Viable and Functional: Long-Term −80 °C Cryopreservation Sustains CD34+ Integrity and Transplant Success
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethics
2.2. Study Population
2.3. Stem Cell Collection and Processing
2.4. Conditioning Regimens and Transplantation
2.5. Viability Assessment
2.6. Engraftment Definitions
2.7. Statistical Analysis
3. Results
3.1. Long-Term Cryostorage Preserves CD34+ Viability Despite Time-Dependent Decline
3.2. Engraftment Dynamics Reflect Disease Biology Rather than Product Integrity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AO | acridine orange |
| ALL | acute lymphoblastic leukemia |
| ANC | absolute neutrophil count |
| CD34+ | cluster of differentiation 34 positive |
| CR | complete remission |
| DMSO | dimethyl sulfoxide |
| EB | ethidium bromide |
| ELN | European LeukemiaNet |
| G-CSF | granulocyte-colony stimulating factor |
| HL | Hodgkin lymphoma |
| HSC | hematopoietic stem cell |
| MM | multiple myeloma |
| NHL | non-Hodgkin lymphoma |
| OS | overall survival |
| PFS | progression-free survival |
| PLT | platelet |
| TBI | total body irradiation |
| T0 | initial collection time point |
| T1 | pre-infusion time point |
| T2 | delayed post-thaw assessment time point |
| WBC | white blood cell |
| 7-AAD | 7-aminoactinomycin D |
References
- Bembnista, E.; Stawicka, P.; Matuszak, P.; Łojko-Dankowska, A.; Dytfeld, D.; Matuszak, M.; Wache, A.; Kaźmierska, K.; Majewska, E.; Lewandowski, K.; et al. Should we Cryopreserve Allogenic Hematopoietic Stem Cell Grafts? Vivo 2025, 39, 870–876. [Google Scholar] [CrossRef]
- Gokarn, A.; Tembhare, P.R.; Syed, H.; Sanyal, I.; Kumar, R.; Parab, S.; Khanka, T.; Punatar, S.; Kedia, S.; Ghogale, S.G.; et al. Long-Term Cryopreservation of Peripheral Blood Stem Cell Harvest Using Low Concentration (4.35%) Dimethyl Sulfoxide with Methyl Cellulose and Uncontrolled Rate Freezing at −80 °C: An Effective Option in Resource-Limited Settings. Transplant. Cell. Ther. 2023, 29, 777.e1–777.e8. [Google Scholar] [CrossRef]
- Wan, B.; Lindo, L.; Mourad, Y.A.; Chung, S.; Forrest, D.; Kuchenbauer, F.; Nantel, S.; Narayanan, S.; Nevill, T.; Power, M.; et al. Outcomes with allogeneic stem cell transplant using cryopreserved versus fresh hematopoietic progenitor cell products. Cytotherapy 2024, 26, 1210–1216. [Google Scholar] [CrossRef]
- Varan, H.D.; Bay, M.; Ozturk, A.; Dalva, K.; İlhan, O. Comparison of the methods evaluating post thawing viability of peripheral blood stem cell graft. Transfus. Apher. Sci. 2019, 58, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.A.; Patel, N.; Kim, G.H.; Chape, L.S.; Agbon, K.G.D.; Angulo Fuentes, M.P.; Barbosa, F.-M.L.; Castellano, R.T.; Gonzalez, G.N.; Mathew, S.; et al. Validation of automated fluorescent-based technology for measuring total nucleated cell viability of hematopoietic progenitor cell products. Transfusion 2022, 62, 848–856. [Google Scholar] [CrossRef]
- Donnenberg, A.D.; Koch, E.K.; Griffin, D.L.; Stanczak, H.M.; Kiss, J.E.; Carlos, T.M.; Buchbarker, D.M.; Yeager, A.M. Viability of cryopreserved BM progenitor cells stored for more than a decade. Cytotherapy 2002, 4, 157–163. [Google Scholar] [CrossRef]
- Cai, Y.; Prochazkova, M.; Kim, Y.-S.; Jiang, C.; Ma, J.; Moses, L.; Martin, K.; Pham, V.; Zhang, N.; Highfill, S.L.; et al. Assessment and comparison of viability assays for cellular products. Cytotherapy 2024, 26, 201–209. [Google Scholar] [CrossRef]
- Rimac, V.; Bojanić, I.; Škifić, M.; Dabelić, S.; Golubić Ćepulić, B. Quality Assessment of Cryopreserved Peripheral Blood Stem Cell Products: Evaluation of Two Methods for Flow Cytometric Viability Testing. Int. J. Lab. Hematol. 2025, 47, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Donmez, A.; Yilmaz, F.; Soyer, N.; Cagirgan, S.; Arik, B.; Tombuloglu, M. The loss of CD34+ cells in peripheral hematopoietic stem cell products cryopreserved by non-controlled rate freezing and stored at −80 °C after overnight storage. Transfus. Apher. Sci. 2014, 51, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gromme, M. Lower cell viability determined by 7-AAD flow cytometry in HPC-A that have been stored overnight at 2–8 degrees prior to cryopreservation. Cytotherapy 2019, 21, S46. [Google Scholar] [CrossRef]
- Makhani, S.S.; Oza, S.P.; Reich-Slotky, R.; Munshi, P.N.; Biran, N.; Donato, M.L.; Siegel, D.S.; Vesole, D.H.; Naam, S.; Rowley, S.D. Sustained Hematopoietic Engraftment Potential after Prolonged Storage of Cryopreserved Hematopoietic Stem Cells Used in Salvage Autologous Stem Cell Transplantation. Transplant. Cell. Ther. 2022, 28, e301–e306. [Google Scholar] [CrossRef] [PubMed]
- Underwood, J.; Rahim, M.; West, C.; Britton, R.; Skipworth, E.; Graves, V.; Sexton, S.; Harris, H.; Schwering, D.; Sinn, A.; et al. How old is too old? In vivo engraftment of human peripheral blood stem cells cryopreserved for up to 18 years—Implications for clinical transplantation and stability programs. World J. Stem Cells 2020, 12, 359–367. [Google Scholar] [CrossRef]
- Attarian, H.; Feng, Z.; Buckner, C.D.; MacLeod, B.; Rowley, S.D. Long-term cryopreservation of bone marrow for autologous transplantation. Bone Marrow Transpl. 1996, 17, 425–430. [Google Scholar]
- Vrhovac, R.; Peric, Z.; Jurenec, S.; Kardum-Skelin, I.; Jelic-Puskaric, B.; Siftar, Z.; Mesaric, J.; Minigo, H.; Jaksic, B. Post-Thaw Viability of Cryopreserved Hematopoietic Progenitor Cell Grafts Influences Patients’ Predisposition towards Infectious Complications in the Post-Transplant Period. Blood 2009, 114, 4666. [Google Scholar] [CrossRef]
- Alswied, A.; Daniel, D.; Chen, L.N.; Alqahtani, T.; West-Mitchell, K.A. CD34+ cell yield among healthy donors: Large-scale model development and validation. J. Clin. Apher. 2024, 39, e22135. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.R.; Anderson, L.; Keeney, M.; Nayar, R.; Chin-Yee, I.A.N. The ISHAGE Guidelines for CD34+ Cell Determination by Flow Cytometry. J. Hematother. 1996, 5, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Chin-Yee, I.; Weir, K.; Popma, J.; Nayar, R.; Sutherland, D.R. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. Cytometry 1998, 34, 61–70. [Google Scholar] [CrossRef]
- Sureda, A.; Carpenter, P.A.; Bacigalupo, A.; Bhatt, V.R.; de la Fuente, J.; Ho, A.; Kean, L.; Lee, J.W.; Sánchez-Ortega, I.; Savani, B.N.; et al. Harmonizing definitions for hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: A report on behalf of the EBMT, ASTCT, CIBMTR, and APBMT. Bone Marrow Transpl. 2024, 59, 832–837. [Google Scholar] [CrossRef]
- Valcárcel, D.; Sánchez-Ortega, I.; Sureda, A. Graft Failure; Springer International Publishing: Berlin/Heidelberg, Germany, 2024; pp. 365–372. [Google Scholar]
- Norton, J.; Campo, L.; Hagen, P.; Tsai, S.B.; Stiff, P.J. In Vivo Potency of Hematopoietic Stem Cells after Long-Term Storage at -80C in DMSO + HES Cryprotectant Mixture: Comparing Engraftment Kinetics and Efficacy Data in First and Second Autologous Stem Cell Transplantation in Myeloma. Blood 2023, 142 (Suppl. S1), 6779. [Google Scholar] [CrossRef]
- Jeung, K.J.; Kang, M.S.; Kwon, K.D.; Kim, K.H.; Lee, J.C.; Lee, S.C.; Kim, H.J.; Bae, S.B.; Kim, C.K.; Lee, N.S.; et al. Influential Factors for Engraftment in Autologous Peripheral Hematopoietic Stem Cell Transplantation (APBSCT). Korean J. Hematol. 2007, 42, 301–308. [Google Scholar] [CrossRef]
- Giai, V.; Tamiazzo, S.; Leoncino, S.; Mele, L.; Ciriello, M.M.; Monaco, F.; Bernocco, E.; Levis, A.; Guaschino, R.; Pini, M.; et al. Graft Purity and Composition Significantly Impact the Engraftment of Autologous Stem Cell Transplants. Blood 2016, 128, 5733. [Google Scholar] [CrossRef]
- Andrić, B.; Vujić, D.; Šerbić, O.; Radonjić, Z.; Simić, M.; Kuzmanović, M. Predictive factors for engraftment kinetics of autologous hematopoietic stem cells in children. Hematol. Transfus. Cell Ther. 2024, 46, S291–S298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, X.; Chen, X.-H.; Gao, L.; Gao, L.; Kong, P.-Y.; Peng, X.-G.; Sun, A.-H.; Wang, Q.-Y. Factors influencing engraftment in HLA-haploidentical/mismatch related transplantation with combined granulocyte-colony stimulating factor-mobilized peripheral blood and bone marrow for patients with leukemia. Transfus. Apher. Sci. 2011, 44, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, L.C.; Lee, E.M.; Shin, S.H.; Kim, Y.S.; Moon, J.-H.; Lee, W.S.; Shin, H.-J.; Kim, M.H.; Ye, B.J.; et al. Predictive factors for rapid neutrophil and platelet engraftment after allogenic peripheral blood stem cell transplantation in patients with acute leukemia. Ann. Hematol. 2013, 92, 1685–1693. [Google Scholar] [CrossRef]
- Ersal, T.; Özkocaman, V.; Yalçın, C.; Orhan, B.; Candar, Ö.; Çubukçu, S.; Koca, T.G.; Pınar, İ.E.; Hunutlu, F.Ç.; Özkalemkaş, F. The effect of cryopreservation on engraftment kinetics in fully matched allogeneic stem cell transplantation: Real-life data and literature review. Transfus. Apher. Sci. 2023, 62, 103821. [Google Scholar] [CrossRef]
- Keyzner, A.; Azzi, J.; Jakubowski, R.; Sinitsyn, Y.; Tindle, S.; Shpontak, S.; Kwon, D.; Isola, L.; Iancu-Rubin, C. Cryopreservation of Allogeneic Hematopoietic Cell Products During COVID-19 Pandemic: Graft Characterization and Engraftment Outcomes. Transplant. Proc. 2023, 55, 1799–1809. [Google Scholar] [CrossRef]
- Plemel, J.R.; Caprariello, A.V.; Keough, M.B.; Henry, T.J.; Tsutsui, S.; Chu, T.H.; Schenk, G.J.; Klaver, R.; Yong, V.W.; Stys, P.K. Unique spectral signatures of the nucleic acid dye acridine orange can distinguish cell death by apoptosis and necroptosis. J. Cell Biol. 2017, 216, 1163–1181. [Google Scholar] [CrossRef]
- Huang, Y.; Patel, S.; Pierce, M.; Lin, B.; Chan, L. Characterization and comparison of acridine orange/propidium iodide and acridine/DAPI viability detection methods for cell and gene therapy development. J. Immunol. 2023, 210 (Suppl. S1), 249.05. [Google Scholar] [CrossRef]
- Hussain, H.; Raj, L.S.; Ahmad, S.; Razak, M.F.A.; Wan Mohamud, W.N.; Bakar, J.; Ghazali, H.M. Determination of cell viability using acridine orange/propidium iodide dual-spectrofluorometry assay. Cogent Food Agric. 2019, 5, 1582398. [Google Scholar] [CrossRef]
- McCullough, J.; Haley, R.; Clay, M.; Hubel, A.; Lindgren, B.; Moroff, G. Long-term storage of peripheral blood stem cells frozen and stored with a conventional liquid nitrogen technique compared with cells frozen and stored in a mechanical freezer. Transfusion 2010, 50, 808–819. [Google Scholar] [CrossRef]
- Fountain, D.; Ralston, M.; Higgins, N.; Gorlin, J.; Uhl, L.; Wheeler, C.; Antin, J.; Churchill, W.; Benjamin, R. Liquid nitrogen freezers: A potential source of microbial contamination of hematopoietic stem cell components. Transfusion 1997, 37, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Tanhehco, Y.C. Effect of temporary storage of cryopreserved cellular therapy products at −80° celsius on cell recovery and viability. Transfus. Apher. Sci. 2024, 63, S94. [Google Scholar] [CrossRef] [PubMed]

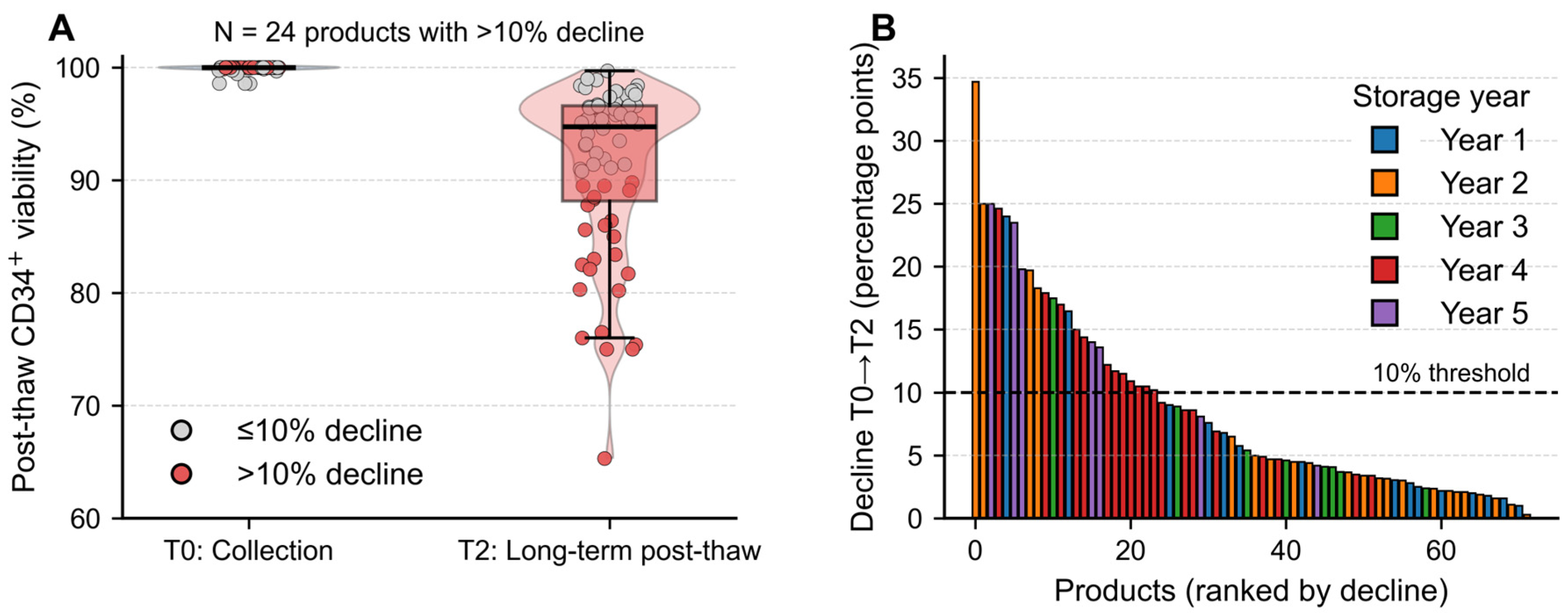
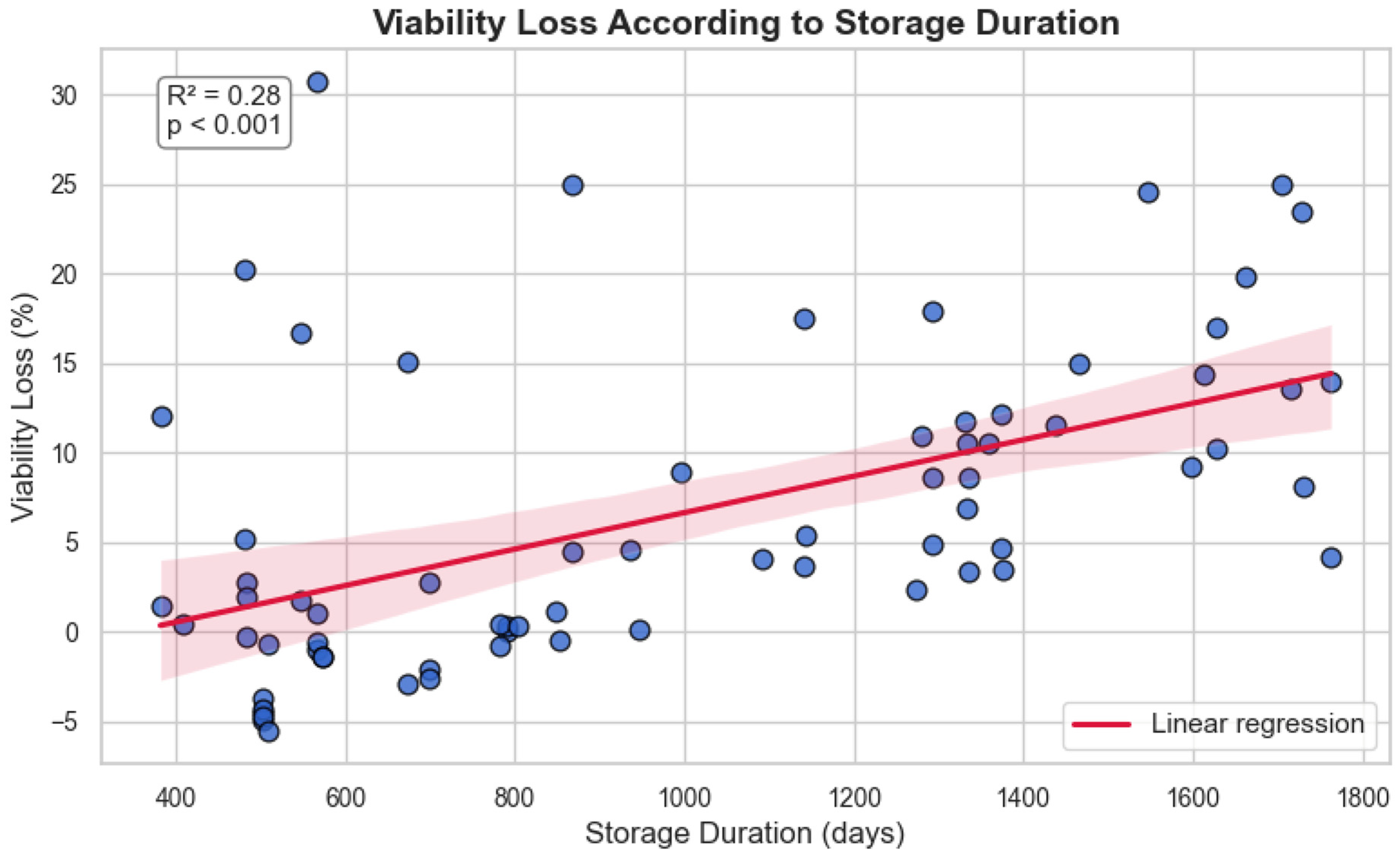

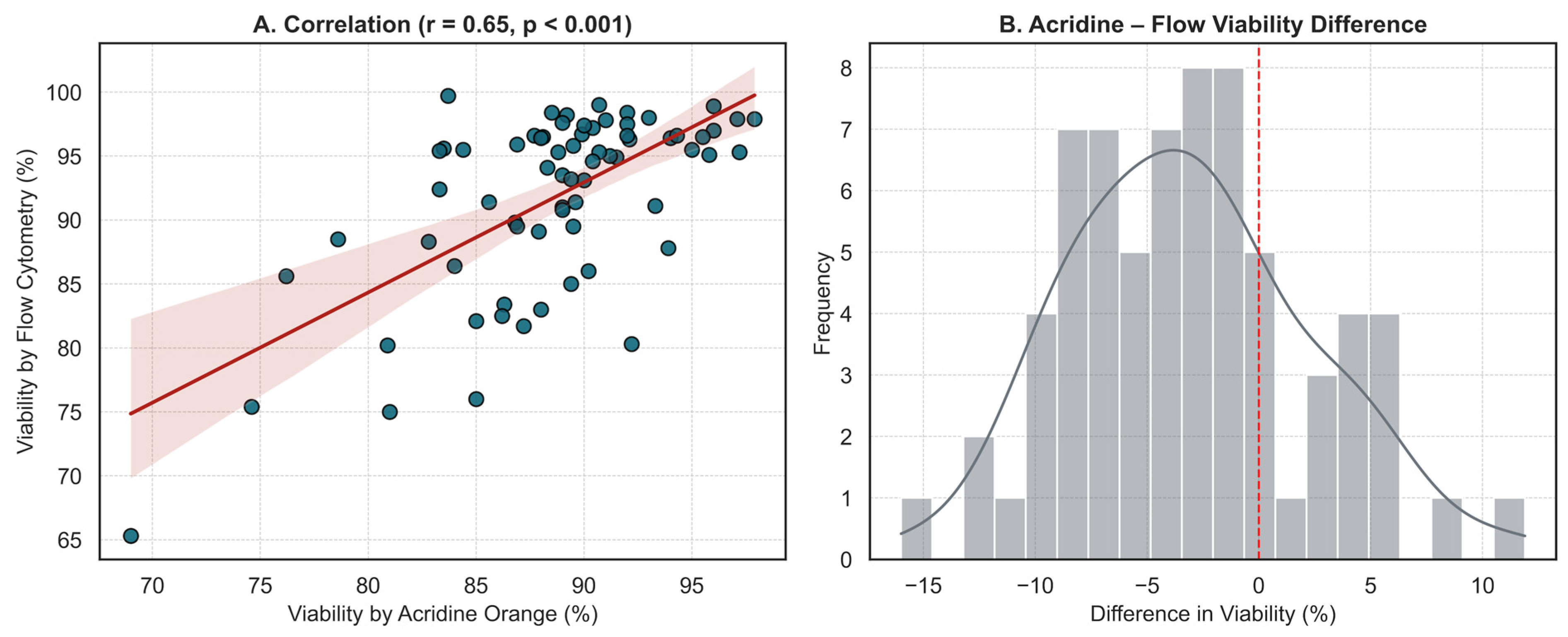
| Characteristic | n (%) or Median (Range) |
|---|---|
| Gender | |
| Female | 10 (40%) |
| Male | 15 (60%) |
| Age (years) | 54 (19–63) |
| Body Mass Index (kg/m2) | 25.9 (18.6–38.3) |
| Diagnosis | |
| Hodgkin lymphoma (HL) | 1 (4%) |
| Non-Hodgkin lymphoma (NHL) | 2 (8%) |
| Multiple myeloma (MM) | 13 (52%) |
| Acute lymphoblastic leukemia (ALL) | 8 (32%) |
| Chronic myelomonocytic leukemia (CMML) | 1 (4%) |
| Mobilization Regimen | |
| Unstimulated bone marrow | 1 (4%) |
| G-CSF | 19 (76%) |
| Etoposide + G-CSF | 4 (16%) |
| Plerixafor + G-CSF | 1 (4%) |
| Transplantation Status | |
| Transplant performed | 20 (80%) |
| Not performed | 5 (20%) |
| Conditioning Regimen and Intensity * | |
| Melphalan 200 mg/m2 (Mel-200) | 10 (50%) |
| Melphalan 140 mg/m2 (Mel-140) | 2 (10%) |
| Busulfan + Cyclophosphamide (Bu-Cy) | 5 (25%) |
| Etoposide + Cyclophosphamide + TBI | 3 (15%) |
| Myeloablative | 20 (100%) |
| Radiotherapy | 3 (12%) |
| Number of Prior Treatment Lines | |
| 1 line | 10 (40%) |
| 2 lines | 8 (32%) |
| 3 lines | 4 (16%) |
| 4 lines | 1 (4%) |
| 5 lines | 2 (8%) |
| Neutrophil engraftment > 0.5 × 109/L (days) | 13.5 (7–23) |
| Platelet engraftment > 20 × 109/L (days) | 13.5 (7–30) |
| Platelet engraftment > 50 × 109/L (days) | 16.5 (13–37) |
| Parameter | Median (Range) |
|---|---|
| Storage duration at −80 °C (days) | 868 (382–1763) |
| Total CD34+ cells collected (µL) | 1770.5 (18–3591) |
| Viable CD34+ cells collected (µL) | 1242 (18–3591) |
| Total cell viability (%) | 100 (95.2–100) |
| CD34+ cell viability (%) | 100 (98.6–100) |
| CD34+ cell dose (×106/kg recipient body weight) | 8.5 (3.3–14.0) |
| Harvest WBC count (×109/L) | 180.5 (45.3–381.0) |
| Harvest neutrophil count (×109/L) | 72.9 (0.01–263.0) |
| Harvest lymphocyte count (×109/L) | 51.4 (8.97–191.0) |
| Harvest monocyte count (×109/L) | 27.9 (7.58–135.0) |
| Mononuclear cell to total WBC ratio | 0.60 (0.23–0.99) |
| Characteristic | CD34+ Cell Dose (×106/kg) | p | Neutropenia Duration (Days) | p | ANC > 0.5 × 109/L (Days) | p | PLT > 20 × 109/L (Days) | p | PLT > 50 × 109/L (Days) | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | 0.7868 | 0.786 | 0.6692 | 0.9623 | 1.000 | |||||
| Female | 5.4 (4.2–6.7) | 11 (7–36) | 13.5 (11–23) | 13 (10–21) | 16.5 (13–36) | |||||
| Male | 6.0 (3.4–8.1) | 14 (6–27) | 13.5 (7–23) | 13.5 (7–30) | 17.5 (13–37) | |||||
| Comorbidity | 0.8364 | 0.2809 | 0.0967 | 0.4557 | 0.4941 | |||||
| Absent | 6.0 (3.5–8.1) | 27 (6–27) | 19.5 (11–23) | 12 (11–17) | 16 (13–26) | |||||
| Present | 5.4 (3.4–6.8) | 10 (6–36) | 12.5 (7–23) | 14 (7–30) | 17 (13–37) | |||||
| Diagnosis | 0.105 | <0.001 | <0.001 | 0.501 | 0.558 | |||||
| Multiple Myeloma | 4.8 (3.4–6.8) | 8.5 (6–18) | 11 (7–19) | 12.5 (7–30) | 17 (13–37) | |||||
| Acute Lymphoblastic Leukemia | 6.0 (4.2–8.1) | 27 (12–36) | 19.5 (15–23) | 14.5 (11–21) | 16 (13–30) | |||||
| CR Status | 0.636 | 0.296 | 0.341 | 0.012 | 0.161 | |||||
| Yes | 5.9 (3.4–6.8) | 14.5 (6–36) | 14.5 (10–23) | 14.5 (10–30) | 19 (13–37) | |||||
| No | 4.8 (3.5–8.1) | 8.5 (6–27) | 11.5 (7–22) | 11 (7–12) | 15.5 (13–16) | |||||
| Fresh Product | 0.1483 | 0.0144 | 0.0179 | 0.5708 | 0.2790 | |||||
| Yes | 6.0 (5.9–6.1) | 27 (12–36) | 17 (15–23) | 14.5 (12–17) | 14 (13–26) | |||||
| No | 4.8 (3.4–8.1) | 9 (6–28) | 11.5 (7–23) | 12.5 (7–30) | 17 (13–37) |
| Parameter | Frozen Group (Patients, n = 6), Median (Range) | Fresh Group (Patients, n = 14), Median (Range) | p |
|---|---|---|---|
| Total CD34+ cells collected (µL) | 2870.0 (45.0–3591.0) | 2050.0 (18.0–2700.0) | 0.2857 |
| Viable CD34+ cells collected (µL) | 2866.0 (45.0–3591.0) | 1368.0 (18.0–2685.0) | 0.1773 |
| Total cell viability (%) | 96.2 (95.7–96.8) | 96.5 (93.5–96.9) | 0.7110 |
| CD34+ cell viability (%) | 100.0 (99.7–100.0) | 100.0 (98.6–100.0) | 0.8135 |
| CD34+ cell dose (×106/kg recipient body weight) | 6.0 (5.9–6.1) | 4.8 (3.42–8.1) | 0.1483 |
| Harvest WBC count (×109/L) | 237.0 (65.5–314.0) | 171.9 (45.3–381.0) | 0.3425 |
| Harvest neutrophil count (×109/L) | 119.5 (0.01–263.0) | 61.8 (0.02–102.0) | 0.0326 |
| Harvest lymphocyte count (×109/L) | 79.9 (51.4–137.0) | 53.9 (22.8–191.0) | 0.1297 |
| Harvest monocyte count (×109/L) | 46.4 (12.2–86.0) | 64.1 (8.3–135.0) | 0.3530 |
| Mononuclear cell to total WBC ratio | 0.60 (0.37–0.99) | 0.61 (0.47–0.99) | 0.7103 |
| Parameter | Correlation Coefficient (ρ) | p-Value | N |
|---|---|---|---|
| Post-thaw viability (Flow cytometry) | −0.087 | 0.6784 | 25 |
| CD34+ cell viability (%) | −0.181 | 0.3877 | 25 |
| Total cell viability (%) | 0.176 | 0.5853 | 25 |
| CD34+ cell dose (×106/kg recipient body weight) | −0.342 | 0.14 | 20 |
| Neutropenia duration (days) | −0.761 | <0.001 | 20 |
| ANC > 0.5 × 109/L (days) | −0.75 | <0.001 | 20 |
| PLT > 20 × 109/L (days) | −0.022 | 0.931 | 18 |
| PLT > 50 × 109/L (days) | 0.066 | 0.808 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinar, I.E.; Sahin, M.; Gursoy, V.; Ersal, T.; Budak, F.; Ozkocaman, V.; Ozkalemkas, F. Viable and Functional: Long-Term −80 °C Cryopreservation Sustains CD34+ Integrity and Transplant Success. J. Clin. Med. 2025, 14, 7032. https://doi.org/10.3390/jcm14197032
Pinar IE, Sahin M, Gursoy V, Ersal T, Budak F, Ozkocaman V, Ozkalemkas F. Viable and Functional: Long-Term −80 °C Cryopreservation Sustains CD34+ Integrity and Transplant Success. Journal of Clinical Medicine. 2025; 14(19):7032. https://doi.org/10.3390/jcm14197032
Chicago/Turabian StylePinar, Ibrahim Ethem, Muge Sahin, Vildan Gursoy, Tuba Ersal, Ferah Budak, Vildan Ozkocaman, and Fahir Ozkalemkas. 2025. "Viable and Functional: Long-Term −80 °C Cryopreservation Sustains CD34+ Integrity and Transplant Success" Journal of Clinical Medicine 14, no. 19: 7032. https://doi.org/10.3390/jcm14197032
APA StylePinar, I. E., Sahin, M., Gursoy, V., Ersal, T., Budak, F., Ozkocaman, V., & Ozkalemkas, F. (2025). Viable and Functional: Long-Term −80 °C Cryopreservation Sustains CD34+ Integrity and Transplant Success. Journal of Clinical Medicine, 14(19), 7032. https://doi.org/10.3390/jcm14197032






