Arthroscopic Management of Patellar Instability in Skeletally Immature Patients: Current Concepts and Future Directions
Abstract
1. Introduction
2. Methods
3. Epidemiology and Etiology of Patellar Instability in Skeletally Immature Patients
4. Diagnostic Approach to Patellar Instability
4.1. Clinical Evaluation
4.2. Radiographic Evaluation
4.3. Updated Dejour Classification and the Menu à la Carte
4.4. MRI-Based Exploration
4.5. Advanced and Emerging Imaging Modalities
4.6. Diagnostic Arthroscopy
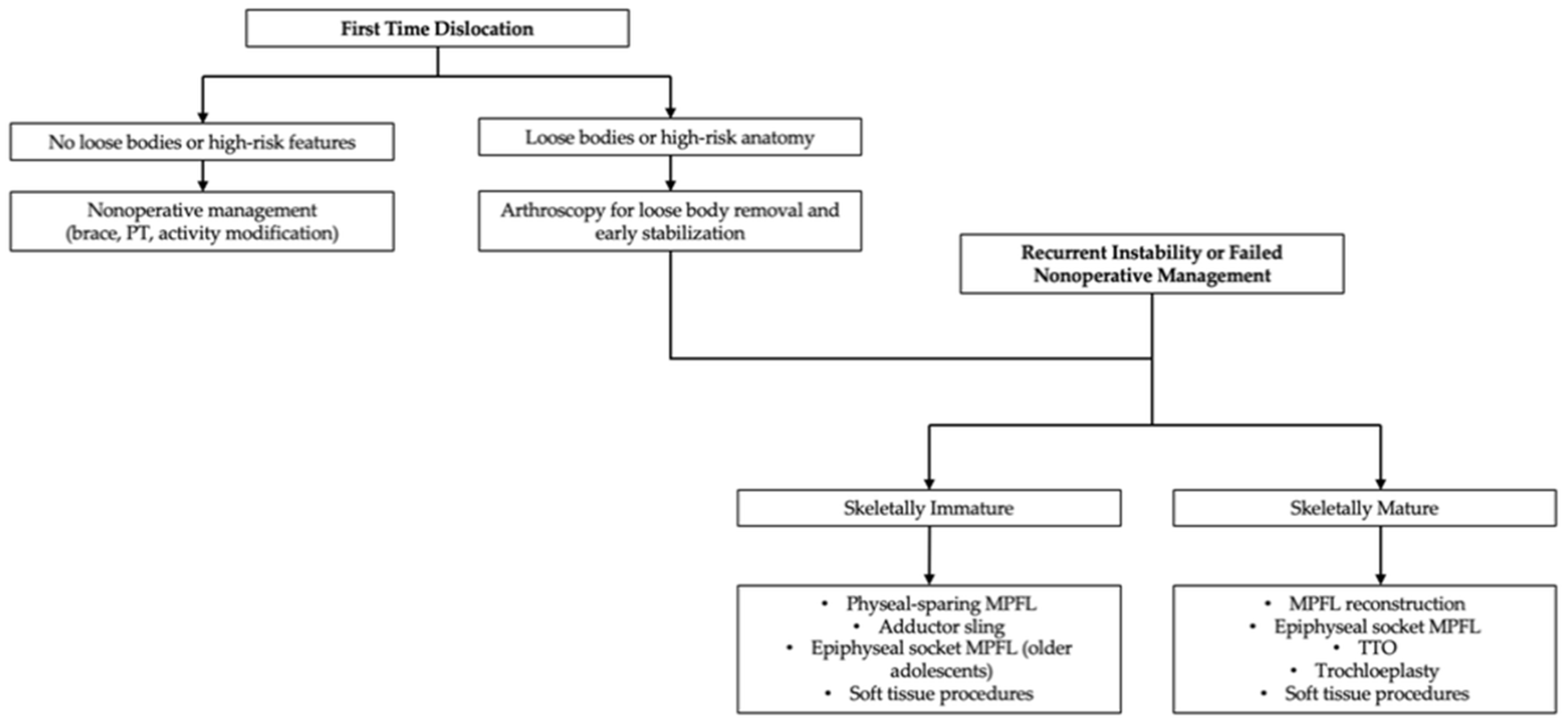
4.7. Indications for Surgical Intervention and Decision-Making
5. Surgical Techniques
5.1. Medial Patellafeoral Ligament Reconstruction
5.2. Tibial Tubercle Osteotomy: Caution in Skeletally Immature Patients
5.3. Trochleoplasty
5.4. Soft Tissue Procedures: Lateral Release and Imbrication
6. Innovations and Future Directions
6.1. Robot-Assisted Navigation
6.2. Bioengineered Scaffolds for Cartilage Restoration
6.3. 3D Modeling and Patient-Specific Surgical Planning
7. Knowledge Gaps and Research Needs
7.1. Lack of Pediatric-Specific Outcome Measures
7.2. Need for Long-Term Studies on Skeletally Immature Patients
7.3. Standardization of Indications and Rehabilitation Protocols
8. Clinical Considerations and Challenges
9. Rehabilitation
10. Strengths and Limitations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TT-TG | Tibial tuberosity to trochlear groove |
| TT-PCL | Tibial tuberosity to posterior cruciate ligament |
| MPFL | Medial patellofemoral ligament |
| 3D | Three-dimensional |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| TTO | Tibial tubercle osteotomy |
| 2D | Two-dimensional |
| PROMs | Patient-reported outcome measures |
| AP | Anteroposterior |
References
- Waterman, B.R.; Belmont, P.J.; Owens, B.D. Patellar Dislocation in the United States: Role of Sex, Age, Race, and Athletic Participation. J. Knee Surg. 2012, 25, 51–58. [Google Scholar] [CrossRef]
- Stefancin, J.J.; Parker, R.D. First-Time Traumatic Patellar Dislocation: A Systematic Review. Clin. Orthop. Relat. Res. 2007, 455, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lewallen, L.W.; McIntosh, A.L.; Dahm, D.L. Predictors of Recurrent Instability after Acute Patellofemoral Dislocation in Pediatric and Adolescent Patients. Am. J. Sports Med. 2013, 41, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Höhne, S.; Gerlach, K.; Irlenbusch, L.; Schulz, M.; Kunze, C.; Finke, R. Patella Dislocation in Children and Adolescents. Z. Orthop. Unf. 2017, 155, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.; Bowman, K.F.; Walsh, C.; Sabb, B.J.; Vanderhave, K.L. Magnetic Resonance Imaging of Acute Patellar Dislocation in Children: Patterns of Injury and Risk Factors for Recurrence. J. Pediatr. Orthop. 2012, 32, 145–155. [Google Scholar] [CrossRef]
- Barzan, M.; Maine, S.; Modenese, L.; Lloyd, D.G.; Carty, C.P. Patellofemoral Joint Alignment Is a Major Risk Factor for Recurrent Patellar Dislocation in Children and Adolescents: A Systematic Review. J. ISAKOS 2018, 3, 287–297. [Google Scholar] [CrossRef]
- Hasler, C.C.; Studer, D. Patella Instability in Children and Adolescents. EFORT Open Rev. 2016, 1, 160–166. [Google Scholar] [CrossRef]
- Jaquith, B.P.; Parikh, S.N. Predictors of Recurrent Patellar Instability in Children and Adolescents after First-Time Dislocation. J. Pediatr. Orthop. 2017, 37, 484–490. [Google Scholar] [CrossRef]
- Migliorini, F.; Marsilio, E.; Cuozzo, F.; Oliva, F.; Eschweiler, J.; Hildebrand, F.; Maffulli, N. Chondral and Soft Tissue Injuries Associated to Acute Patellar Dislocation: A Systematic Review. Life 2021, 11, 1360. [Google Scholar] [CrossRef]
- Grantham, W.J.; Aman, Z.S.; Brady, A.W.; Rosenberg, S.I.; Turnbull, T.L.; Storaci, H.W.; Dornan, G.J.; LaPrade, R.F. Medial Patellotibial Ligament Reconstruction Improves Patella Tracking When Combined with Medial Patellofemoral Reconstruction: An in Vitro Kinematic Study. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 2501–2509. [Google Scholar] [CrossRef]
- Madry, H.; Grün, U.W.; Knutsen, G. Cartilage Repair and Joint Preservation: Medical and Surgical Treatment Options. Dtsch. Arztebl. Int. 2011, 108, 669. [Google Scholar] [PubMed]
- Sahin, E.; Tandogan, R.; Liebensteiner, M.; Demey, G.; Kayaalp, A. Management of Patellar Instability in Skeletally Immature Patients. EFORT Open Rev. 2024, 9, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Stavinoha, T.J.; Shea, K.G. Physeal Sparing Approaches for MPFL Reconstruction. Curr. Rev. Musculoskelet. Med. 2023, 16, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Popkin, C.A.; Bayomy, A.F.; Trupia, E.P.; Chan, C.M.; Redler, L.H. Patellar Instability in the Skeletally Immature. Curr. Rev. Musculoskelet. Med. 2018, 11, 172–181. [Google Scholar] [CrossRef]
- Sheng, X.; Guo, L.; Jiang, J.; Liu, Z.; Chen, Y.; Wang, X.; Zhang, X.; Zhao, L.; Wu, M.; Geng, B.; et al. Robot-Assisted Medial Patellofemoral Ligament Reconstruction in the Treatment of Recurrent Patellar Dislocation Can Improve Tunnel Accuracy but Yields Similar Outcome Compared with Traditional Technique. Arthrosc. J. Arthrosc. Relat. Surg. 2025, 41, 2999–3009. [Google Scholar] [CrossRef]
- Fritz, B.; Fucentese, S.F.; Zimmermann, S.M.; Tscholl, P.M.; Sutter, R.; Pfirrmann, C.W.A. 3D-Printed Anatomic Models of the Knee for Evaluation of Patellofemoral Dysplasia in Comparison to Standard Radiographs and Computed Tomography. Eur. J. Radiol. 2020, 127, 109011. [Google Scholar] [CrossRef]
- Kristin, E.Y.; Beitler, B.; Cooperman, D.R.; Frumberg, D.; Schneble, C.; McLaughlin, W.; Fulkerson, J.P. Three-Dimensional Reproductions for Surgical Decision-Making in the Treatment of Recurrent Patella Dislocation. Arthrosc. Tech. 2023, 12, e807–e811. [Google Scholar]
- Liang, Q.; Liao, C.; Zhao, Z.; Li, J.; Zhan, H.; Liu, P.; Kang, X.; Ren, B.; Tian, B.; Zhang, L.; et al. Robot-Assisted Individualized Medial Patellofemoral Ligament Reconstruction in Skeletally Immature Patients with Recurrent Patellar Dislocation: A Single-Center Retrospective Study. Orthop. J. Sports Med. 2025, 13, 23259671251339496. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Karampikas, V.; Zikopoulos, A.; Sioutis, S.; Mastrokalos, D.; Koulalis, D.; Scarlat, M.M.; Hernigou, P. Orthobiologics: A Review. Int. Orthop. 2023, 47, 1645–1662. [Google Scholar] [CrossRef]
- McFarlane, K.H.; Coene, R.P.; Feldman, L.; Miller, P.E.; Heyworth, B.E.; Kramer, D.E.; Kocher, M.S.; Yen, Y.-M.; Milewski, M.D. Increased Incidence of Acute Patellar Dislocations and Patellar Instability Surgical Procedures across the United States in Paediatric and Adolescent Patients. J. Child. Orthop. 2021, 15, 149–156. [Google Scholar] [CrossRef]
- Poorman, M.; Talwar, D.; SanJuan, J.; Baldwin, K.; Sutliff, N.; Franklin, C.C. Increasing Hospital Admissions for Patellar Instability: A National Database Study from 2004–2017. Orthop. J. Sports Med. 2019, 7, 2325967119S00145. [Google Scholar] [CrossRef]
- VandenBerg, C.D.; Sarkisova, N.; Pace, J.L.; Rhodes, J.; Perea, S.H.; Green, D.W. Current Practice Trends in the Surgical Management of Patellofemoral Instability: A Survey of the Paediatric Research in Sports Medicine (PRiSM) Society. J. Child. Orthop. 2021, 15, 571–576. [Google Scholar] [CrossRef]
- Lewallen, L.; McIntosh, A.; Dahm, D. First-Time Patellofemoral Dislocation: Risk Factors for Recurrent Instability. J. Knee Surg. 2015, 28, 303–310. [Google Scholar] [CrossRef] [PubMed]
- DeVries, C.A.; Bomar, J.D.; Pennock, A.T. Prevalence of Trochlear Dysplasia and Associations with Patellofemoral Pain and Instability in a Skeletally Mature Population. J. Bone Jt. Surg. 2021, 103, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Won, S.H.; Park, M.S.; Sung, K.H. Normative Values of Tibial Tubercle–Trochlear Groove Distance and Tibial Tubercle–Posterior Cruciate Ligament Distance in Children. Am. J. Sports Med. 2023, 51, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Daynes, J.; Hinckel, B.B.; Farr, J. Tibial Tuberosity—Posterior Cruciate Ligament Distance. J. Knee Surg. 2016, 29, 471–477. [Google Scholar] [CrossRef]
- Su, P.; Jian, N.; Mao, B.; Zhang, Z.; Li, J.; Fu, W. Defining the Role of TT-TG and TT-PCL in the Diagnosis of Lateralization of the Tibial Tubercle in Recurrent Patellar Dislocation. BMC Musculoskelet. Disord. 2021, 22, 52. [Google Scholar] [CrossRef]
- Brady, J.M.; Rosencrans, A.S.; Shubin Stein, B.E. Use of TT-PCL versus TT-TG. Curr. Rev. Musculoskelet. Med. 2018, 11, 261–265. [Google Scholar] [CrossRef]
- Seitlinger, G.; Scheurecker, G.; Högler, R.; Labey, L.; Innocenti, B.; Hofmann, S. Tibial Tubercle–Posterior Cruciate Ligament Distance: A New Measurement to Define the Position of the Tibial Tubercle in Patients with Patellar Dislocation. Am. J. Sports Med. 2012, 40, 1119–1125. [Google Scholar] [CrossRef]
- Rethlefsen, S.A.; Nguyen, D.T.; Wren, T.A.L.; Milewski, M.D.; Kay, R.M. Knee Pain and Patellofemoral Symptoms in Patients with Cerebral Palsy. J. Pediatr. Orthop. 2015, 35, 519–522. [Google Scholar] [CrossRef]
- Becher, C.; Fleischer, B.; Rase, M.; Schumacher, T.; Ettinger, M.; Ostermeier, S.; Smith, T. Effects of Upright Weight Bearing and the Knee Flexion Angle on Patellofemoral Indices Using Magnetic Resonance Imaging in Patients with Patellofemoral Instability. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, O.; Steensen, R.N.; Rullkoetter, P.J.; Fitzpatrick, C.K. Computational Approach to Correcting Joint Instability in Patients with Recurrent Patellar Dislocation. J. Orthop. Res. 2020, 38, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, C.S.; Stein, B.E.S.; Matuz, D.; Henry, J.H. Immediate Surgical Repair of the Medial Patellar Stabilizers for Acute Patellar Dislocation: A Review of Eight Cases. Am. J. Sports Med. 2000, 28, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.J.; Bell, R.H.; Anisette, G. Acute Patellar Dislocations. Am. J. Sports Med. 1986, 14, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Palmu, S.; Kallio, P.E.; Donell, S.T.; Helenius, I.; Nietosvaara, Y. Acute Patellar Dislocation in Children and Adolescents: A Randomized Clinical Trial. J. Bone Jt. Surg. 2008, 90, 463–470. [Google Scholar] [CrossRef]
- Patel, N.B.; Oak, S.R.; Rogers, K.; Crawford, E.A. The Onset and Development of Patella Alta in Children with Patellar Instability. J. Pediatr. Orthop. 2023, 43, 447–452. [Google Scholar] [CrossRef]
- Insall, J.; Salvati, E. Patella Position in the Normal Knee Joint. Radiology 1971, 101, 101–104. [Google Scholar] [CrossRef]
- Kurowecki, D.; Shergill, R.; Cunningham, K.M.; Peterson, D.C.; Takrouri, H.S.R.; Habib, N.O.; Ainsworth, K.E. A Comparison of Sagittal MRI and Lateral Radiography in Determining the Insall–Salvati Ratio and Diagnosing Patella Alta in the Pediatric Knee. Pediatr. Radiol. 2022, 52, 527–532. [Google Scholar] [CrossRef]
- Suomalainen, J.-S.; Regalado, G.; Joukainen, A.; Kääriäinen, T.; Könönen, M.; Manninen, H.; Sipola, P.; Kokki, H. Effects of Knee Flexion and Extension on the Tibial Tuberosity–Trochlear Groove (TT–TG) Distance in Adolescents. J. Exp. Orthop. 2018, 5, 31. [Google Scholar] [CrossRef]
- Dejour, D.H.; Mazy, D.; Pineda, T.; Cance, N.; Dan, M.J.; de Sanctis, E.G. Patellar Instability: Current Approach. EFORT Open Rev. 2025, 10, 378–387. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Cram, T.R.; James, E.W.; Rasmussen, M.T. Trochlear Dysplasia and the Role of Trochleoplasty. Clin. Sports Med. 2014, 33, 531–545. [Google Scholar] [CrossRef]
- Kazley, J.M.; Banerjee, S. Classifications in Brief: The Dejour Classification of Trochlear Dysplasia. Clin. Orthop. Relat. Res. 2019, 477, 2380–2386. [Google Scholar] [CrossRef]
- Huntington, L.S.; Webster, K.E.; Devitt, B.M.; Feller, J.A. Risk Assessment and Management of Primary Patellar Dislocation Is Complex and Multifactorial: A Survey of Australian Knee Surgeons. J. ISAKOS 2021, 6, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.J.; Elrick, B.P.; Carpenter, M.L.; Raji, Y.; McQuivey, K.S.; Sherman, S.L.; Frank, R.M. Tibial Tubercle Osteotomy: Indications, Outcomes, and Complications. Curr. Rev. Musculoskelet. Med. 2024, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Dejour, D.H.; Mesnard, G.; de Sanctis, E. Updated Treatment Guidelines for Patellar Instability: “Un Menu à La Carte”. J. Exp. Orthop. 2021, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Babyn, P.; Astori, I.; White, L.; Doria, A.; Cole, W. MRI of Traumatic Patellar Dislocation in Children. Pediatr. Radiol. 2006, 36, 1163–1170. [Google Scholar] [CrossRef]
- Askenberger, M.; Arendt, E.A.; Ekström, W.; Voss, U.; Finnbogason, T.; Janarv, P.-M. Medial Patellofemoral Ligament Injuries in Children with First-Time Lateral Patellar Dislocations: A Magnetic Resonance Imaging and Arthroscopic Study. Am. J. Sports Med. 2016, 44, 152–158. [Google Scholar] [CrossRef]
- Wilson, A.; Afarin, A.; Shaw, C.; Shirley, E.; Pierce, J.; Slakey, J.B. Magnetic Resonance Imaging Findings After Acute Patellar Dislocation in Children. Orthop. J. Sports Med. 2013, 1, 2325967113512460. [Google Scholar] [CrossRef]
- Mallinos, A.; Jones, K. The Double-Edged Sword: Anterior Cruciate Ligament Reconstructions on Adolescent Patients—Growth Plate Surgical Challenges and Future Considerations. J. Clin. Med. 2024, 13, 7522. [Google Scholar] [CrossRef]
- Shamrock, A.G.; Day, M.A.; Duchman, K.R.; Glass, N.; Westermann, R.W. Medial Patellofemoral Ligament Reconstruction in Skeletally Immature Patients: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2019, 7, 2325967119855023. [Google Scholar] [CrossRef]
- Mariani, S.; La Marra, A.; Arrigoni, F.; Necozione, S.; Splendiani, A.; Di Cesare, E.; Barile, A.; Masciocchi, C. Dynamic Measurement of Patello-Femoral Joint Alignment Using Weight-Bearing Magnetic Resonance Imaging (WB-MRI). Eur. J. Radiol. 2015, 84, 2571–2578. [Google Scholar] [CrossRef]
- Hansen, P.; Harving, M.; Øhlenschlæger, T.; Brinch, S.; Lavard, P.; Krogsgaard, M.; Boesen, M. Comparison between Conventional MRI and Weight-Bearing Positional MRI Reveals Important Differences in Radiological Measurements of the Patellofemoral Joint. Skelet. Radiol. 2023, 52, 1525–1534. [Google Scholar] [CrossRef]
- Schneble, C.A.; Yu, K.; Venkadesan, M.; Cooperman, D.; Beitler, B.; Sieberer, J.; Fulkerson, J. Three-Dimensional Imaging of the Patellofemoral Joint Improves Understanding of Trochlear Anatomy and Pathology and Planning of Realignment. Arthrosc. J. Arthrosc. Relat. Surg. 2025, 41, 130–140. [Google Scholar] [CrossRef]
- Pennock, A.T.; Chang, A.; Doan, J.; Bomar, J.D.; Edmonds, E.W. 3D Knee Trochlear Morphology Assessment by Magnetic Resonance Imaging in Patients with Normal and Dysplastic Trochleae. J. Pediatr. Orthop. 2020, 40, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.J.; Elias, J.J.; Williams, A.A.; Demehri, S.; Cosgarea, A.J. Characterization of Patellar Maltracking Using Dynamic Kinematic CT Imaging in Patients with Patellar Instability. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.G.; Ostlere, S.J.; Pal, C.; Phillips, A.; Reid, H.; Dodd, C. Assessment of Patellar Maltracking Using Combined Static and Dynamic MRI. Eur. Radiol. 2000, 10, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, K.; Mrozek, T.; Jancewicz, P.; Sar, M.; Hermanowicz, J.; Szajwa, L.; Góralczyk, A. All-Arthroscopic Management of Lateral Patellar Instability. Arthrosc. Tech. 2024, 13, 103055. [Google Scholar] [CrossRef]
- Vollnberg, B.; Koehlitz, T.; Jung, T.; Scheffler, S.; Hoburg, A.; Khandker, D.; Hamm, B.; Wiener, E.; Diederichs, G. Prevalence of Cartilage Lesions and Early Osteoarthritis in Patients with Patellar Dislocation. Eur. Radiol. 2012, 22, 2347–2356. [Google Scholar] [CrossRef]
- Tanaka, M.J.; Mirochnik, K.; Esfahani, S.A.; Lubberts, B.; Waryasz, G.; Bhimani, R. Arthroscopic Patellofemoral Measurements Can Reliably Assess Patellar Instability. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 902–910. [Google Scholar] [CrossRef]
- Mazzolaa, C.; Mantovani, D. Patellofemoral Malalignment and Chondral Damage: Current Concepts. Joints 2013, 1, 27. [Google Scholar]
- Hinckel, B.; Smith, J.; Tanaka, M.J.; Matsushita, T.; Martinez-Cano, J.P. Patellofemoral Instability Part 1 (When to Operate and Soft Tissue Procedures): State of the Art. J. ISAKOS 2025, 10, 100278. [Google Scholar] [CrossRef]
- Jain, N.P.; Khan, N.; Fithian, D.C. A Treatment Algorithm for Primary Patellar Dislocations. Sports Health 2011, 3, 170–174. [Google Scholar] [CrossRef]
- Straume-Næsheim, T.M.; Randsborg, P.-H.; Mikaelsen, J.R.; Sivertsen, E.A.; Devitt, B.; Granan, L.-P.; Årøen, A. Recurrent Lateral Patella Dislocation Affects Knee Function as Much as ACL Deficiency–However Patients Wait Five Times Longer for Treatment. BMC Musculoskelet. Disord. 2019, 20, 318. [Google Scholar] [CrossRef]
- Kutschke, M.J.; Albright, J.A.; Winschel, J.M.; He, E.W.; Cruz, A.I.; Daniels, A.H.; Owens, B.D. Increased Risk of Patellofemoral Instability Events and Surgical Management in Patients with Joint Hypermobility Syndromes: A Matched Cohort Analysis. Arthrosc. Sports Med. Rehabil. 2024, 6, 100995. [Google Scholar] [CrossRef] [PubMed]
- Nomura, E.; Inoue, M.; Osada, N. Anatomical Analysis of the Medial Patellofemoral Ligament of the Knee, Especially the Femoral Attachment. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hendawi, T.; Godshaw, B.; Flowers, C.; Stephens, I.; Haber, L.; Waldron, S. Autograft vs Allograft Comparison in Pediatric Medial Patellofemoral Ligament Reconstruction. Ochsner J. 2019, 19, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Tscholl, P.M.; Ernstbrunner, L.; Pedrazzoli, L.; Fucentese, S.F. The Relationship of Femoral Tunnel Positioning in Medial Patellofemoral Ligament Reconstruction on Clinical Outcome and Postoperative Complications. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 2410–2416. [Google Scholar] [CrossRef]
- Stephen, J.M.; Kaider, D.; Lumpaopong, P.; Deehan, D.J.; Amis, A.A. The Effect of Femoral Tunnel Position and Graft Tension on Patellar Contact Mechanics and Kinematics after Medial Patellofemoral Ligament Reconstruction. Am. J. Sports Med. 2014, 42, 364–372. [Google Scholar] [CrossRef]
- Alm, L.; Krause, M.; Mull, C.; Frosch, K.-H.; Akoto, R. Modified Adductor Sling Technique: A Surgical Therapy for Patellar Instability in Skeletally Immature Patients. Knee 2017, 24, 1282–1288. [Google Scholar] [CrossRef]
- Uppstrom, T.J.; Price, M.; Black, S.; Gausden, E.; Haskel, J.; Green, D.W. Medial Patellofemoral Ligament (MPFL) Reconstruction Technique Using an Epiphyseal Femoral Socket with Fluoroscopic Guidance Helps Avoid Physeal Injury in Skeletally Immature Patients. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3536–3542. [Google Scholar] [CrossRef]
- Zhang, Q.; Ying, L.; Han, D.; Ye, L.; Tung, T.-H.; Liang, J.; Liu, P.; Zhou, X. Arthroscopic Reconstruction of the Medial Patellofemoral Ligament in Skeletally Immature Patients Using the Modified Sling Procedure: A Novel Technique for MPFL Reconstruction. J. Orthop. Surg. Res. 2023, 18, 334. [Google Scholar] [CrossRef]
- Redler, L.H.; Wright, M.L. Surgical Management of Patellofemoral Instability in the Skeletally Immature Patient. J. Am. Acad. Orthop. Surg. 2018, 26, e405–e415. [Google Scholar] [CrossRef]
- Ladenhauf, H.N.; Berkes, M.B.; Green, D.W. Medial Patellofemoral Ligament Reconstruction Using Hamstring Autograft in Children and Adolescents. Arthrosc. Tech. 2013, 2, e151–e154. [Google Scholar] [CrossRef]
- Bremond, N.; Prima, R.; Rabattu, P.-Y.; Accadbled, F.; Chotel, F.; Konkel, M.; Eid, A.; Philippe, C.; Godinho, A.; Turati, M.; et al. Isolated MPFL Reconstruction with Soft Tissue Femoral Fixation Technique in 54 Skeletally Immature Patients: Clinical Outcomes at 2 Years Follow-up. A French Multicenter Retrospective Study. Orthop. Traumatol. Surg. Res. 2023, 109, 103530. [Google Scholar] [CrossRef]
- Song, J.-G.; Kang, S.-B.; Oh, S.-H.; Han, J.-H.; Shah, D.; Park, H.-J.; Kholmurodov, U.T.; Nha, K.-W. Medial Soft-Tissue Realignment versus Medial Patellofemoral Ligament Reconstruction for Recurrent Patellar Dislocation: Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.A. MPFL Reconstruction for PF Instability. The Soft (Tissue) Approach. Rev. Chir. Orthopédique Traumatol. 2009, 95, 371–374. [Google Scholar] [CrossRef]
- Morgan, V.K.; Warrier, A.A.; Credille, K.; Wang, Z.; Elias, T.; Haneberg, E.; Hevesi, M.; Yanke, A.B. Medial Patellofemoral Ligament Reconstruction in Skeletally Immature Patients: A Systematic Review of Outcomes by Fixation Technique. Orthop. J. Sports Med. 2025, 13, 23259671251322724. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.; Criseo, N.; Annibaldi, A.; Carrozzo, A.; Pagnotta, S.M.; Cantagalli, M.R.; Orlandi, P.; Daggett, M. Medial Patellofemoral Ligament Reconstruction Using Gracilis Tendon Graft and “All Suture” Knotless Anchors for Patellar Fixation. Arthrosc. Tech. 2023, 12, e2329–e2334. [Google Scholar] [CrossRef]
- Migliorini, F.; Driessen, A.; Quack, V.; Schenker, H.; Tingart, M.; Eschweiler, J. Patellar Fixation Graft via Suture Anchors versus Tunnel Techniques during Isolated MPFL Reconstruction for Recurrent Patellofemoral Instability: A Systematic Review of the Literature. Arch. Orthop. Trauma. Surg. 2020, 140, 1201–1210. [Google Scholar] [CrossRef]
- Deasey, M.J.; Moran, T.E.; Lesevic, M.; Burnett, Z.R.; Diduch, D.R. Small, Short, Oblique Patellar Tunnels for Patellar Fixation Do Not Increase Fracture Risk or Complications in MPFL Reconstruction: A Retrospective Cohort Study. Orthop. J. Sports Med. 2020, 8, 2325967120954430. [Google Scholar] [CrossRef]
- Burrus, M.T.; Werner, B.C.; Conte, E.J.; Diduch, D.R. Troubleshooting the Femoral Attachment during Medial Patellofemoral Ligament Reconstruction: Location, Location, Location. Orthop. J. Sports Med. 2015, 3, 2325967115569198. [Google Scholar] [CrossRef]
- Stephen, J.M.; Kittl, C.; Williams, A.; Zaffagnini, S.; Marcheggiani Muccioli, G.M.; Fink, C.; Amis, A.A. Effect of Medial Patellofemoral Ligament Reconstruction Method on Patellofemoral Contact Pressures and Kinematics. Am. J. Sports Med. 2016, 44, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.J.; Jones, K.C.; Lalonde, M.K.; Gabra, J.N.; Rezvanifar, S.C.; Cosgarea, A.J. Allowing One Quadrant of Patellar Lateral Translation during Medial Patellofemoral Ligament Reconstruction Successfully Limits Maltracking without Overconstraining the Patella. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.K.; Grawe, B.; Magnussen, R.A.; Ceasar, A.; Parikh, S.N.; Wall, E.J.; Colosimo, A.J.; Kaeding, C.C.; Myer, G.D. Outcomes after Isolated Medial Patellofemoral Ligament Reconstruction for the Treatment of Recurrent Lateral Patellar Dislocations: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2016, 44, 2993–3005. [Google Scholar] [CrossRef] [PubMed]
- Desio, S.M.; Burks, R.T.; Bachus, K.N. Soft Tissue Restraints to Lateral Patellar Translation in the Human Knee. Am. J. Sports Med. 1998, 26, 59–65. [Google Scholar] [CrossRef]
- McCarthy, M.A.; Bollier, M.J. Medial Patella Subluxation: Diagnosis and Treatment. Iowa Orthop. J. 2015, 35, 26. [Google Scholar]
- Christoforakis, J.; Bull, A.M.J.; Strachan, R.K.; Shymkiw, R.; Senavongse, W.; Amis, A.A. Effects of Lateral Retinacular Release on the Lateral Stability of the Patella. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 273–277. [Google Scholar] [CrossRef]
- Levy, B.J.; Jimenez, A.E.; Fitzsimmons, K.P.; Pace, J.L. Medial Patellofemoral Ligament Reconstruction and Lateral Retinacular Lengthening in the Skeletally Immature Patient. Arthrosc. Tech. 2020, 9, e737–e745. [Google Scholar] [CrossRef]
- Sanguanjit, P.; Rujiraphum, P.; Apivatgaroon, A.; Chernchujit, B. Medium to Long-Term Outcomes of Medial Patellofemoral Ligament Reconstruction Using the Superficial Quadriceps versus a Hamstring Autograft in Patellar Instability Patients. Sci. Rep. 2023, 13, 13353. [Google Scholar] [CrossRef]
- Duke, A.J.; Dai, A.; Botros, D.; Leatherwood, W.; Montemurro, N.J.; Richardson, M.; Grossman, M. A Patella-Sided Tensioning Technique for Medial Patellofemoral Ligament Reconstruction. Arthrosc. Tech. 2023, 12, e483–e489. [Google Scholar] [CrossRef]
- Lorbach, O.; Zumbansen, N.; Kieb, M.; Efe, T.; Pizanis, A.; Kohn, D.; Haupert, A. Medial Patellofemoral Ligament Reconstruction: Impact of Knee Flexion Angle during Graft Fixation on Dynamic Patellofemoral Contact Pressure—A Biomechanical Study. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1072–1082. [Google Scholar] [CrossRef]
- Zirbes, C.F.; Henriquez, A.; Amanah, A.; Therien, A.D.; Perez-Espina, S.; Dorrestein, E.; Zheng, D.; Lilly, J.; Luo, E.J.; Fox, M.A.; et al. Physeal-Sparing Soft Tissue Realignment in Pediatric Patellofemoral Instability Patients: A Review of Treatment Options and Outcomes. J. Clin. Med. 2025, 14, 1116. [Google Scholar] [CrossRef]
- Husen, M.; Milbrandt, T.A.; Shah, V.; Krych, A.J.; Stuart, M.J.; Saris, D.B.F. Medial Patellofemoral Ligament Reconstruction Using Allografts in Skeletally Immature Patients. Am. J. Sports Med. 2023, 51, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mou, L.; Zhang, S.; Liu, W.; Remila, A.; Han, M.; Xiang, W.; Fang, R. 3D-Printed Individualized Navigation Template versus the Fluoroscopic Guide to Defining the Femoral Tunnel for Medial Patellofemoral Ligament Reconstruction: A Retrospective Study. Medicine 2023, 102, e32729. [Google Scholar] [CrossRef] [PubMed]
- Liles, J.; Johnston, T.; Hu, J.; Riboh, J.C. Physeal-Sparing Medial Patellofemoral Ligament Reconstruction with Suture Anchor for Femoral Graft Fixation. Arthrosc. Tech. 2020, 9, e889–e895. [Google Scholar] [CrossRef] [PubMed]
- Makovicka, J.L.; Hartigan, D.E.; Patel, K.A.; Tummala, S.V.; Chhabra, A. Medial Patellofemoral Ligament Reconstruction Using All-Soft Suture Anchors for Patellar Fixation. Arthrosc. Tech. 2018, 7, e231–e237. [Google Scholar] [CrossRef]
- Uboldi, F.; Gaetano, A.C.S.O.T.; Pini-CTO, M.; Pieroni, A.; Puglia, F.; Ferrua, P.; Priano, D.; Memeo, A. Medial Patellofemoral Ligament Surgery in the Pediatric Population: A Literature Review on the Current State of the Art. Joints 2024, 2, e869. [Google Scholar]
- Alshomrani, Y. A Landscape of Recent Literature on the Predictors of Success and Failure in Medial Patellofemoral Ligament Reconstruction. Orthop. Rev. 2025, 17, 138208. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, H.; Liang, Q. Clinical Outcomes after Medial Patellofemoral Ligament Reconstruction Using Transosseous Sutures versus Suture Anchors: A Prospective Nonrandomized Controlled Trial. Orthop. J. Sports Med. 2020, 8, 2325967120917112. [Google Scholar] [CrossRef]
- Vellios, E.E.; Trivellas, M.; Arshi, A.; Beck, J.J. Recurrent Patellofemoral Instability in the Pediatric Patient: Management and Pitfalls. Curr. Rev. Musculoskelet. Med. 2020, 13, 58–68. [Google Scholar] [CrossRef]
- Marsh, J.S.; Daigneault, J.P.; Sethi, P.; Polzhofer, G.K. Treatment of Recurrent Patellar Instability with a Modification of the Roux-Goldthwait Technique. J. Pediatr. Orthop. 2006, 26, 461–465. [Google Scholar] [CrossRef]
- Capella, M.; Sabatini, L.; Camazzola, D.; Risitano, S.; D’Antonio, D.; Rea, A.; Massè, A. C-Arm Guidance During Thin Flap Arthroscopic Trochleoplasty for Chronic Patellar Instability. Arthrosc. Tech. 2023, 12, e1579–e1588. [Google Scholar] [CrossRef] [PubMed]
- Blønd, L.; Barfod, K.W. Trochlear Shape and Patient-Reported Outcomes after Arthroscopic Deepening Trochleoplasty and Medial Patellofemoral Ligament Reconstruction: A Retrospective Cohort Study Including MRI Assessments of the Trochlear Groove. Orthop. J. Sports Med. 2023, 11, 23259671231171376. [Google Scholar] [CrossRef] [PubMed]
- Hysing-Dahl, T.; Inderhaug, E. Rehabilitation after Surgery for Patellar Instability. J. Exp. Orthop. 2024, 11, e12062. [Google Scholar] [CrossRef] [PubMed]
- Riedl, G.; Holzer, L.A.; Smekal, V. Comparable Postoperative Outcomes in Patients Treated with Either Open or Arthroscopic Trochleoplasty for Patella Dysplasia. Knee Surg. Relat. Res. 2024, 36, 41. [Google Scholar] [CrossRef]
- Ferrua, P.; Compagnoni, R.; Calanna, F.; Randelli, P.S.; Dejour, D. Good Patient Satisfaction with Low Complications Rate after Trochleoplasty in Patellofemoral Instability. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3444–3450. [Google Scholar] [CrossRef]
- Høj, S.; Lundegaard, J.K.; Blønd, L.; Lavard, P.; Rechter, A.S.; Dippmann, C.; Barfod, K.W. Open and Arthroscopic Deepening Trochleoplasty Improves Post-Operative Outcomes: A Systematic Review of the Literature Reveals Lack of Comparability between Techniques. Knee Surg. Sports Traumatol. Arthrosc. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.; Kumbar, S.G.; Nukavarapu, S.P. Engineered Osteochondral Scaffolds with Bioactive Cartilage Zone for Enhanced Articular Cartilage Regeneration. Ann. Biomed. Eng. 2025, 53, 597–611. [Google Scholar] [CrossRef]
- Beitler, B.G.; Kunsel, K.; Kristin, E.Y.; Wang, A.; Tommasini, S.M.; Wiznia, D.H.; Fulkerson, J.P. Three-Dimensional Printing of Models of Patellofemoral Joint Articular Cartilage in Patients with Patella Instability for Observing Joint Congruity. Arthrosc. Tech. 2023, 12, e1853–e1858. [Google Scholar] [CrossRef]
- Shea, C.; Rustad, A.; Anchustegui, N.G.; Troyer, S.; Dingel, A.; Shea, K.; Ganley, T.J.; Milbrandt, T.A. MPFL Surgical Simulation with Femoral Drill Hole Allowing for Adjustable Graft Tension. Orthop. J. Sports Med. 2019, 7, 2325967119S00044. [Google Scholar] [CrossRef]
- Watson, N.A.D.; Duchman, K.R.; Bollier, M.J.; Grosland, N.M. A Finite Element Analysis of Medial Patellofemoral Ligament Reconstruction. Iowa Orthop. J. 2015, 35, 13. [Google Scholar]
- Elias, J.J.; Kelly, M.J.; Smith, K.E.; Gall, K.A.; Farr, J. Dynamic Simulation of the Effects of Graft Fixation Errors during Medial Patellofemoral Ligament Reconstruction. Orthop. J. Sports Med. 2016, 4, 2325967116665080. [Google Scholar] [CrossRef]
- Morris, E.J.; Gray, K.; Gibbons, P.J.; Grayson, J.; Sullivan, J.; Amorim, A.B.; Burns, J.; McKay, M.J. Evaluating the Use of PROMs in Paediatric Orthopaedic Registries. Children 2023, 10, 1552. [Google Scholar] [CrossRef]
- Kunze, K.N.; Madjarova, S.; Jayakumar, P.; Nwachukwu, B.U. Challenges and Opportunities for the Use of Patient-Reported Outcome Measures in Orthopaedic Pediatric and Sports Medicine Surgery. J. Am. Acad. Orthop. Surg. 2023, 31, e898–e905. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.; Carsen, S.; Vasireddi, A.; Mulpuri, K. Use of Patient-Reported Outcome Measures in Pediatric Orthopaedic Literature. J. Pediatr. Orthop. 2018, 38, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Khormaee, S.; Kramer, D.E.; Yen, Y.-M.; Heyworth, B.E. Evaluation and Management of Patellar Instability in Pediatric and Adolescent Athletes. Sports Health 2015, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.A.F.; Pinto, R.A.P.; Antunes, A.J.A.M.; de Oliveira, P.A.R. Patellofemoral Instability in Skeletally Immature Patients. Porto Biomed. J. 2017, 2, 120–123. [Google Scholar] [CrossRef]
- Massachusetts General Brigham Sports Medicine Pediatric Rehabilitation Protocol for Medial Patellofemoral Ligament (MPFL) Reconstruction 2024. Available online: https://www.massgeneral.org/assets/mgh/pdf/orthopaedics/sports-medicine/physical-therapy/pediatric-mpfl-protocol.pdf (accessed on 2 October 2025).
- Lampros, R.E.; Wiater, A.L.; Tanaka, M.J. Rehabilitation and Return to Sport after Medial Patellofemoral Complex Reconstruction. Arthrosc. Sports Med. Rehabil. 2022, 4, e133–e140. [Google Scholar] [CrossRef]
- Meynard, P.; Malatray, M.; Sappey-Marinier, E.; Magnussen, R.A.; Bodiou, V.; Lustig, S.; Servien, E. Medial Patellofemoral Ligament Reconstruction for Recurrent Patellar Dislocation Allows a Good Rate to Return to Sport. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1865–1870. [Google Scholar] [CrossRef]
- Hysing-Dahl, T.; Magnussen, L.H.; Faleide, A.G.H.; Inderhaug, E. Feasibility of Return to Sports Assessment 6 Months after Patellar Instability Surgery. BMC Musculoskelet. Disord. 2023, 24, 662. [Google Scholar] [CrossRef]
- Saper, M.G.; Fantozzi, P.; Bompadre, V.; Racicot, M.; Schmale, G.A. Return-to-Sport Testing after Medial Patellofemoral Ligament Reconstruction in Adolescent Athletes. Orthop. J. Sports Med. 2019, 7, 2325967119828953. [Google Scholar] [CrossRef]
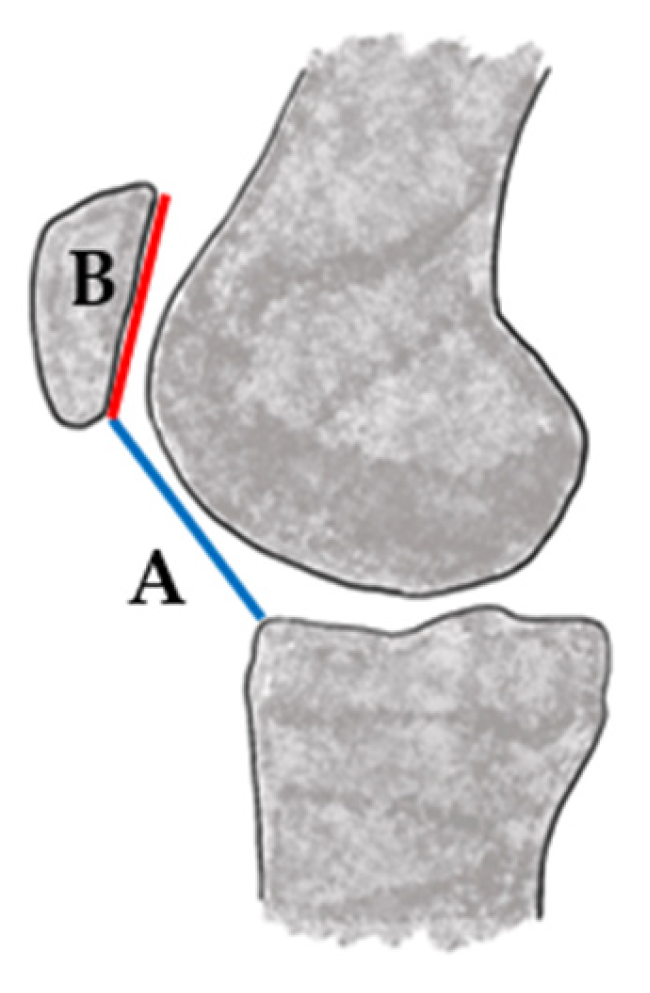
| Classification/ Measure | Risk Factor | Method | Threshold for Abnormality | Notes | Limitations |
|---|---|---|---|---|---|
| Caton–Deschamps Index [36] | Patella alta | Ratio of distance from inferior patellar pole to tibial plateau divided by the length of the patellar articular surface on lateral radiograph | Greater than 1.2 indicates patella alta | Preferred in pediatric populations due to growth plate visibility Sensitive to knee flexion angle at the time of imaging; does not account for trochlear morphology or dynamic engagement | |
| Insall–Salvati Ratio [38] | Patella alta | Ratio of patellar tendon length to patellar length | Greater than 1.2 indicates patella alta | Common in adults, but less reliable in immature knees. Some success in skeletally immature populations [38] Inconsistent reproducibility in pediatric knees | |
| TT-TG Distance [25] | Lateralization to tibial tuberacle | Measured on axial MRI or computer tomography (CT) as horizontal distance between tibial tubercle and trochlear groove | Greater than 20 mm considered abnormal | Greater than 20 mm associated with instability; 15–20 mm may be borderline and context-dependent in pediatric cases [39] Values vary with imaging modality (MRI vs. CT) and knee flexion angle | |
| Dejour Classification (2025) [40] | Trochlear dysplasia | Integrated assessment using lateral radiographs and MRI; emphasizes functional evaluation of patellofemoral tracking | Redefined dysplasia categories with emphasis on dynamic morphology; linked directly to updated menu à la carte treatment framework. | Supersedes earlier A-D system; provides improved risk stratification and clearer surgical indications (trochleoplasty reserves for select high-grade dysplasia, not skeletally immature patients) [40] | Requires advanced imaging; some parameters prone to inter-observer variability |
| TT-PCL [26,27,28,29] | Rotational alignment, lateralization of the tibial tubercle | Measured on axial MRI or CT as the distance from the tibial tubercle to the posterior cruciate ligament insertion | Greater than 20 mm is typically considered abnormal [29] | Used alongside TT-TG distance to evaluate rotational malalignment and refine surgical planning, especially important in skeletally immature patients | Normative pediatric values not well established |
| Parameter | Definition | Imaging Plane | Threshold | Clinical Significance |
|---|---|---|---|---|
| Spur height | Bony supratrochlear spur height | Sagittal | >5 mm | Indicates high-grade trochlear dysplasia |
| Cranial trochlear orientation | Orientation of proximal trochlea vs. posterior condyles | Axial | Positive value | Reflects cranial malorientation; predicts patellar maltracking |
| Trochlear groove height | Angle between trochlear groove and posterior condylar axis | Coronal | >11° | Excessive obliquity indicates severe dysplasia |
| Patellar height index | Ratio of patellar length to articular contact length | Sagittal | >1.16 | Identifies patella alta, risk of delayed trochlear engagement |
| Sagittal patellofemoral engagement | Ratio of engaged patella length to total patellar length | Sagittal | <0.38 | Low value = poor engagement, instability risk |
| TT-TG distance | Horizontal distance tibial tubercle to trochlear groove | Axial | ≥14 mm | Pathologic lateralization, informs consideration for TTO |
| Site | Fixation | Indications | Advantages | Limitations/Risks | Ease of Use | Evidence |
|---|---|---|---|---|---|---|
| Femur | Short epiphyseal socket + interface screws | Epiphyseal anatomy permits short tunnel distal to physis | Anatomic fixation; firm stability | Risk of physeal breach; hardware in small epiphysis Moderate Level IV | ||
| Femur | Short socket + cortical button/adjustable loop | Need for fine tension control; physeal respect | Micro-adjustable tension; preserves epiphysis | Requires cortical bridge; hardware prominence Moderate Level IV [13,95] | ||
| Femur | Adductor sling (soft tissue loop) | Younger patients; desire to avoid drilling | No bone tunnels; fully physeal sparing | Creep/lengthening; soft tissue isometry dependent Moderate Level IV [69,70,71,72] | ||
| Femur | Epiphyseal suture anchors | Tunnel-free physeal sparing | Minimal bone removal | Pull-out risk; limited long-tern outcomes | Easy-Moderate | Level III–IV [95,96] |
| Patella | Medial anchors (2 low profile) | Most pediatric cases | Minimizes fracture risk; small sockets | Anchor pull-out; cost | Easy-Moderate | Level III–IV [78,79,97,98] |
| Patella | Protected unicortical tunnels | When anchors unavailable | Strong fixation; inexpensive | Risk of fracture if bicortical or oversized | Moderate | Level IV [98,99] |
| Patella | Full-thickness transverse tunnels | Historically used | High stiffness | High fracture risk in pediatrics | Moderate | Consensus to avoid [80] |
| Technique | Indication | Skeletally Immature | Advantages | Limitations | Level of Evidence |
|---|---|---|---|---|---|
| Physeal-sparing MPFL | Recurrent instability, open physes | Preferred | Preserves growth plates; good outcomes | Technical complexity; limited long-term data | Level III–IV [43,65,66,67,68] |
| Adductor sling | Younger children, avoid tunnels | Yes | No bone drilling; physeal safe | Graft creep risk | Level IV [69,70,71,72] |
| Epiphyseal socket MPFL | Older adolescents; nearing maturity | Selective | Anatomic fixation Risk of epiphyseal breach Level IV [13,69,70] | ||
| TTO | TT-TG > 20 mm, patella alta | Contraindicated | Corrects alignment | Growth disturbance risk | Level III [44,49,100,101] |
| Trochleoplasty | Severe trochlear dysplasia | Contraindicated | Corrects bony dysplasia | Contraindicated in open physes | Level III [102,103,104,105,106,107] |
| Soft tissue procedures | Adjunctive instability | Yes | Minimally invasive | Variable outcomes | Level IV [14,61,72,74,75] |
| Innovation | Evidence Strength | Clinical Availability | Cost | Learning Curve |
|---|---|---|---|---|
| Robot-assisted navigation [18] | Small single-center retrospective studies (n = 20–40; <2 years follow up) | Available in tertiary centers only | High capital/equipment cost | Requires specialized training |
| Bioengineers Scaffolds [108] | Mostly preclinical and animal models | Experimental, not widely available | Variable, currently high | Straightforward arthroscopic integration if validated |
| 3D Modeling and Patient-Specific Planning [53,109,110,111,112] | Small retrospective series (n < 50); promising feasibility | Growing availability at academic centers | Moderate (software) | Moderate learning curve, aids planning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallinos, A.; Jones, K. Arthroscopic Management of Patellar Instability in Skeletally Immature Patients: Current Concepts and Future Directions. J. Clin. Med. 2025, 14, 7085. https://doi.org/10.3390/jcm14197085
Mallinos A, Jones K. Arthroscopic Management of Patellar Instability in Skeletally Immature Patients: Current Concepts and Future Directions. Journal of Clinical Medicine. 2025; 14(19):7085. https://doi.org/10.3390/jcm14197085
Chicago/Turabian StyleMallinos, Alexandria, and Kerwyn Jones. 2025. "Arthroscopic Management of Patellar Instability in Skeletally Immature Patients: Current Concepts and Future Directions" Journal of Clinical Medicine 14, no. 19: 7085. https://doi.org/10.3390/jcm14197085
APA StyleMallinos, A., & Jones, K. (2025). Arthroscopic Management of Patellar Instability in Skeletally Immature Patients: Current Concepts and Future Directions. Journal of Clinical Medicine, 14(19), 7085. https://doi.org/10.3390/jcm14197085






