dissection Compared with Superficial Parotidectomy—A 10-Year Retrospective Cohort Study. Sci. World J. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Borsetto, D.; Iocca, O.; De Virgilio, A.; Boscolo-Rizzo, P.; Phillips, V.; Nicolai, P.; Spriano, G.; Fussey, J.; Di Maio, P. Elective Neck Dissection in Primary Parotid Carcinomas: A Systematic Review and Meta-analysis. J. Oral Pathol. Med. 2021, 50, 136–144. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Serpa, M.S.; Viveiros, S.K.; Sena, D.A.C.; De Carvalho Pinho, R.F.; De Abreu Guimarães, L.D.; De Sousa Andrade, E.S.; Dias Pereira, J.R.; Silveira, M.M.F.D.; Sobral, A.P.V.; et al. Salivary Gland Tumors in a Brazilian Population: A 20-Year Retrospective and Multicentric Study of 2292 Cases. J. Cranio-Maxillofac. Surg. 2018, 46, 2227–2233. [Google Scholar] [CrossRef]
- Skalova, A.; Bishop, J.A.; Mehrotra, R.; Thompson, L.D.R. Salivary gland tumours. In WHO Classification of Tumours, 5th ed.; Head and Neck Tumours Part A; WHO Classification of Tumours Editorial Board Eds; International Agency for Research on Cancer: Lyon, France, 2024; pp. 159–250. [Google Scholar]
- Mengi, E. Salivary Gland Tumors: A 15-Year Experience of a Universıty Hospital in Turkey. North. Clin. Istanb. 2020, 7, 366–371. [Google Scholar] [CrossRef]
- Jin, X.; Jing, X.; Lew, M.; Pantanowitz, L. Concordance of Second Opinion Diagnosis in Salivary Gland Cytopathology: Experience from a Single Academic Institution. Diagn. Histopathol. 2023, 29, 380–385. [Google Scholar] [CrossRef]
- Barca, I.; Novembre, D.; Cordaro, R.; Lo Faro, C.; Colangeli, W.; Boschetti, C.E.; Giudice, A.; Cristofaro, M.G. Myoepithelioma of the Parotid Gland: A Case Report with Review of the Literature. Oral Maxillofac. Surg. Cases 2020, 6, 100131. [Google Scholar] [CrossRef]
- Vrinceanu, D.; Dumitru, M.; Bratiloveanu, M.; Marinescu, A.; Serboiu, C.; Manole, F.; Palade, D.O.; Costache, A.; Costache, M.; Patrascu, O. Parotid Gland Tumors: Molecular Diagnostic Approaches. Int. J. Mol. Sci. 2024, 25, 7350. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Shetty, S. Classification of Parotidectomy: A Proposed Modification to the European Salivary Gland Society Classification System. Eur. Arch. Otorhinolaryngol. 2017, 274, 3175–3181. [Google Scholar] [CrossRef]
- Abu-Ghanem, Y.; Mizrachi, A.; Popovtzer, A.; Abu-Ghanem, N.; Feinmesser, R. Recurrent Pleomorphic Adenoma of the Parotid Gland: Institutional Experience and Review of the Literature. J. Surg. Oncol. 2016, 114, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Kawata, R.; Higashino, M.; Nishikawa, S.; Inui, T.; Terada, T.; Haginomori, S.; Kurisu, Y.; Hirose, Y. Recurrent Benign Pleomorphic Adenoma of the Parotid Gland: Facial Nerve Identification and Risk Factors for Facial Nerve Paralysis at Re-Operation. Auris Nasus Larynx 2019, 46, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Fusconi, M.; Cialente, F.; De Soccio, G.; Ralli, M.; Minni, A.; Agolli, G.; De Vincentiis, M.; Remacle, M.; Petrone, P.; et al. Rupture of the Pleomorphic Adenoma of the Parotid Gland: What to Know before, during and after Surgery. J. Clin. Med. 2021, 10, 5368. [Google Scholar] [CrossRef]
- Espinosa, C.A.; Fernández-Valle, Á.; Lequerica-Fernández, P.; De Villalaín, L.; De Vicente, J.C. Clinicopathologic and Surgical Study of Pleomorphic Adenoma of the Parotid Gland: Analysis of Risk Factors for Recurrence and Facial Nerve Dysfunction. J. Oral Maxillofac. Surg. 2018, 76, 347–354. [Google Scholar] [CrossRef]
- Patel, D.K.; Morton, R.P. Demographics of Benign Parotid Tumours: Warthin’s Tumour versus Other Benign Salivary Tumours. Acta Otolaryngol. 2016, 136, 83–86. [Google Scholar] [CrossRef] [PubMed]
- van Weert, S.; Valstar, M.; Lissenberg-Witte, B.; Bloemena, E.; Smit, L.; van der Wal, J.; Vergeer, M.; Smeele, L.; Leemans, C.R. Prognostic Factors in Acinic Cell Carcinoma of the Head and Neck: The Amsterdam Experience. Oral Oncol. 2022, 125, 105698. [Google Scholar] [CrossRef] [PubMed]
- Rajbhar, R.; Ekatpure, D.; Kolhe, A. Comprehensive Five-Year Study on Salivary Gland Tumors: Demographic, Clinical, and Histopathological Insights. Adv. Oral Maxillofac. Surg. 2025, 17, 100523. [Google Scholar] [CrossRef]
- Thielker, J.; Grosheva, M.; Ihrler, S.; Wittig, A.; Guntinas-Lichius, O. Contemporary Management of Benign and Malignant Parotid Tumors. Front. Surg. 2018, 5, 39. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Stubbs, V.; Chen, J.; Yalamanchi, P.; Cannady, S.; Brant, J.; Newman, J. Mucoepidermoid Carcinoma of the Parotid Gland: A National Cancer Database Study. Am. J. Otolaryngol. 2018, 39, 321–326. [Google Scholar] [CrossRef]
- Dhanani, R.; Iftikhar, H.; Awan, M.S.; Zahid, N.; Momin, S.N.A. Role of Fine Needle Aspiration Cytology in the Diagnosis of Parotid Gland Tumors: Analysis of 193 Cases. Int. Arch. Otorhinolaryngol. 2020, 24, e508–e512. [Google Scholar] [CrossRef]
- Germano, C.; Borriello, G.; Corazzelli, G.; Abbate, V.; Troise, S.; Dell’Aversana Orabona, G.; Piombino, P.; Romano, A.; Collà Ruvolo, C.; Bonavolontà, P. Mitigating Complications Rate in Parotid Gland Tumor Surgery by Reconstruction Technique with SurgiMend®: A Retrospective, Single-Center, Observational Study on 300 Consecutive Patients. J. Craniomaxillofac. Surg. 2025, 53, 1129–1134. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Silver, C.E.; Thielker, J.; Bernal-Sprekelsen, M.; Bradford, C.R.; De Bree, R.; Kowalski, L.P.; Olsen, K.D.; Quer, M.; Rinaldo, A.; et al. Management of the Facial Nerve in Parotid Cancer: Preservation or Resection and Reconstruction. Eur. Arch. Otorhinolaryngol. 2018, 275, 2615–2626. [Google Scholar] [CrossRef]
- Cirignaco, G.; Monarchi, G.; Betti, E.; Paglianiti, M.; Catarzi, L.; Tel, A.; Vaira, L.A.; Balercia, P.; Consorti, G. Outcome of Facial Nerve Integrity After Parotid Gland Surgery With and Without Intraoperative Monitoring: A Ten-Year Retrospective Study. J. Clin. Med. 2025, 14, 1156. [Google Scholar] [CrossRef]
- Tsang, P.P.M.; Gupta, G.; Mukhopadhyay, S. Metastatic Tumours to the Parotid: A 20-Year Single Institutional Experience with an Emphasis on 14 Unusual Presentations. Ann. Diagn. Pathol. 2024, 73, 152386. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Li, W. Clinical Parameters Predictors of Malignant Transformation of Recurrent Parotid Pleomorphic Adenoma. Sci. Rep. 2023, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Jiwani, R.; White, R.; Bakalov, V.; Moll, R.; Liput, J.; Greenberg, L. Advances in Targeted and Systemic Therapy for Salivary Gland Carcinomas: Current Options and Future Directions. Curr. Oncol. 2025, 32, 232. [Google Scholar] [CrossRef] [PubMed]
- Park, G.C.; Cho, K.; Kang, J.; Roh, J.; Choi, S.; Kim, S.Y.; Nam, S.Y. Relationship between Histopathology of Pleomorphic Adenoma in the Parotid Gland and Recurrence after Superficial Parotidectomy. J. Surg. Oncol. 2012, 106, 942–946. [Google Scholar] [CrossRef]
- Liu, H.; Wen, W.; Huang, H.; Liang, Y.; Tan, X.; Liu, S.; Liu, C.; Hu, M. Recurrent Pleomorphic Adenoma of the Parotid Gland: Intraoperative Facial Nerve Monitoring during Parotidectomy. Otolaryngol. Head. Neck Surg. 2014, 151, 87–91. [Google Scholar] [CrossRef]
- Andreasen, S.; Therkildsen, M.H.; Bjørndal, K.; Homøe, P. Pleomorphic Adenoma of the Parotid Gland 1985–2010: A Danish Nationwide Study of Incidence, Recurrence Rate, and Malignant Transformation. Head. Neck 2016, 38, E1364–E1369. [Google Scholar] [CrossRef]
- Pfisterer, M.J.; Vazquez, A.; Mady, L.J.; Khan, M.N.; Baredes, S.; Eloy, J.A. Squamous Cell Carcinoma of the Parotid Gland: A Population-Based Analysis of 2545 Cases. Am. J. Otolaryngol. 2014, 35, 469–475. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, J.; You, Y.; Yang, X.; Wang, Y. Primary Squamous Cell Carcinoma of the Parotid Gland: Clinicopathological Characteristics, Treatment, and Prognosis. Int. J. Oral. Maxillofac. Surg. 2021, 50, 151–157. [Google Scholar] [CrossRef]
- Scherl, C.; Kato, M.G.; Erkul, E.; Graboyes, E.M.; Nguyen, S.A.; Chi, A.C.; Morgan, P.F.; Day, T.A. Outcomes and Prognostic Factors for Parotid Acinic Cell Carcinoma: A National Cancer Database Study of 2362 Cases. Oral. Oncol. 2018, 82, 53–60. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, L.; Zhang, C.; Xia, R.; Tian, Z.; Li, J. Clinicopathologic Features and Prognostic Factors of Widely Invasive Carcinoma Ex Pleomorphic Adenoma of Parotid Gland: A Clinicopathologic Analysis of 126 Cases in a Chinese Population. J. Oral. Maxillofac. Surg. 2020, 78, 2247–2257. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Wang, Q.; Chen, Y.; Gao, T.; Han, R.; Li, N.; Zhang, K. The Clinicopathological Features of Parotid Lymphoma. Int. J. Clin. Exp. Pathol. 2020, 13, 2050–2057. [Google Scholar]
- Girardi, F.M.; Wagner, V.P.; Martins, M.D.; Abentroth, A.L.; Hauth, L.A. Better Outcome for Parotid versus Neck Metastasis of Head and Neck Cutaneous Squamous Cell Carcinoma: A New Report on Reemerging Data. Braz. J. Otorhinolaryngol. 2021, 87, 389–395. [Google Scholar] [CrossRef]
- Gontarz, M.; Urbańska, M.; Bargiel, J.; Gąsiorowski, K.; Marecik, T.; Szczurowski, P.; Zapała, J.; Wyszyńska-Pawelec, G. Metastatic Malignancies in the Parotid Gland: A Retrospective Study. J. Craniomaxillofac Surg. 2024, 52, 1334–1340. [Google Scholar] [CrossRef]
- Troch, M.; Formanek, M.; Streubel, B.; Müllauer, L.; Chott, A.; Raderer, M. Clinicopathological Aspects of Mucosa-associated Lymphoid Tissue (MALT) Lymphoma of the Parotid Gland: A Retrospective Single-center Analysis of 28 Cases. Head. Neck 2011, 33, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.J.; Ciarleglio, M.M.; Cong, X.; Otremba, M.D.; Judson, B.L. Parotid Gland Lymphoma: Prognostic Analysis of 2140 Patients. Laryngoscope 2013, 123, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H. Salivary neoplasms: Overview of a 35-year experience with 2,807 patients. Head. Neck Surg. 1986, 8, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, R.; Yatabe, Y.; Yamamoto, Y.; Shimoi, T.; Iwata, S.; Goto, Y.; Mizushima, Y.; Kawai, A.; Higashi, T. Proposal for a New Classification of Rare Cancers Adopting Updated Histological Tumor Types. Pathol. Int. 2025, 75, 291–309. [Google Scholar] [CrossRef]
- Moore, M.G.; Yueh, B.; Lin, D.T.; Bradford, C.R.; Smith, R.V.; Khariwala, S.S. Controversies in the Workup and Surgical Management of Parotid Neoplasms. Otolaryngol. Head. Neck Surg. 2021, 164, 27–36. [Google Scholar] [CrossRef]
- Ata-Ali, J.; Zurriaga, O.; Alberich, C. Incidence and survival rates for malignant salivary gland tumors. J. Oral. Sci. 2016, 58, 67–73. [Google Scholar] [CrossRef]
- Tanzawa, A.; Saito, K.; Ota, M.; Takahashi, K.; Ohno, I.; Hanazawa, T.; Uzawa, K.; Takiguchi, Y. Salivary Gland-Type Cancers: Cross-Organ Demographics of a Rare Cancer. Int. J. Clin. Oncol. 2024, 29, 755–763. [Google Scholar] [CrossRef]
- Lee, D.H.; Jung, E.K.; Lee, J.K.; Lim, S.C. Comparative Analysis of Benign and Malignant Parotid Gland Tumors: A Retrospective Study of 992 Patients. Am. J. Otolaryngol. 2023, 44, 103690. [Google Scholar] [CrossRef] [PubMed]
- Hornung, B.; Constantinidis, J.; Thimsen, V.; Agaimy, A.; Koch, M.; Gostian, A.-O.; Sievert, M.; Müller, S.; Iro, H.; Mantsopoulos, K. Pleomorphic Adenoma of the Parotid Gland and the Parapharyngeal Space: Two Diametrically Opposing Surgical Philosophies for the Same Histopathologic Entity? J. Clin. Med. 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Przewoźny, T.; Stankiewicz, C. Neoplasms of the Parotid Gland in Northern Poland, 1991–2000: An Epidemio-logic Study. Eur. Arch. Otorhinolaryngol. 2004, 261, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.C.; Zhan, K.Y.; White-Gilbertson, S.J.; Day, T.A. Predictors of Nodal Metastasis in Parotid Malignancies: A National Cancer Data Base Study of 22,653 Patients. Otolaryngol. Head. Neck Surg. 2016, 154, 121–130. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Wendt, T.G.; Buentzel, J.; Esser, D.; Böger, D.; Mueller, A.H.; Piesold, J.-U.; Schultze-Mosgau, S.; Schlattmann, P.; Schmalenberg, H. Incidence, Treatment, and Outcome of Parotid Carcinoma, 1996–2011: A Population-Based Study in Thuringia, Germany. J. Cancer Res. Clin. Oncol. 2015, 141, 1679–1688. [Google Scholar] [CrossRef]
- Busch, A.; Bauer, L.; Wardelmann, E.; Rudack, C.; Grünewald, I.; Stenner, M. Prognostic Relevance of Epithelial–Mesenchymal Transition and Proliferation in Surgically Treated Primary Parotid Gland Cancer. J. Clin. Pathol. 2017, 70, 403–409. [Google Scholar] [CrossRef]
- Cunha, J.-L.-S.; Fraga, V.-R.-A.; de Lima, W.-P.; Andrade, A.O.; Gordón-Núñez, M.-A.; Nonaka, C.-F.-W.; Alves, P.-M.; Júnior, R.-L.-C.A. Salivary Gland Tumors: A 13-Year Clinicopathologic Retrospective Study in a Brazilian Northeast Population. J. Clin. Exp. Dent. 2023, 15, e88–e95. [Google Scholar] [CrossRef]
- Ghaderi, H.; Kruger, E.; Ahmadvand, S.; Mohammadi, Y.; Khademi, B.; Ghaderi, A. Epidemiological Profile of Salivary Gland Tumors in Southern Iranian Population: A Retrospective Study of 405 Cases. J. Cancer Epidemiol. 2023, 2023, 8844535. [Google Scholar] [CrossRef]
- Papadogeorgakis, N. Partial Superficial Parotidectomy as the Method of Choice for Treating Pleomorphic Ade-nomas of the Parotid Gland. Br. J. Oral. Maxillofac. Surg. 2011, 49, 447–450. [Google Scholar] [CrossRef]
- Su, C.-C.; Li, C.-F.; Lin, C.-N. Unreported Cytologic Characteristics of Oncocytes in Warthin’s Tumors. Tzu Chi Med. J. 2010, 22, 137–140. [Google Scholar] [CrossRef]
- Venkatesh, S.; Srinivas, T.; Hariprasad, S. Parotid Gland Tumors: 2-Year Prospective Clinicopathological Study. Ann. Maxillofac. Surg. 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.; Aslam, M.N.; Stall, J.N.; Udager, A.M.; Chiosea, S.; McHugh, J.B. Clinicopathologic and Im-munophenotypic Characterization of 25 Cases of Acinic Cell Carcinoma with High-Grade Transformation. Head. Neck Pathol. 2015, 10, 152–160. [Google Scholar] [CrossRef] [PubMed]
| Benign (n = 161) No. (%) | Malignant (n = 60) No. (%) | ||
|---|---|---|---|
| Onset age (years) | 0–20 | 6 (3.7) | 0 (0) |
| 21–30 | 11 (6.8) | 0 (0) | |
| 31–40 | 23 (14.3) | 1 (1.7) | |
| 41–50 | 32 (19.9) | 4 (6.7) | |
| 51–60 | 32 (19.9) | 16 (26.7) | |
| 61–70 | 35 (21.7) | 15 (25) | |
| 71–80 | 19 (11.8) | 16 (26.7) | |
| 81–90 | 3 (1.9) | 7 (11.7) | |
| >91 | 0 (0) | 1 (1.7) | |
| Gender | Male | 73 (45.3) | 44 (73.3) |
| Female | 88 (54.7) | 16 (26.7) | |
| Residence | Rural | 71 (44) | 34 (56.7) |
| Urban | 90 (56) | 26 (43.3) | |
| Surgery | Biopsy of the salivary gland or duct | 8 (5) | 6 (10) |
| Total parotidectomy with facial nerve resection sacrifice | 8 (5) | 12 (20) | |
| Total parotidectomy with facial nerve preservation | 27 (16.7) | 10 (16.7) | |
| Partial parotidectomy | 96 (59.6) | 8 (13.3) | |
| Lymph node biopsy | 2 (1.2) | 1 (1.7) | |
| Radical excision of cervical lymph nodes | 1 (0.60) | 0 (0) | |
| Other procedures | 19 (11.8) | 23 (38.3) |
| Clinicopathological Criteria | Pleomorphic Adenoma (n = 74) No. (%) | Basal Cell Adenoma (n = 8) No. (%) | Warthin Tumor (n = 23) No. (%) | p Value * | |
|---|---|---|---|---|---|
| Age groups (years) | 0–20 | 4 (5.4) | 0 (0) | 0 (0) | p = 0.014 |
| 21–30 | 8 (10.8) | 0 (0) | 0 (0) | ||
| 31–40 | 15 (20.3) | 0 (0) | 2 (8.7) | ||
| 41–50 | 13 (17.6) | 1 (12.5) | 5 (21.7) | ||
| 51–60 | 19 (25.7) | 0 (0) | 5 (21.7) | ||
| 61–70 | 11 (14.9) | 5 (62.5) | 7 (30.4) | ||
| 71–80 | 4 (5.4) | 1 (12.5) | 4 (17.4) | ||
| 81–90 | 0 (0) | 1 (12.5) | 0(0) | ||
| Gender | Male | 31 (41.9) | 4 (50) | 21 (91.3) | p < 0.001 |
| Female | 43 (58.1) | 4 (50) | 2 (8.7) | ||
| Surgery | Partial parotidectomy | 49 (66.2) | 5 (62.5) | 16 (69.6) | p = 0.054 |
| Total parotidectomy with facial nerve preservation | 18 (24.3) | 0 (0) | 3 (13) | ||
| Total parotidectomy with partial facial nerve sacrifice | 2 (2.7) | 2 (25) | 0 (0) | ||
| Other procedures | 5 (6.8) | 1 (12.5) | 4 (17.4) | ||
| Clinicopathological Criteria | Primary Tumors (n = 25) No. (%) | MTS (n = 26) No. (%) | p Value * | |
|---|---|---|---|---|
| Onset age, years | 11–20 | 1 (4) | 0 (0) | p = 0.811 |
| 31–40 | 1 (4) | 3 (11.5) | ||
| 41–50 | 1 (4) | 1 (3.8) | ||
| 51–60 | 5 (20) | 4 (15.4) | ||
| 61–70 | 8 (32) | 5 (19.2) | ||
| 71–80 | 6 (24) | 9 (34.6) | ||
| >80 | 3 (12) | 4 (15.4) | ||
| Gender | Male | 12 (48) | 15 (57.7) | p = 0.488 |
| Female | 13 (52) | 11 (42.3) | ||
| Residence | Rural | 16 (64) | 15 (57.7) | p = 0.645 |
| Urban | 9 (36) | 11 (42.3) | ||
| Size | <2 cm | 2 (8) | 7 (26.9) | p = 0.170 |
| 2–4 cm | 9 (36) | 5 (19.2) | ||
| >4 cm | 14 (56) | 14 (53.8) | ||
| Surgery | Partial parotidectomy | 10 (40) | 7 (26.9) | p = 0.401 |
| Total parotidectomy with facial nerve preservation | 4 (16) | 3 (11.5) | ||
| Total parotidectomy with facial nerve sacrifice | 5 (20) | 11 (42.3) | ||
| Other procedures | 6 (24) | 5 (19.2) | ||
| Evolution | Aggravated, discharged upon request | 0 (0) | 1 (3.8) | p = 0.289 |
| Improved | 1 (4) | 3 (11.5) | ||
| Stationary | 1 (4) | 3 (11.5) | ||
| Healed | 23 (92) | 19 (73.1) | ||
| Malignant Tumor Size | p Value * | ||||
|---|---|---|---|---|---|
| <2 cm No. (%) | 2–4 cm No. (%) | >4 cm No. (%) | |||
| Gender | Male | 8 (88.9) | 5 (35.7) | 14 (50) | p = 0.036 |
| Female | 1 (11.1) | 9 (64.3) | 14 (50) | ||
| Location | Right | 8 (88.9) | 3 (21.4) | 13 (46.4) | p = 0.007 |
| Left | 1 (11.1) | 11 (78.6) | 15 (53.6) | ||
| Surgery | Biopsy of the salivary gland or duct | 2 (22.2) | 0 (0) | 1 (3.6) | p = 0.057 |
| Partial parotidectomy | 0 (0) | 3 (21.4) | 4 (14.3) | ||
| Total parotidectomy with facial nerve preservation | 5 (55.6) | 7 (50) | 5 (17.9) | ||
| Total parotidectomy with facial nerve resection sacrifice | 1 (11.1) | 2 (14.3) | 13 (46.4) | ||
| Other procedures | 1 (11.1) | 2 (14.3) | 5 (17.9) | ||
| Evolution | Aggravated, discharged upon request | 0 (0) | 0 (0) | 1 (3.6) | p = 0.052 |
| Improved | 1 (11.1) | 0 (0) | 3 (10.7) | ||
| Stationary | 3 (33.3) | 0 (0) | 1 (3.6) | ||
| Healed | 5 (55.6) | 14 (100) | 23 (82.1) | ||
| Partial Parotidectomy No. (%) | Total Parotidectomy with Facial Nerve Preservation No. (%) | Total Parotidectomy with Facial Nerve Sacrifice No. (%) | Other Procedures No. (%) | p Value * | ||
|---|---|---|---|---|---|---|
| Gender | Male | 46 (52.9) | 13 (46.4) | 13 (65) | 11 (52.4) | p = 0.650 |
| Female | 41 (47.1) | 15 (53.6) | 7 (35) | 10 (47.6) | ||
| Total | 87 (100) | 28 (100) | 20 (100) | 21 (100) | ||
| Size | <2 cm | 13 (14.9) | 5 (17.9) | 1 (5) | 3 (14.3) | p = 0.13 |
| 2–4 cm | 49 (56.3) | 14 (50) | 4 (20) | 8 (38.1) | ||
| >4 cm | 25 (28.7) | 9 (32.1) | 15 (75) | 10 (47.6) | ||
| Total | 87 (100) | 28(100) | 20 (100) | 21 (100) | ||
| Evolution | Aggravated, discharged upon request | 0 (0) | 0 (0) | 1 (5) | 0 (0) | p < 0.001 |
| Improved | 1 (1.1) | 0 (0) | 3 (15) | 0 (0) | ||
| Stationary | 1 (1.1) | 0 (0) | 0 (3.6) | 4 (19) | ||
| Healed | 85 (97.7) | 28 (100) | 16 (80) | 17 (81) | ||
| Total | 87 (100) | 28 (100) | 20 (100) | 21 (100) |
| Histopathological Type (n) | |||||
|---|---|---|---|---|---|
| Benign | Malignant | Total | |||
| Clinical diagnosis | Benign | 101 | 15 | 116 | Sensitivity 70.6% Specificity 96.2% |
| Malignant | 4 | 36 | 40 | ||
| Total | 105 | 51 | 156 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, L.-B.; Ghiciuc, C.-M.; Costan, V.V.; Ungureanu, C.; Ianole, V.; Apostol, D.-G.C. Benign and Malignant Parotid Gland Tumors: Insights from a Five-Year Northeast Romanian Population. J. Clin. Med. 2025, 14, 7087. https://doi.org/10.3390/jcm14197087
Ungureanu L-B, Ghiciuc C-M, Costan VV, Ungureanu C, Ianole V, Apostol D-GC. Benign and Malignant Parotid Gland Tumors: Insights from a Five-Year Northeast Romanian Population. Journal of Clinical Medicine. 2025; 14(19):7087. https://doi.org/10.3390/jcm14197087
Chicago/Turabian StyleUngureanu, Loredana-Beatrice, Cristina-Mihaela Ghiciuc, Victor Vlad Costan, Carmen Ungureanu, Victor Ianole, and Delia-Gabriela Ciobanu Apostol. 2025. "Benign and Malignant Parotid Gland Tumors: Insights from a Five-Year Northeast Romanian Population" Journal of Clinical Medicine 14, no. 19: 7087. https://doi.org/10.3390/jcm14197087
APA StyleUngureanu, L.-B., Ghiciuc, C.-M., Costan, V. V., Ungureanu, C., Ianole, V., & Apostol, D.-G. C. (2025). Benign and Malignant Parotid Gland Tumors: Insights from a Five-Year Northeast Romanian Population. Journal of Clinical Medicine, 14(19), 7087. https://doi.org/10.3390/jcm14197087







