Completely Fluoroless, “Apron-Less” Approach to Supraventricular Tachycardia Ablation Compared to Traditional Fluoroscopy Guided Ablation: Feasibility, Safety and Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
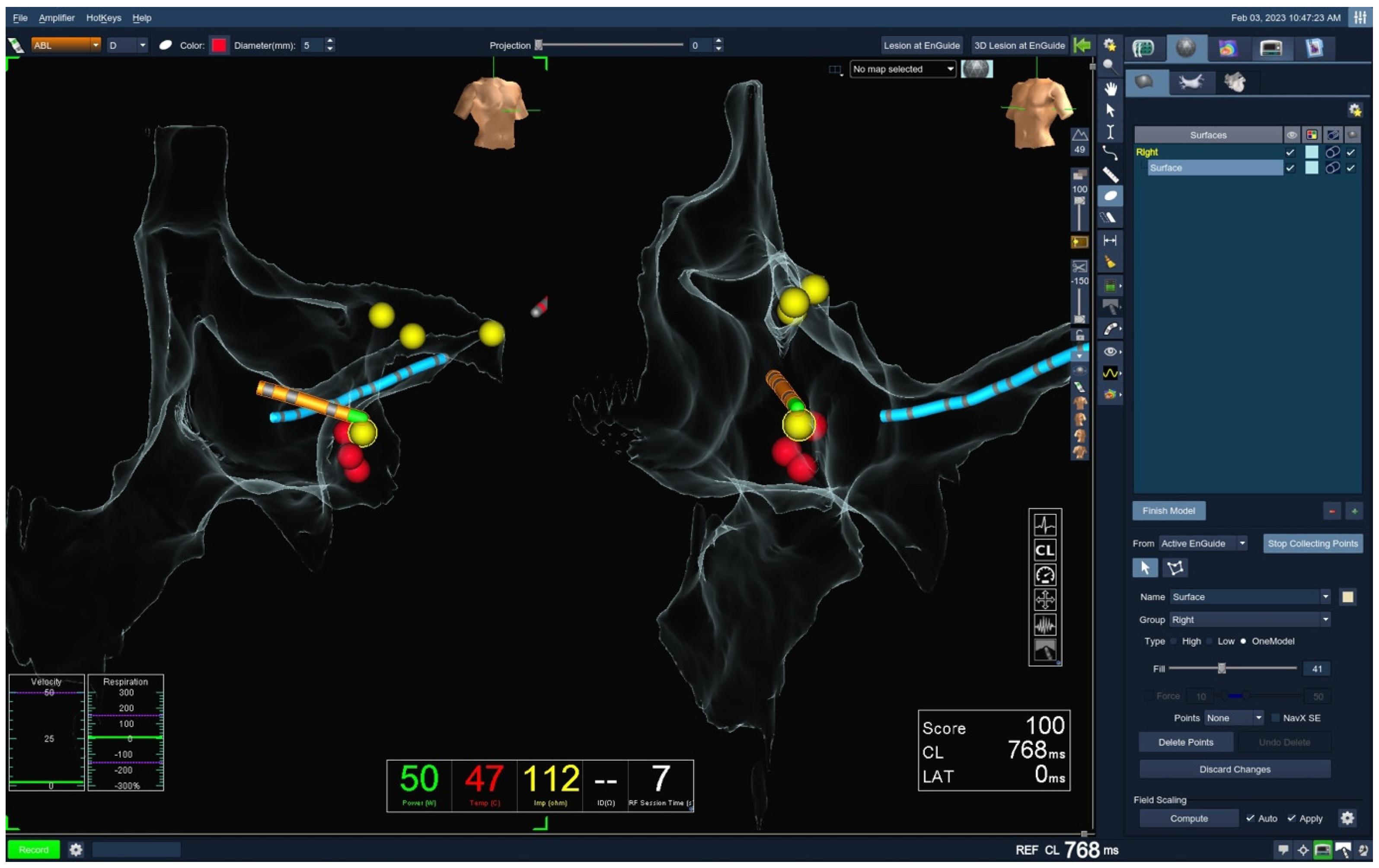
2.2. Ablation Protocol
2.3. Follow-Up
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Procedural Characteristics
3.3. Complications and Outcomes
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three dimensional |
| AADs | Antiarrhythmic drugs |
| ACT | Activated clotting time |
| AE | Atrioesophageal |
| AF | Atrial fibrillation |
| AFL | Atrial flutter |
| ALARA | As low as reasonably achievable |
| AT | Atrial tachycardia |
| AV | Arteriovenous |
| AV | Atrioventricular |
| AVNRT | Atrioventricular nodal reentrant tachycardia |
| AVRT | Atrioventricular reentrant tachycardia |
| BMI | Body mass index |
| COPD | Chronic obstructive pulmonary disease |
| CS | Coronary sinus |
| CTI | Cavotricuspid isthmus |
| DOAC | Direct oral anticoagulants |
| EAMS | Electroanatomical mapping systems |
| ECG | Electrocardiogram |
| EP | Electrophysiology |
| HFrEF | Heart failure with reduced ejection fraction |
| ICD | Implantable cardioverter–defibrillator |
| ICE | Intracardiac echocardiography |
| IVC | Inferior vena cava |
| LA | Left atrium |
| LVEF | Left ventricular ejection fraction |
| MF | Minimal fluoroscopy |
| OSAS | Obstructive sleep apnea syndrome |
| PM | Pacemaker |
| RA | Right atrium |
| RF | Radiofrequency |
| SVC | Superior vena cava |
| SVT | Supraventricular tachycardia |
| SVTs | Supraventricular tachycardias |
| TSP | Transseptal puncture |
| TV | Tricuspid valve |
| UFH | Unfractionated heparin |
| VKA | Vitamin K antagonist |
| ZF | Zero fluoroscopy |
References
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia—The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC): Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2020, 41, 655–720, Erratum in Eur. Heart J. 2020, 41, 4258. [Google Scholar] [CrossRef] [PubMed]
- Zei, P.C.; Quadros, K.K.; Clopton, P.; Thosani, A.; Ferguson, J.; Brodt, C.; O’Riordan, G.; Ramsis, M.; Mitra, R.; Baykaner, T. Safety and Efficacy of Minimal- versus Zero-fluoroscopy Radiofrequency Catheter Ablation for Atrial Fibrillation: A Multicenter, Prospective Study. J. Innov. Card. Rhythm. Manag. 2020, 11, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Jaco, J.W.; Miller, D.L. Measuring and monitoring radiation dose during fluoroscopically guided procedures. Tech. Vasc. Interv. Radiol. 2010, 13, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Giaccardi, M.; Del Rosso, A.; Guarnaccia, V.; Ballo, P.; Mascia, G.; Chiodi, L.; Colella, A. Near-zero x-ray in arrhythmia ablation using a 3-dimensional electroanatomic mapping system: A multicenter experience. Heart Rhythm. 2016, 13, 150–156. [Google Scholar] [CrossRef]
- Casella, M.; Dello Russo, A.; Pelargonio, G.; Del Greco, M.; Zingarini, G.; Piacenti, M.; Di Cori, A.; Casula, V.; Marini, M.; Pizzamiglio, F.; et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: The NO-PARTY multicentre randomized trial. Europace 2016, 18, 1565–1572. [Google Scholar] [CrossRef]
- Casella, M.; Pelargonio, G.; Dello Russo, A.; Riva, S.; Bartoletti, S.; Santangeli, P.; Scarà, A.; Sanna, T.; Proietti, R.; Di Biase, L.; et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX™ mapping system: Personal experience and review of the literature. J. Interv. Card. Electrophysiol. 2011, 31, 109–118. [Google Scholar] [CrossRef]
- Papez, A.L.; Al-Ahdab, M.; Dick, M., 2nd; Fischbach, P.S. Impact of a computer assisted navigation system on radiation exposure during pediatric ablation procedures. J. Interv. Card. Electrophysiol. 2007, 19, 121–127. [Google Scholar] [CrossRef]
- Yang, L.; Sun, G.; Chen, X.; Chen, G.; Yang, S.; Guo, P.; Wang, Y.; Wang, D.W. Meta-Analysis of Zero or Near-Zero Fluoroscopy Use During Ablation of Cardiac Arrhythmias. Am. J. Cardiol. 2016, 118, 1511–1518. [Google Scholar] [CrossRef]
- Canpolat, U.; Faggioni, M.; Della Rocca, D.G.; Chen, Q.; Ayhan, H.; Vu, A.A.; Mohanty, S.; Trivedi, C.; Gianni, C.; Bassiouny, M. State of Fluoroless Procedures in Cardiac Electrophysiology Practice. J. Innov. Card. Rhythm. Manag. 2020, 11, 4018–4029. [Google Scholar] [CrossRef]
- Fernández-Gómez, J.M.; Moriña-Vázquez, P.; Morales Edel, R.; Venegas-Gamero, J.; Barba-Pichardo, R.; Carranza, M.H. Exclusion of fluoroscopy use in catheter ablation procedures: Six years of experience at a single center. J. Cardiovasc. Electrophysiol. 2014, 25, 638–644. [Google Scholar] [CrossRef]
- Alvarez, M.; Tercedor, L.; Almansa, I.; Ros, N.; Galdeano, R.S.; Burillo, F.; Santiago, P.; Peñas, R. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009, 6, 1714–1720. [Google Scholar] [CrossRef]
- Debreceni, D.; Janosi, K.; Vamos, M.; Komocsi, A.; Simor, T.; Kupo, P. Zero and Minimal Fluoroscopic Approaches During Ablation of Supraventricular Tachycardias: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 856145. [Google Scholar] [CrossRef] [PubMed]
- Roguin, A.; Goldstein, J.; Bar, O.; Goldstein, J.A. Brain and neck tumors among physicians performing interventional procedures. Am. J. Cardiol. 2013, 111, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Casella, M.; Dello Russo, A.; Russo, E.; Catto, V.; Pizzamiglio, F.; Zucchetti, M.; Majocchi, B.; Riva, S.; Vettor, G.; Dessanai, M.A.; et al. X-Ray Exposure in Cardiac Electrophysiology: A Retrospective Analysis in 8150 Patients Over 7 Years of Activity in a Modern, Large-Volume Laboratory. J. Am. Heart Assoc. 2018, 7, e008233. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.W.; Tra, Y.; Garratt, K.N.; Powell, W.; Lopez-Cruz, G.; Chambers, C.; Goldstein, J.A. Society for Cardiovascular Angiography and Interventions. Occupational health hazards of interventional cardiologists in the current decade: Results of the 2014 SCAI membership survey. Catheter. Cardiovasc. Interv. 2015, 86, 913–924. [Google Scholar] [CrossRef]
- Birnie, D.; Healey, J.S.; Krahn, A.D.; Ahmad, K.; Crystal, E.; Khaykin, Y.; Chauhan, V.; Philippon, F.; Exner, D.; Thibault, B.; et al. Prevalence and risk factors for cervical and lumbar spondylosis in interventional electrophysiologists. J. Cardiovasc. Electrophysiol. 2011, 22, 957–960. [Google Scholar] [CrossRef]
- Rubenstein, D.S.; Holmes, B.B.; Manfredi, J.A.; McKillop, M.S.; Netzler, P.C.; Ward, C.C. Aegrescit medendo: Orthopedic disability in electrophysiology—Call for fluoroscopy elimination—Review and commentary. J. Interv. Card. Electrophysiol. 2022, 64, 239–253, Erratum in J. Interv. Card. Electrophysiol. 2022, 64, 255. [Google Scholar] [CrossRef]
- Maskoun, W.; Pino, M.I.; Ayoub, K.; Llanos, O.L.; Almomani, A.; Nairooz, R.; Hakeem, A.; Miller, J. Incidence of Atrial Fibrillation After Atrial Flutter Ablation. JACC Clin. Electrophysiol. 2016, 2, 682–690. [Google Scholar] [CrossRef]
- Rotter, M.; Takahashi, Y.; Sanders, P.; Haïssaguerre, M.; Jaïs, P.; Hsu, L.F.; Sacher, F.; Pasquié, J.L.; Clementy, J.; Hocini, M. Reduction of fluoroscopy exposure and procedure duration during ablation of atrial fibrillation using a novel anatomical navigation system. Eur. Heart J. 2005, 26, 1415–1421. [Google Scholar] [CrossRef]
- Bergonti, M.; Dello Russo, A.; Sicuso, R.; Ribatti, V.; Compagnucci, P.; Catto, V.; Gasperetti, A.; Zucchetti, M.; Cellucci, S.; Vettor, G.; et al. Long-Term Outcomes of Near-Zero Radiation Ablation of Paroxysmal Supraventricular Tachycardia: A Comparison with Fluoroscopy-Guided Approach. JACC Clin. Electrophysiol. 2021, 7, 1108–1117. [Google Scholar] [CrossRef]
- Giaccardi, M.; Mascia, G.; Paoletti Perini, A.; Giomi, A.; Cartei, S.; Milli, M. Long-term outcomes after “Zero X-ray” arrhythmia ablation. J. Interv. Card. Electrophysiol. 2019, 54, 43–48. [Google Scholar] [CrossRef]
- Prolič Kalinšek, T.; Šorli, J.; Jan, M.; Šinkovec, M.; Antolič, B.; Klemen, L.; Žižek, D.; Pernat, A. Conventional fluoroscopy-guided versus zero-fluoroscopy catheter ablation of supraventricular tachycardias. BMC Cardiovasc. Disord. 2022, 22, 98. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Proietti, R.; Wang, X.; Ouyang, F.; Ma, C.S.; Yu, R.H.; Zhao, C.; Ma, K.; Qiu, J.; et al. Zero-fluoroscopy approach for ablation of supraventricular tachycardia using the Ensite NavX system: A multicenter experience. BMC Cardiovasc. Disord. 2020, 20, 48. [Google Scholar] [CrossRef]
- Bocz, B.; Debreceni, D.; Janosi, K.-F.; Turcsan, M.; Simor, T.; Kupo, P. Electroanatomical Mapping System-Guided vs. Intracardiac Echocardiography-Guided Slow Pathway Ablation: A Randomized, Single-Center Trial. J. Clin. Med. 2023, 12, 5577. [Google Scholar] [CrossRef] [PubMed]
- Luani, B.; Basho, M.; Ismail, A.; Rauwolf, T.; Kaese, S.; Tobli, N.; Samol, A.; Pankraz, K.; Schmeisser, A.; Wiemer, M.; et al. Catheter navigation by intracardiac echocardiography enables zero-fluoroscopy linear lesion formation and bidirectional cavotricuspid isthmus block in patients with typical atrial flutter. Cardiovasc. Ultrasound 2023, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Debreceni, D.; Janosi, K.F.; Turcsan, M.; Toth, D.; Bocz, B.; Simor, T.; Kupo, P. Feasibility and safety of cavotricuspid isthmus ablation using exclusive intracardiac echocardiography guidance: A proof-of-concept, observational trial. Front. Cardiovasc. Med. 2023, 10, 1244137. [Google Scholar] [CrossRef] [PubMed]
- Velagic, V.; Mugnai, G.; Prepolec, I.; Pasara, V.; Milinković, A.; Nekić, A.; Bogdanic, J.E.; Posavec, J.P.; Puljević, D.; de Asmundis, C.; et al. Feasibility and safety of reprocessing of intracardiac echocardiography catheters for electrophysiology procedures—A large single center experience. Cardiovasc. Ultrasound 2023, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Hanninen, M.; Yeung-Lai-Wah, N.; Massel, D.; Gula, L.J.; Skanes, A.C.; Yee, R.; Klein, G.J.; Manlucu, J.; Leong-Sit, P. Cryoablation versus RF ablation for AVNRT: A meta-analysis and systematic review. J. Cardiovasc. Electrophysiol. 2013, 24, 1354–1360. [Google Scholar] [CrossRef]
- Gaita, F.; Guerra, P.G.; Battaglia, A.; Anselmino, M. The dream of near-zero X-rays ablation comes true. Eur. Heart J. 2016, 37, 2749–2755. [Google Scholar] [CrossRef]

| Characteristic | Fluoroless | Control | p Value |
|---|---|---|---|
| Age, years (mean ± SD) | 50.9 ± 16.5 | 51.9 ± 16.1 | 0.540 |
| Gender, male, n (%) | 95 (47.5) | 117 (58.5) | 0.031 |
| BMI (mean ± SD) | 26.7 ± 4.8 | 27.5 ± 6.0 | 0.142 |
| Arterial hypertension, n (%) | 80 (40.0) | 90 (45.0) | 0.319 |
| Diabetes mellitus, n (%) | 18 (9.0) | 19 (9.5) | 0.851 |
| Coronary artery disease, n (%) | 9 (4.5) | 13 (6.5) | 0.367 |
| Chronic kidney disease, n (%) | 8 (4.0) | 7 (3.5) | 0.808 |
| COPD, n (%) | 4 (2.0) | 8 (4.0) | 0.238 |
| Hyperlipidemia, n (%) | 58 (29.0) | 58 (29.0) | 0.949 |
| HFrEF, n (%) | 11 (5.5) | 14 (7.0) | 0.426 |
| OSAS, n (%) | 2 (1.0) | 5 (2.5) | 0.250 |
| Valvular heart disease, n (%) | 11 (5.5) | 13 (6.5) | 0.646 |
| PM/ICD implanted, n (%) | 3 (1.5) | 9 (4.5) | 0.010 |
| AADs, n (%) | I 28 (14.0) | I 27 (13.5) | 0.900 |
| II 103 (51.5) | II 110 (55.0) | 0.470 | |
| III 14 (7.0) | III 22 (11.0) | 0.280 | |
| IV 8 (4.0) | IV 4 (2.0) | 0.245 | |
| Anticoagulation, n (%) | VKA 6 (3.0) | VKA 12 (6.0) | 0.148 |
| DOAC 49 (24.5) | DOAC 51 (25.5) | 0.885 | |
| LVEF, % (mean ± SD) | 57.6 ± 10.9 | 56.9 ± 11.8 | 0.538 |
| Characteristic | Fluoroless | Control | p Value |
|---|---|---|---|
| Indication, n (%) | AVNRT 112 (56.0) | AVNRT 100 (50.0) | 0.232 |
| AVRT 33 (16.5) | AVRT 31 (15.5) | 0.425 | |
| AT 11 (5.5) | AT 2 (1.0) | 0.012 | |
| AFL 44 (22.0) | AFL 67 (33.5) | 0.012 | |
| Duration, min (mean ± SD) | 59.0 ± 25.8 | 72.7 ± 34.0 | <0.001 |
| Fluoroscopy duration, min (mean ± SD) | 0 | 11.4 ± 9.5 | <0.001 |
| Fluoroscopy total, mGy (mean ± SD) | 0 | 19.6 ± 26.9 | <0.001 |
| Fluoroscopy dose, Gy/cm2 (mean ± SD) | 0 | 174.8 ± 252.9 | <0.001 |
| Ablation duration, s (mean ± SD) | 418.7 ± 898.8 | 558.4 ± 646.6 | 0.075 |
| Acute success, n (%) | 200 (100.0) | 200 (100.0) | 0.152 |
| TSP, n (%) | 32 (16.0) | 21 (10.5) | 0.200 |
| Fluoroless | Control | p Value | |
|---|---|---|---|
| Arrhythmia recurrence, n (%) | 12 (6.0) | 10 (5.0) | 0.670 |
| Time to recurrence, days (mean ± SD) | 127.5 ± 41.4 | 140.0 ± 24.0 | 0.410 |
| Complications, n (%) | 0 (0) | Total AV-block 1 (0.5) | 0.313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekic, A.; Pasara, V.; Prepolec, I.; Bilic-Pavlinovic, A.; Cala, A.; Kardum, D.; Katic, Z.; Pezo-Nikolic, B.; Puljevic, D.; Milicic, D.; et al. Completely Fluoroless, “Apron-Less” Approach to Supraventricular Tachycardia Ablation Compared to Traditional Fluoroscopy Guided Ablation: Feasibility, Safety and Clinical Outcomes. J. Clin. Med. 2025, 14, 7076. https://doi.org/10.3390/jcm14197076
Nekic A, Pasara V, Prepolec I, Bilic-Pavlinovic A, Cala A, Kardum D, Katic Z, Pezo-Nikolic B, Puljevic D, Milicic D, et al. Completely Fluoroless, “Apron-Less” Approach to Supraventricular Tachycardia Ablation Compared to Traditional Fluoroscopy Guided Ablation: Feasibility, Safety and Clinical Outcomes. Journal of Clinical Medicine. 2025; 14(19):7076. https://doi.org/10.3390/jcm14197076
Chicago/Turabian StyleNekic, Andrija, Vedran Pasara, Ivan Prepolec, Ana Bilic-Pavlinovic, Ana Cala, Domagoj Kardum, Zvonimir Katic, Borka Pezo-Nikolic, Davor Puljevic, Davor Milicic, and et al. 2025. "Completely Fluoroless, “Apron-Less” Approach to Supraventricular Tachycardia Ablation Compared to Traditional Fluoroscopy Guided Ablation: Feasibility, Safety and Clinical Outcomes" Journal of Clinical Medicine 14, no. 19: 7076. https://doi.org/10.3390/jcm14197076
APA StyleNekic, A., Pasara, V., Prepolec, I., Bilic-Pavlinovic, A., Cala, A., Kardum, D., Katic, Z., Pezo-Nikolic, B., Puljevic, D., Milicic, D., & Velagic, V. (2025). Completely Fluoroless, “Apron-Less” Approach to Supraventricular Tachycardia Ablation Compared to Traditional Fluoroscopy Guided Ablation: Feasibility, Safety and Clinical Outcomes. Journal of Clinical Medicine, 14(19), 7076. https://doi.org/10.3390/jcm14197076