Colchicine in Contemporary Pharmacotherapy: Mechanistic Insights and Clinical Horizons
Abstract
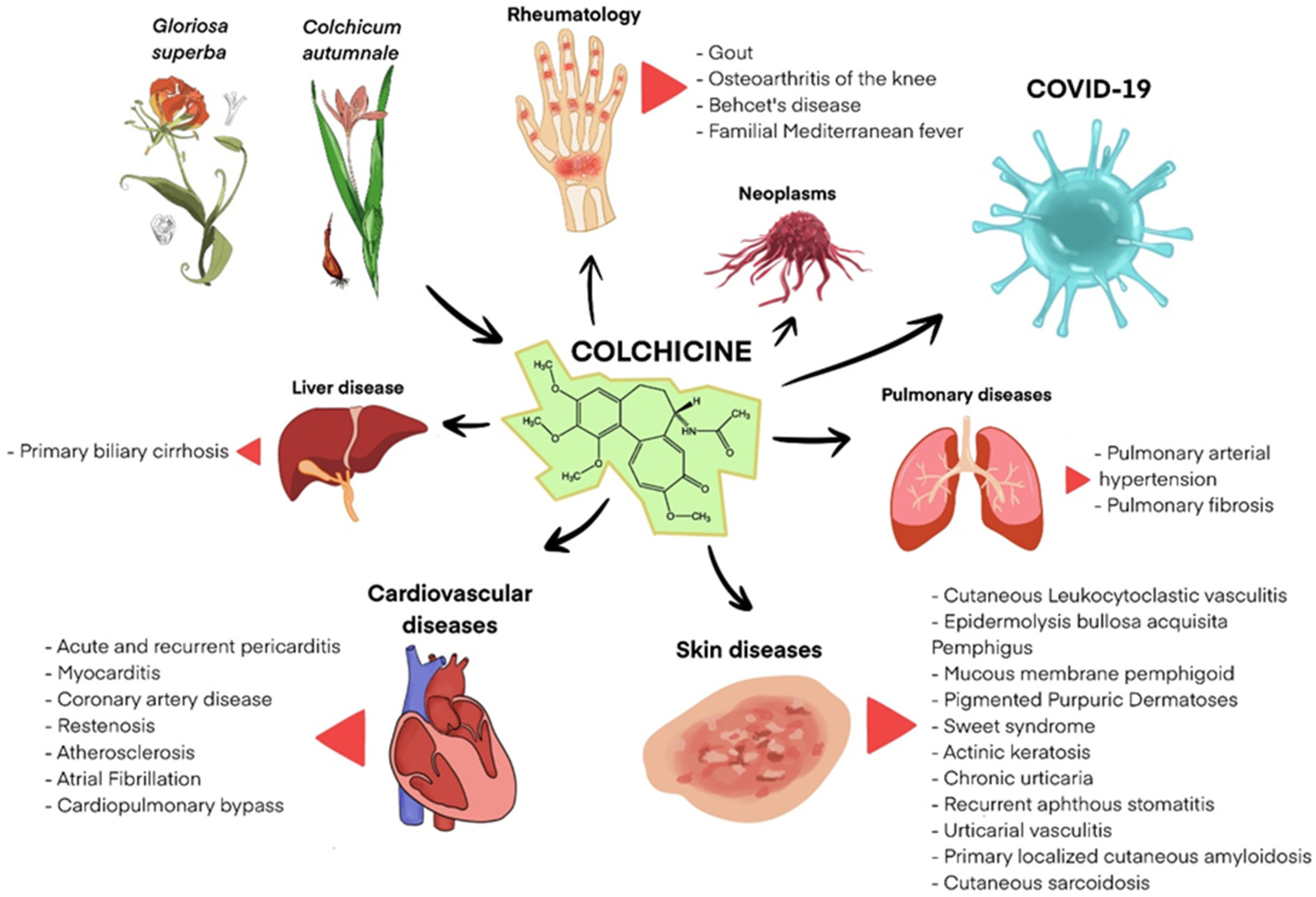
1. Introduction
2. Pharmacokinetics of Colchicine
3. Colchicine in Cardiovascular Diseases
3.1. Acute and Recurrent Pericarditis
3.2. Coronary Artery Disease
3.3. Atrial Fibrillation
3.4. Myocarditis
3.5. Cardiopulmonary Bypass
4. Colchicine in Dermatologic Diseases
4.1. Cutaneous Leukocytoclastic vasculitis
4.2. Behçet’s Syndrome
4.3. Epidermolysis Bullosa Acquisita (EBA)
4.4. Pemphigus
4.5. Mucous Membrane Pemphigoid (MMP)
4.6. Pigmented Purpuric Dermatoses (PPD)
4.7. Sweet’s Syndrome
4.8. Actinic Keratosis
4.9. Chronic Urticaria
4.10. Recurrent Aphthous Stomatitis (RAS)
4.11. Urticarial Vasculitis
4.12. Primary Localized Cutaneous Amyloidosis (PLCA)
4.13. Cutaneous Sarcoidosis
5. Colchicine in COVID-19
6. Risks and How to Minimize Them
7. Colchicine in Guidelines
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angelidis, C.; Kotsialou, Z.; Kossyvakis, C.; Vrettou, A.R.; Zacharoulis, A.; Kolokathis, F.; Kekeris, V.; Giannopoulos, G. Colchicine Pharmacokinetics and Mechanism of Action. Curr. Pharm. Des. 2018, 24, 659–663. [Google Scholar] [CrossRef]
- Zhang, F.S.; He, Q.Z.; Qin, C.H.; Little, P.J.; Weng, J.P.; Xu, S.W. Therapeutic potential of colchicine in cardiovascular medicine: A pharmacological review. Acta Pharmacol. Sin. 2022, 43, 2173–2190. [Google Scholar] [CrossRef]
- Malkinson, F.D. Colchicine: New Uses of an Old, Old Drug. Arch. Dermatol. 1982, 118, 453. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Varma, J.K. Molecular mechanism of colchicine action: Induced local unfolding of beta-tubulin. Biochemistry 1993, 32, 13560–13565. Available online: https://www.semanticscholar.org/paper/Molecular-mechanism-of-colchicine-action%3A-induced-Sackett-Varma/2d7ee3faf2bb5a6139fc7f085e7d1d77f22a36b7 (accessed on 21 February 2024). [CrossRef] [PubMed]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine--update on mechanisms of action and therapeutic uses. Arthritis Rheum. 2015, 45, 341–350. Available online: https://www.semanticscholar.org/paper/Colchicine--Update-on-mechanisms-of-action-and-Leung-Hui/6d441e01a80eb6fd218b17d091a8e944b4a20153 (accessed on 21 February 2024). [CrossRef] [PubMed]
- Bondt, E.D.E.; Betrains, A.; Vanderschueren, S. Colchicine, terug van nooit weggeweest. Reumatologie 2022, 5. Available online: https://www.semanticscholar.org/paper/Colchicine%2C-terug-van-nooit-weggeweest-Bondt-Betrains/4c9fff33cf0557b4840da750a570aa830751fb26 (accessed on 21 February 2024). [CrossRef]
- Dasgeb, B.; Kornreich, D.; McGuinn, K.; Okon, L.; Brownell, I.; Sackett, D.L. Colchicine: An ancient drug with novel applications. Br. J. Dermatol. 2018, 178, 350–356. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Beerkens, F.J.; Shah, B.; Giannopoulos, G.; Vrachatis, D.A.; Giotaki, S.G.; Siasos, G.; Nicolas, J.; Arnott, C.; Patel, S.; et al. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation 2022, 145, 61–78. [Google Scholar] [CrossRef]
- Chen, T.; Liu, G.; Yu, B. Colchicine for Coronary Artery Disease: A Review. Front. Cardiovasc. Med. 2022, 9, 892588. Available online: https://www.frontiersin.org/articles/10.3389/fcvm.2022.892588 (accessed on 2 March 2024). [CrossRef]
- Sabouraud, A.; Rochdi, M.; Urtizberea, M.; Christen, M.; Achtert, G.; Scherrmann, J. Pharmacokinetics of colchicine: A review of experimental and clinical data. Z. Gastroenterol. 1992, 30, 35–39. Available online: https://www.semanticscholar.org/paper/Pharmacokinetics-of-colchicine%3A-a-review-of-and-Sabouraud-Rochdi/8a76957efa4005747e9215811eba885fda6a7c8b (accessed on 30 July 2024).
- Niel, E.; Scherrmann, J.M. Colchicine today. Jt. Bone Spine 2006, 73, 672–678. [Google Scholar] [CrossRef]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [PubMed]
- Rodríguez de la Serna, A.; Guindo Soldevila, J.; Martí Claramunt, V.; Bayés de Luna, A. Colchicine for recurrent pericarditis. Lancet 1987, 26, 1517. [Google Scholar] [CrossRef] [PubMed]
- Guindo, J.; Rodriguez de la Serna, A.; Ramió, J.; de Miguel Diaz, M.A.; Subirana, M.T.; Perez Ayuso, M.J.; Cosín, J.; Bayés de Luna, A. Recurrent pericarditis. Relief with colchicine. Circulation 1990, 82, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Adler, Y.; de Luna, A.B.; Imazio, M. Colchicine in Pericarditis. Eur. Heart J. 2017, 38, 1706–1709. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Cemin, R.; Ferrua, S.; Maggiolini, S.; Beqaraj, F.; Demarie, D.; Forno, D.; Ferro, S.; Maestroni, S.; et al. A Randomized Trial of Colchicine for Acute Pericarditis. N. Engl. J. Med. 2013, 369, 1522–1528. [Google Scholar] [CrossRef]
- Imazio, M.; Bobbio, M.; Cecchi, E.; Demarie, D.; Demichelis, B.; Pomari, F.; Moratti, M.; Gaschino, G.; Giammaria, M.; Ghisio, A.; et al. Colchicine in Addition to Conventional Therapy for Acute Pericarditis. Circulation 2005, 112, 2012–2016. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.105.542738 (accessed on 3 March 2024). [CrossRef]
- Imazio, M.; Brucato, A.; Trinchero, R.; Spodick, D.; Adler, Y. Colchicine for pericarditis: Hype or hope? Eur. Heart J. 2009, 30, 532–539. [Google Scholar] [CrossRef]
- Adler, Y.; Ravid, M.; Avidan, B.; Zemer, D.; Ehrenfeld, M.; Shemesh, J.; Tomer, Y.; Shoenfeld, Y. Usefulness of colchicine in preventing recurrences of pericarditis. Am. J. Cardiol. 1994, 73, 916–917. [Google Scholar] [CrossRef] [PubMed]
- Millaire, A.; De Groote, P.; Decoulx, E.; Goullard, L.; Ducloux, G. Treatment of recurrent pericarditis with colchicine. Eur. Heart J. 1994, 15, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.; The, S.H.; Xu, X.F.; Ireland, M.A.; Lenderink, T. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Opstal, T.S.; van Broekhoven, A.; Fiolet, A.T.; Mosterd, A.; Eikelboom, J.W.; Nidorf, S.M.; Thompson, P.L.; Budgeon, C.A.; Bartels, L.; de Nooijer, R.; et al. Long-Term Efficacy of Colchicine in Patients with Chronic Coronary Disease: Insights from LoDoCo2. Circulation 2022, 145, 626–628. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.121.058233 (accessed on 9 September 2024). [CrossRef]
- Tong, D.C.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; Stub, D.; et al. Colchicine in Patients with Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation 2020, 142, 1890–1900. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G.; Penson, P.E.; Henein, M.Y.; Banach, M.; Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group; International Lipid Expert Panel (ILEP). Efficacy and safety of colchicine in patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2022, 88, 1520–1528. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Ben-Chetrit, E.; Ridker, P.M. Low-dose colchicine for atherosclerosis: Long-term safety. Eur. Heart J. 2024, 45, 1596–1601. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Pullara, A.; Adler, Y.; Barosi, A.; Caforio, A.L.; Cemin, R.; Chirillo, F.; Comoglio, C.; et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: The COPPS-2 randomized clinical trial. JAMA 2014, 312, 1016–1023. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Kossyvakis, C.; Efremidis, M.; Panagopoulou, V.; Kaoukis, A.; Raisakis, K.; Bouras, G.; Angelidis, C.; Theodorakis, A.; et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: A randomized controlled study. J. Am. Coll. Cardiol. 2012, 60, 1790–1796. [Google Scholar] [CrossRef]
- Benali, K.; Barre, V.; Hermida, A.; Galand, V.; Milhem, A.; Philibert, S.; Boveda, S.; Bars, C.; Anselme, F.; Maille, B.; et al. Recurrences of Atrial Fibrillation Despite Durable Pulmonary Vein Isolation: The PARTY-PVI Study. Circ. Arrhythm Electrophysiol. 2023, 16, e011354. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Fu, Y.; Su, Q.; Jin, M.; Guo, L.; Miao, C.; Zhu, S.; Zhuang, J.; Zhang, Z.; Hong, J. Colchicine for prevention of post-operative atrial fibrillation: Meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 1032116. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Wang, M.K.; Popova, E.; Chan, M.T.; Landoni, G.; Cata, J.P.; Reimer, C.; McLean, S.R.; Srinathan, S.K.; Reyes, J.C.; et al. Effect of colchicine on perioperative atrial fibrillation and myocardial injury after non-cardiac surgery in patients undergoing major thoracic surgery (COP-AF): An international randomised trial. Lancet 2023, 402, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.; Andreis, A.; Avondo, S.; Vaira, M.P.; Imazio, M. Colchicine for Cardiovascular Medicine: A Systematic Review and meta-analysis. Future Cardiol. 2022, 18, 647–659. [Google Scholar] [CrossRef]
- Li, Y.W.; Chen, S.X.; Yang, Y.; Zhang, Z.H.; Zhou, W.B.; Huang, Y.N.; Huang, Z.Q.; He, J.Q.; Chen, T.F.; Wang, J.F.; et al. Colchicine Inhibits NETs and Alleviates Cardiac Remodeling after Acute Myocardial Infarction. Cardiovasc. Drugs Ther. 2024, 38, 31–41. [Google Scholar] [CrossRef]
- Kavgacı, A.; Incedere, F.; Terlemez, S.; Kula, S. Successful treatment of two cases of acute myocarditis with colchicine. Cardiol. Young. 2023, 33, 1741–1742. [Google Scholar] [CrossRef]
- Wan, S.; LeClerc, J.L.; Vincent, J.L. Inflammatory Response to Cardiopulmonary Bypass: Mechanisms Involved and Possible Therapeutic Strategies. Chest 1997, 112, 676–692. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Pan, T.; Zhang, H.T.; Zhong, K.; Li, Z.S.; Jiang, X.; Pan, J.; Wang, D.J. Evaluation of low-dose colchicine in patients with cardiopulmonary bypass: Study protocol for a randomised controlled trial. BMJ Open 2022, 12, e050577. [Google Scholar] [CrossRef]
- Dastoli, S.; Nistico, S.P.; Morrone, P.; Patruno, C.; Leo, A.; Citraro, R.; Gallelli, L.; Russo, E.; De Sarro, G.; Bennardo, L. Colchicine in Managing Skin Conditions: A Systematic Review. Pharmaceutics 2022, 14, 294. [Google Scholar] [CrossRef]
- Fraticelli, P.; Benfaremo, D.; Gabrielli, A. Diagnosis and management of leukocytoclastic vasculitis. Intern. Emerg. Med. 2021, 16, 831–841. [Google Scholar]
- Callen, J.P. Colchicine is effective in controlling chronic cutaneous leukocytoclastic vasculitis. J. Am. Acad. Dermatol. 1985, 13, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Plotnick, S.; Huppert, A.S.; Kantor, G. Colchicine and leukocytoclastic vasculitis. Arthritis Rheum. 1989, 32, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Sais, G.; Vidaller, A.; Jucglà, A.; Gallardo, F.; Peyrí, J. Colchicine in the Treatment of Cutaneous Leukocytoclastic Vasculitis: Results of a Prospective, Randomized Controlled Trial. Arch. Dermatol. 1995, 131, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Elbirt, D.; Asher, I.; Sthoeger, Z.M. [Behçet’s disease—Clinical presentation, diagnostic and therapeutic approach]. Harefuah 2002, 141, 462–467, 497. [Google Scholar]
- Emmi, G.; Bettiol, A.; Hatemi, G.; Prisco, D. Behçet’s syndrome. Lancet Lond. Engl. 2024, 403, 1093–1108. [Google Scholar] [CrossRef]
- Matsumura, N.; Mizushima, Y. Leucocyte movement and colchicine treatment in Behcet’s disease. Lancet Lond. Engl. 1975, 2, 813. [Google Scholar] [CrossRef]
- Yurdakul, S.; Mat, C.; Tüzün, Y.; Özyazgan, Y.; Hamuryudan, V.; Uysal, Ö.; Şenocak, M.; Yazici, H. A double-blind trial of colchicine in Behçet’s syndrome. Arthritis Rheum. 2001, 44, 2686–2692. [Google Scholar] [CrossRef]
- Davatchi, F.; Sadeghi Abdollahi, B.; Tehrani Banihashemi, A.; Shahram, F.; Nadji, A.; Shams, H.; Chams-Davatchi, C. Colchicine versus placebo in Behçet’s disease: Randomized, double-blind, controlled crossover trial. Mod. Rheumatol. 2009, 19, 542–549. [Google Scholar] [CrossRef]
- Wang, Z.; Zu, X.; Xiong, S.; Mao, R.; Qiu, Y.; Chen, B.; Zeng, Z.; Chen, M.; He, Y. The Role of Colchicine in Different Clinical Phenotypes of Behcet Disease. Clin. Ther. 2023, 45, 162–176. [Google Scholar] [CrossRef]
- Hatemi, G.; Christensen, R.; Bang, D.; Bodaghi, B.; Celik, A.F.; Fortune, F.; Gaudric, J.; Gul, A.; Kötter, I.; Leccese, P.; et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann. Rheum. Dis. 2018, 77, 808–818. [Google Scholar] [CrossRef]
- Lunzer, R.; Delle-Karth, G.; Zeitlinger, M.; Prager, M.; Pracher, L.M. Colchicine-Phoenix from the ashes. Wien. Klin. Wochenschr. 2025, 137, 1–33. [Google Scholar] [CrossRef]
- Miyamoto, D.; Gordilho, J.O.; Santi, C.G.; Porro, A.M. Epidermolysis bullosa acquisita. An. Bras. Dermatol. 2022, 97, 409–423. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.H.; Kim, S.C. Epidermolysis bullosa acquisita: A retrospective clinical analysis of 30 cases. Acta Derm. Venereol. 2011, 91, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Komine, M.; Suzuki, M.; Murata, S.; Hirano, T.; Ishii, N.; Hashimoto, T.; Ohtsuki, M. Oral colchicine monotherapy for epidermolysis bullosa acquisita: Mechanism of action and efficacy. J. Dermatol. 2016, 43, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Dainichi, T.; Ohyama, B.; Yasumoto, S.; Oono, T.; Iwatsuki, K.; Elfert, S.; Fritsch, A.; Bruckner-Tuderman, L.; Hashimoto, T. A case of epidermolysis bullosa acquisita with clinical features of Brunsting-Perry pemphigoid showing an excellent response to colchicine. J. Am. Acad. Dermatol. 2009, 61, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Kridin, K.; Schmidt, E. Epidemiology of Pemphigus. JID Innov. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Hodak, E.; Lapidoth, M.; David, M. Effect of colchicine in the subcorneal pustular dermatosis type of IgA pemphigus. J. Am. Acad. Dermatol. 1999, 40, 91–94. [Google Scholar] [CrossRef]
- Mathachan, S.R.; Arora, P.; Sardana, K.; Paliwal, P. Pemphigus Foliaceous with Prominent Neutrophilic Pustules: A Rare Variant Responsive to Colchicine. Indian J. Dermatol. 2022, 67, 481. [Google Scholar] [CrossRef]
- Du, G.; Patzelt, S.; van Beek, N.; Schmidt, E. Mucous membrane pemphigoid. Autoimmun. Rev. 2022, 21, 103036. [Google Scholar] [CrossRef]
- Chaidemenos, G.; Sidiropoulos, T.; Katsioula, P.; Koussidou-Eremondi, T. Colchicine in the management of mucous membrane pemphigoid. Dermatol Ther. 2011, 24, 443–445. [Google Scholar] [CrossRef]
- Fribourg, E.; Chuy, V.; Fenelon, M.; Catros, S.; Fricain, J.C. Is There a Role for Colchicine in the Treatment of Oral Mucous Membrane Pemphigoid? Oral Dis. 2025, 31, 1237–1240. [Google Scholar] [CrossRef]
- Kimak, A.; Żebrowska, A. Therapeutic Approach in Pigmented Purpuric Dermatoses-A Scoping Review. Int. J. Mol. Sci. 2024, 25, 2644. [Google Scholar] [CrossRef]
- Martínez Pallás, I.; Conejero del Mazo, R.; Lezcano Biosca, V. Dermatosis purpúricas pigmentadas. Revisión de la literatura científica. Actas Dermo-Sifiliográf. 2020, 111, 196–204. [Google Scholar] [CrossRef]
- Cavalcante, M.L.L.L.; Masuda, P.Y.; Brito FFde Pinto, A.C.V.D.; Itimura, G.; Nunes, A.J.F. Schamberg’s disease: Case report with therapeutic success by using colchicine. An. Bras. Dermatol. 2017, 92, 246–248. [Google Scholar] [CrossRef]
- Bhandari, M.; Khullar, G.; Sharma, S. Granulomatous pigmented purpuric dermatosis favorably treated with the combination of colchicine and calcium dobesilate. Dermatol. Ther. 2020, 33, e13843. [Google Scholar] [CrossRef]
- Calabrese, L.; Satoh, T.K.; Aoki, R.; Rubegni, G.; Neulinger-Mũnoz, M.; Stadler, P.C.; French, L.E.; Rubegni, P. Sweet syndrome: An update on clinical aspects, pathophysiology, and treatment. Ital. J. Dermatol. Venereol. 2024, 159, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Suehisa, S.; Tagami, H. Treatment of acute febrile neutrophilic dermatosis (Sweet’s syndrome) with colchicine. Br. J. Dermatol. 1981, 105, 483. [Google Scholar] [CrossRef] [PubMed]
- Amouri, M.; Masmoudi, A.; Ammar, M.; Boudaya, S.; Khabir, A.; Boudawara, T.; Turki, H. Sweet’s syndrome: A retrospective study of 90 cases from a tertiary care center. Int. J. Dermatol. 2016, 55, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Chiewchanvit, S.; Jamjanya, S.; Rattanathammethee, T.; Mahanupab, P.; Tovanabutra, N.; Chuamanochan, M. Bullous Sweet syndrome in a patient with acute myeloid leukemia treated with midostaurin: Rapid response to acitretin and colchicine-A case report. Dermatol. Ther. 2021, 34, e15171. [Google Scholar] [CrossRef]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of care for the management of actinic keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Faghihi, G.; Elahipoor, A.; Iraji, F.; Behfar, S.; Abtahi-Naeini, B. Topical Colchicine Gel versus Diclofenac Sodium Gel for the Treatment of Actinic Keratoses: A Randomized, Double-Blind Study. Adv. Med. 2016, 2016, 5918393. [Google Scholar] [CrossRef]
- Akar, A.; Bülent Taştan, H.; Erbil, H.; Arca, E.; Kurumlu, Z.; Gür, A.R. Efficacy and safety assessment of 0.5% and 1% colchicine cream in the treatment of actinic keratoses. J. Dermatol. Treat. 2001, 12, 199–203. [Google Scholar] [CrossRef]
- Siddiqui, A.; Bai, A.; Kumar, H.; Mandhwani, Y.R.; Bai, A.; Bai, P.; Kumar, N.; Muskan, F.N.; Jatoi, S.; Ali, Z.; et al. Chronic urticaria and vitamin D supplementations: A systematic review. Eur. J. Med. Res. 2025, 30, 691. [Google Scholar] [CrossRef]
- Nabavizadeh, S.H.; Babaeian, M.; Esmaeilzadeh, H.; Mortazavifar, N.; Alyasin, S. Efficacy of the colchicine add-on therapy in patients with autoimmune chronic urticaria. Dermatol. Ther. 2021, 34, e15119. [Google Scholar] [CrossRef]
- Criado, R.F.J.; Criado, P.R.; Martins, J.E.C.; Valente, N.Y.S.; Michalany, N.S.; Vasconcellos, C. Urticaria unresponsive to antihistaminic treatment: An open study of therapeutic options based on histopathologic features. J. Dermatol. Treat. 2008, 19, 92–96. [Google Scholar] [CrossRef]
- Lau, C.B.; Smith, G.P. Recurrent aphthous stomatitis: A comprehensive review and recommendations on therapeutic options. Dermatol. Ther. 2022, 35, e15500. [Google Scholar] [CrossRef]
- Fontes, V.; Machet, L.; Huttenberger, B.; Lorette, G.; Vaillant, L. [Recurrent aphthous stomatitis: Treatment with colchicine. An open trial of 54 cases]. Ann. Dermatol. Venereol. 2002, 129, 1365–1369. [Google Scholar]
- Pakfetrat, A.; Mansourian, A.; Momen-Heravi, F.; Delavarian, Z.; Momen-Beitollahi, J.; Khalilzadeh, O.; Basir-Shabestari, S. Comparison of colchicine versus prednisolone in recurrent aphthous stomatitis: A double-blind randomized clinical trial. Clin. Investig. Med. 2010, 33, E189–E195. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.W.; Chung, K.B.; Bang, D.; Kim, D.Y. Safety, Efficacy, and Drug Survival of Colchicine in Recurrent Aphthous Stomatitis in a Real-World Setting. Ann. Dermatol. 2022, 34, 22–27. [Google Scholar] [CrossRef]
- Alsahaf, S.; Alkurdi, K.A.; Challacombe, S.J.; Tappuni, A.R. Topical betamethasone and systemic colchicine for treatment of recurrent aphthous stomatitis: A randomised clinical trial. BMC Oral Health 2023, 23, 709. [Google Scholar]
- Wang, R.X.; Newman, S.A. Urticarial Vasculitis. Immunol. Allergy Clin. 2024, 44, 483–502. [Google Scholar] [CrossRef]
- Wiles, J.C.; Hansen, R.C.; Lynch, P.J. Urticarial vasculitis treated with colchicine. Arch. Dermatol. 1985, 121, 802–805. [Google Scholar] [CrossRef]
- Asherson, R.A.; Buchanan, N.; Kenwright, S.; Fletcher, C.M.; Hughes, G.R. The normocomplementemic urticarial vasculitis syndrome-report of a case and response to colchicine. Clin. Exp. Dermatol. 1991, 16, 424–427. [Google Scholar] [CrossRef]
- Jachiet, M.; Flageul, B.; Deroux, A.; Le Quellec, A.; Maurier, F.; Cordoliani, F.; Godmer, P.; Abasq, C.; Astudillo, L.; Belenotti, P.; et al. The clinical spectrum and therapeutic management of hypocomplementemic urticarial vasculitis: Data from a French nationwide study of fifty-seven patients. Arthritis Rheumatol. 2015, 67, 527–534. [Google Scholar] [CrossRef]
- Fujii, M.; Kishibe, M.; Ishida-Yamamoto, A. Case of hypocomplementemic urticarial vasculitis with Sjögren’s syndrome successfully treated with oral corticosteroid and colchicine. J. Dermatol. 2021, 48, e112–e113. [Google Scholar] [CrossRef]
- Wang, Q.X.; Ye, Q.; Zhou, K.Y.; Luo, S.Y.; Fang, S. Systematic review and meta-analysis of treatments and outcomes in primary localized cutaneous amyloidosis. Clin. Exp. Dermatol. 2025, 50, 1343–1354. [Google Scholar] [CrossRef]
- Weidner, T.; Illing, T.; Elsner, P. Primary Localized Cutaneous Amyloidosis: A Systematic Treatment Review. Am. J. Clin. Dermatol. 2017, 18, 629–642. [Google Scholar] [CrossRef]
- Chakravarty, K.; Chanda, M. Role of colchicine in primary localised cutaneous amyloidosis. Indian J. Dermatol. Venereol. Leprol. 1995, 61, 268–269. [Google Scholar]
- Kaltoft, B.; Schmidt, G.; Lauritzen, A.F.; Gimsing, P. Primary localised cutaneous amyloidosis–a systematic review. Dan. Med. J. 2013, 60, A4727. [Google Scholar]
- Abdelghaffar, M.; Hwang, E.; Damsky, W. Cutaneous Sarcoidosis. Clin. Chest Med. 2024, 45, 71–89. [Google Scholar] [CrossRef]
- Wise, R.D. Clinical resolution of facial cutaneous sarcoidosis with systemic colchicine and a topical corticosteroid ointment. Compr. Ther. 2008, 34, 105–110. [Google Scholar]
- Pereira, E.G.; Guimarães, T.F.; Bottino, C.B.; D’Acri, A.M.; Lima, R.B.; Martins, C.J. Sarcoidosis and chronic hepatitis C: Treatment with prednisone and colchicine. An. Bras. Dermatol. 2016, 91, 231–234. [Google Scholar] [CrossRef]
- COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease [Internet]. Available online: https://elicit.com/?workflow=table-of-papers&run=db43e818-2fd7-4b74-ad93-beb3d0e4869a (accessed on 26 December 2023).
- Surma, S.; Basiak, M.; Romańczyk, M.; Filipiak, K.J.; Okopień, B. Colchicine—From rheumatology to the new kid on the block: Coronary syndromes and COVID-19. Cardiol. J. 2023, 30, 297–311. Available online: https://journals.viamedica.pl/cardiology_journal/article/view/85217?fbclid=IwAR2z38il-MOettU_g5IXDbOrsrJua9PTL8Shw0uV8vnHlhqxPDS3xfvgHaU (accessed on 26 December 2023). [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Tangianu, F.; Abbate, A.; Dentali, F. Colchicine for COVID-19: Targeting NLRP3 inflammasome to blunt hyperinflammation. Inflamm. Res. 2022, 71, 293–307. [Google Scholar] [CrossRef]
- Pelechas, E.; Drossou, V.; Voulgari, P.V.; Drosos, A.A. COVID-19 in patients with gout on colchicine. Rheumatol. Int. 2021, 41, 1503–1507. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19. Am. J. Cardiol. 2021, 145, 170–172. [Google Scholar] [CrossRef]
- Mansouri, N.; Marjani, M.; Tabarsi, P.; von Garnier, C.; Mansouri, D. Successful Treatment of Covid-19 Associated Cytokine Release Syndrome with Colchicine. A Case Report and Review of Literature. Immunol Invest. 2021, 50, 884–890. [Google Scholar] [CrossRef]
- Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.; Emrich-Filho, L.L.; et al. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021, 7, e001455. [Google Scholar] [CrossRef]
- Chiu, L.; Lo, C.H.; Shen, M.; Chiu, N.; Aggarwal, R.; Lee, J.; Choi, Y.G.; Lam, H.; Prsic, E.H.; Chow, R.; et al. Colchicine use in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0261358. Available online: https://pubmed.ncbi.nlm.nih.gov/34962939/ (accessed on 29 December 2023). [CrossRef]
- Manenti, L.; Maggiore, U.; Fiaccadori, E.; Meschi, T.; Antoni, A.D.; Nouvenne, A.; Ticinesi, A.; Cerundolo, N.; Prati, B.; Delsante, M.; et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS ONE 2021, 16, e0248276. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0248276 (accessed on 29 December 2023). [CrossRef]
- Horby, P.; Campbell, M.; Spata, E.; Emberson, J.; Staplin, N.; Pessoa-Amorim, G.; Peto, L.; Wiselka, M.; Wiffen, L.; Tiberi, S.; et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Respir. Med. 2021, 9, 1419–1426. Available online: https://www.semanticscholar.org/paper/Colchicine-in-patients-admitted-to-hospital-with-a-Horby-Campbell/9d047241bfadb7250dbafca145b10446c71e93f4 (accessed on 29 December 2023). [CrossRef]
- Perricone, C.; Scarsi, M.; Brucato, A.; Pisano, P.; Pigatto, E.; Becattini, C.; Cingolani, A.; Tiso, F.; Prota, R.; Tomasoni, L.R.; et al. Treatment with COLchicine in hospitalized patients affected by COVID-19: The COLVID-19 trial. Eur. J. Intern. Med. 2023, 107, 30–36. [Google Scholar] [CrossRef]
- Mitev, V. Colchicine—The Divine Medicine against COVID-19. J. Pers. Med. 2024, 14, 756. [Google Scholar] [CrossRef]
- Rahman, M.; Datta, P.K.; Islam, K.; Haque, M.; Mahmud, R.; Mallik, U.; Hasan, P.; Haque, M.; Faruq, I.; Sharif, M.; et al. Efficacy of colchicine in patients with moderate COVID-19: A double-blinded, randomized, placebo-controlled trial. PLoS ONE 2022, 17, e0277790. [Google Scholar] [CrossRef]
- Stamp, L.K.; Horsley, C.; Te Karu, L.; Dalbeth, N.; Barclay, M. Colchicine: The good, the bad, the ugly and how to minimize the risks. Rheumatol. Oxf. Engl. 2023, 63, 936–944. [Google Scholar] [CrossRef]
- Hansten, P.D.; Tan, M.S.; Horn, J.R.; Gomez-Lumbreras, A.; Villa-Zapata, L.; Boyce, R.D.; Subbian, V.; Romero, A.; Gephart, S.; Malone, D.C. Colchicine Drug Interaction Errors and Misunderstandings: Recommendations for Improved Evidence-Based Management. Drug Saf. 2023, 46, 223–242. [Google Scholar] [CrossRef]
- Fu, M.; Zhao, J.; Li, Z.; Zhao, H.; Lu, A. Clinical outcomes after colchicine overdose. Medicine 2019, 98, e16580. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z. Progress in the management of acute colchicine poisoning in adults. Intern. Emerg. Med. 2022, 17, 2069–2081. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Andreis, A.; Imazio, M.; Piroli, F.; Avondo, S.; Casula, M.; Paneva, E.; Maria De Ferrari, G. Efficacy and safety of colchicine for the prevention of major cardiovascular and cerebrovascular events in patients with coronary artery disease: A systematic review and meta-analysis on 12 869 patients. Eur. J. Prev. Cardiol. 2021, 28, 1916–1925. [Google Scholar] [CrossRef]

| Trial | Population | Dose | Primary Endpoints | Main Adverse Effects | Conclusions |
|---|---|---|---|---|---|
| LoDoCo (2013) | n = 532, stable CAD | Col 0.5 mg vs. control | HR 0.33 (0.18–0.59), ARR 10.7%, NNT 9 | GI disturbances | Significant benefit |
| LoDoCo2 (2020) | n = 5522, stable CAD | Col 0.5 mg vs. placebo | HR 0.69 (0.57–0.83), ARR 2.8%, NNT 36 | GI effects, possible ↑non-CV death | Significant benefit with safety signal |
| COLCOT (2019) | n = 4745, recent MI | Col 0.5 mg vs. placebo | HR 0.77 (0.61–0.96), ARR 1.6%, NNT 63 | GI disturbances | Modest but significant benefit |
| COPS (2020) | n = 795, ACS | Col 0.5 mg BID/daily vs. placebo | HR 0.65 (0.38–1.09), p = 0.10 | ↑Non-CV mortality signal | No significant benefit, mortality concerns |
| COPPS-2 (2014) | n = 360, cardiac surgery | Col 0.5 mg vs. placebo | ARR 10.0%, NNT 10 | ↑GI effects (NNH = 12) | Benefit for PPS, no benefit for POAF |
| Disease | Type of Studies/Level of Evidence | Sample Size (n) | Effectiveness Assessment and Conclusions (Grade) |
|---|---|---|---|
| Leukocytoclastic Vasculitis | Case reports, series of 13 patients (Callen, 1985) [41]; RCT (Sais, 1995) [43] | single cases; n = 13; RCT: n = 41 | Low—variable efficacy; frequent relapses after discontinuation; one negative RCT |
| Behçet’s Syndrome | case series; RCTs: (2001,2009); | n = 12; RCT: n = 116, n = 169 | Moderate—good result in mucocutaneous and articular manifestations: supported by EULAR recommendations |
| Epidermolysis Bullosa Acquisita | Retrospective series, case reports | n = 30; single cases | Low-effective in mild forms; favorable safety profile; no RCTs |
| Pemphigus (IgA, foliaceus) | Case reports (2–3 patients) | <5 | Very low—only case reports; requires controlled studies |
| Mucous Membrane Pemphigoid | Retrospective series, observational studies | n = 80 | Low-moderate—efficacy in oral involvement; better tolerance than dapsone; 67% response in series; >80% remission in retrospective study |
| Pigmented Purpuric Dermatoses | Case reports | 2 patients | Very low—only cases; favorable outcome but no controlled data |
| Sweet’s Syndrome | retrospective study (n = 90); case reports | n = 90; single cases | Moderate—effective in most patients; rapid improvement; no RCts, but large series supports use |
| Actinic Keratosis | RCTs (vs diclofenac, 2016; 0.5% vs. 1%, 2001) | n = 70, n = 62 | Moderate—effective topical therapy, well tolerated; not included in guidelines |
| Chronic Urticaria | clinical trials | n = 55, n = 22 | Low—partial benefit in some patients; evidence inconsistent |
| Recurrent Aphthous Stomatitis | RCTs (2002, 2010, 2023); retrospective study | n = 54, n = 34, n = 106; n = 150 | Moderate—confirmed efficacy; comparable to steroids; side effects limit use |
| Urticarial Vasculitis | Case reports, retrospective cohort | Single cases; n = 57 | Low—some patients respond; more effective than NSAIDs; RCTs lacking |
| Primary Cutaneous Amyloidosis | Small series, systematic review | n = 15, n = 94 | Low—improved pruritus and pigmentation; no controlled trials and |
| Cutaneous Sarcoidosis | case reports | n = 3, n = 1 | Very low—only case data; possible role as adjunct therapy |
| Aspect | Details |
|---|---|
| Mechanism of Action |
|
| |
| Clinical Evidence Supporting Use |
|
| Challenges and Controversies |
|
| Dose-Dependent Considerations |
|
| Future Perspectives |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołowiec, Ł.; Osiak-Gwiazdowska, J.; Jaśniak, A.; Mucha, W.; Wojtaluk, M.; Czerniecka, W.; Wołowiec, A.; Banach, J.; Grześk, G. Colchicine in Contemporary Pharmacotherapy: Mechanistic Insights and Clinical Horizons. J. Clin. Med. 2025, 14, 7078. https://doi.org/10.3390/jcm14197078
Wołowiec Ł, Osiak-Gwiazdowska J, Jaśniak A, Mucha W, Wojtaluk M, Czerniecka W, Wołowiec A, Banach J, Grześk G. Colchicine in Contemporary Pharmacotherapy: Mechanistic Insights and Clinical Horizons. Journal of Clinical Medicine. 2025; 14(19):7078. https://doi.org/10.3390/jcm14197078
Chicago/Turabian StyleWołowiec, Łukasz, Joanna Osiak-Gwiazdowska, Albert Jaśniak, Weronika Mucha, Małgorzata Wojtaluk, Weronika Czerniecka, Anna Wołowiec, Joanna Banach, and Grzegorz Grześk. 2025. "Colchicine in Contemporary Pharmacotherapy: Mechanistic Insights and Clinical Horizons" Journal of Clinical Medicine 14, no. 19: 7078. https://doi.org/10.3390/jcm14197078
APA StyleWołowiec, Ł., Osiak-Gwiazdowska, J., Jaśniak, A., Mucha, W., Wojtaluk, M., Czerniecka, W., Wołowiec, A., Banach, J., & Grześk, G. (2025). Colchicine in Contemporary Pharmacotherapy: Mechanistic Insights and Clinical Horizons. Journal of Clinical Medicine, 14(19), 7078. https://doi.org/10.3390/jcm14197078






