Optimizing Fetal Surveillance in Fetal Growth Restriction: A Narrative Review of the Role of the Computerized Cardiotocographic Assessment
Abstract
1. Introduction
1.1. Etiology and Pathophysiology of Fetal Growth Restriction
1.2. Clinical Classification and Diagnostic Challenges
1.3. Doppler Surveillance in Fetal Growth Restriction
1.4. Maternal Perception of Fetal Movements
1.5. Role and Interpretation of Cardiotocography
1.5.1. Standard Criteria and Interpretation of Normal Fetal Heart Rate in Cardiotocography
- Baseline Fetal Heart Rate: The baseline heart rate measured in beats per minute. The baseline rate should be between 110 and 160 beats per minute. This range reflects normal autonomic regulation and is considered physiologically stable.
- Baseline Variability: Variability refers to the fluctuations in the baseline FHR, reflecting the interplay between sympathetic and parasympathetic nervous systems. Reassuring variability lies within 5 to 25 beats per minute.
- Accelerations: Accelerations are transient increases in FHR below the baseline, typically by ≥15 bpm lasting for ≥15 s. While their presence is reassuring, their absence does not automatically indicate pathology if other parameters are normal.
- Decelerations: Are decrease in FHR below the baseline that lasted longer than 15 s and had an amplitude greater than 10 bpm. Early deceleration, meaning the start of the deceleration, is simultaneous with the start of uterine contraction, and the end of the deceleration end simultaneous with the contraction, and late decelerations means the start of the deceleration is after the start of the contraction and the end of the deceleration is after the end of the contraction. There should be no decelerations, or only early decelerations, which are typically benign and associated with fetal head compression during contractions. Variable or late decelerations may suggest cord compression or uteroplacental insufficiency and warrant further evaluation.
1.5.2. Standard Criteria and Interpretation of Normal Computerized Cardiotocography
- Baseline Fetal Heart Rate: The baseline heart rate measured in beats per minute. A stable heart rate between 110 and 160 beats per minute is considered normal and reflects adequate autonomic control.
- Short-Term Variation (ST variation): Measure beat to beat variation (expressed in milliseconds) of fetal heart rate which cannot be interpreted by the human eye.
- Short-Term Variability (STV): Measure beat to beat variation (expressed in bpm), sequential epoch-to-epoch variation.
- Long-term variability (LTV) measure minute-by-minute range of pulse intervals and represents the broader oscillations in baseline FHR over time. A normal LTV ranges between 5 and 25 bpm, indicating an intact autonomic nervous system and adequate fetal oxygenation. Abnormally low or high LTV may signal fetal hypoxia, sedation, or altered neurological function.
- Accelerations: The presence of at least one acceleration (a transient increase in FHR of ≥10 bpm lasting ≥15 s) indicates fetal reactivity. While not mandatory, accelerations enhance the reassuring nature of the trace.
- Episodes of High Variation: These are periods of increased FHR variability and are associated with fetal movement. A normal cCTG should include at least one episode of high variation during the monitoring period.
- Absence of Decelerations: A normal cCTG should show no late or prolonged decelerations. Occasional early or brief variable decelerations may be considered acceptable if the overall trace is otherwise normal.
- Fetal Movements (Optional): Detection of fetal movements during the trace is supportive but not essential for a normal classification under the Dawes–Redman system.
1.5.3. Duration of Recording
2. Materials and Methods
3. Results
3.1. Evidence from Recent Studies
- decreased baseline variability;
- absence of accelerations;
- the emergence of late decelerations;
- sustained bradycardia or tachycardia.
| Author, Publication Year of the Study | Study Cohort | Results Related to Computerized Cardiotocography Alterations On Variability, STV, ST Variation and LTV | Correlation with Other Modalities of Surveillance/Management Implications |
|---|---|---|---|
| Hecher, K. 2001 [47] | 110 singleton pregnancies with growth-restricted fetuses after 24 weeks (early and late FGR) | Low ST variation associated with imminent fetal compromise. ST Variation < 3 ms often indicated delivery within 5 days of absent/reversed end-diastolic flow. | Before 32 weeks of gestation, the PI of the DV and STVariation in FHR are crucial indicators for assessing the appropriate timing of delivery. If either parameter shows persistent abnormalities, delivery should be taken into consideration. |
| Anceschi, M.M. 2004 [48] | 24 FGR pregnancies with abnormal Doppler velocimetry, monitored with cCTG before cesarean delivery | STV correlated significantly with umbilical artery blood gases (pH, pCO2). An STV threshold <4.5 ms predicted severe acidosis (pH <7.00) and hypercarbia (pCO2 >80 mmHg) with 100% sensitivity and 70–78% specificity. Other cCTG parameters showed no significant associations. | STV is a reliable marker of fetal acidosis and hypercarbia in FGR with Doppler abnormalities. A cutoff of 4.5 ms may serve as a clinical reference for timing delivery. |
| Soncini, E. 2006 [49] | 50 FGR pregnancies, 186 cCTG recordings evaluated in conjunction with Doppler (UA, MCA, DV). | Absent/reversed end-diastolic flow in UA was linked with reduced STV, variability, and accelerations. Brain-sparing (MCA/UA <1) showed no association with cCTG changes. DV abnormalities were closely related to loss of variability, indicating advanced compromise. In milder cases, physiological maturation of cCTG with gestational age was present, but absent in severe FGR. | cCTG alterations parallel Doppler deterioration, with DV changes most predictive of advanced decompensation. Combined use of cCTG and Doppler (UA, DV) is essential for early recognition of fetal compromise in FGR. |
| Ferrario, M. 2009 [50] | 59 fetuses at 27–34 weeks (17 normal, 19 non-severe FGR, 23 severe FGR) | Signal complexity indices (Lempel–Ziv Complexity, Multiscale Entropy) effectively discriminated severe FGR from both normal and non-severe FGR. Severe FGR showed reduced entropy/complexity, indicating loss of physiological autonomic control. A multiparametric approach (LZC + MSE slope) improved sensitivity and accuracy compared with single measures. | Complexity analysis of fetal heart rate provides early identification of severe FGR and differentiates it from non-severe cases. Multiparametric methods show promise for enhancing prenatal surveillance. |
| Huhn, E.A. 2011 [51] | 74 FGR vs. 161 normal pregnancies, gestational age–matched (retrospective, single-center). | Both STV and PRSA-derived AAC were significantly reduced in FGR compared with controls. AAC demonstrated superior discriminatory capacity over STV, with more balanced sensitivity and specificity. | PRSA-derived AAC provides additional insight into fetal autonomic control and may be a more accurate marker of FGR than STV. Supports further longitudinal validation for outcome prediction. |
| Tagliaferri, S. 2015 [52] | 120 pregnancies ≥30 weeks (59 normal, 61 IUGR), retrospective cross-sectional. | Phase-rectified slope indices (APRS/DPRS) discriminated IUGR from normal more accurately than conventional cCTG parameters (STV, LTI, entropy, Lempel–Ziv). Both indices showed significant correlation with cord blood pH. Predictive value was strongest before 34 weeks. | APRS and DPRS provide superior diagnostic performance over standard cCTG metrics, particularly in early-onset IUGR, and represent promising tools to optimize delivery timing. |
| Lobmaier, S. 2016 [53] | 279 very preterm FGR fetuses (26–32 weeks), secondary analysis of TRUFFLE study | Applied phase-rectified signal averaging (PRSA: AAC, ADC) to raw cCTG data and compared with STV. AAC/ADC declined earlier (72 h before delivery) than STV (<48 h). PRSA indices predicted antenatal death and Apgar <7 more accurately than STV, but neither method predicted neurodevelopment at 2 yrs. | PRSA detects fetal deterioration earlier than STV and may provide superior short-term prognostic value in very preterm FGR. |
| Amorim-Costa, C. 2017 [54] | 11 687 cases were analyzed retrospective, of which 1 986 were identified as small for gestational age (SGA) < p10, and 543 FGR < p3 | SGA fetuses exhibited a lower average long-term variability (LTV), which was the parameter showing the most statistically significant difference, as well as reduced average STV and fewer accelerations. Baseline FHR was lower in the SGA group compared to normal fetuses starting from 34 weeks of gestation. The number of decelerations remained consistently similar between both groups. | Differences most evident between 28–35 weeks; deceleration frequency remained similar across groups. |
| Strolux, L. 2017 [55] | 1163 FGR < 3 th centiles and 1163 control cases | FGR fetuses show a lower percentage of high variability (active sleep) compared to the normal population | Combining HRV markers with inferred fetal sleep states improved early-onset FGR detection. |
| Frusca, T. 2017 [56] | 511 cases, TRUFFLE study (The Trial of Umbilical and Fetal Flow in Europe) | <32 weeks, DV Doppler abnormalities sometimes preceded STV reduction; waiting until cCTG became abnormal increased risk of fetal death or neurological issue. >32 weeks abnormal CTG parameters precede DV abnormalities | Delivery between 26–28+6 Weeks: - DV absent a wave associated with cCTG-STVariation <2.6 milliseconds at 26+0–28+6 weeks and <3 milliseconds at 29+0–31+6 weeks. -decelerations Delivery after 32 weeks: -Spontaneous repeated persistent deceleration -UA modifications ARED flow may always prompt delivery >32 weeks and AED >34 weeks. |
| Visser, G.H.A. 2017 [57] | 310 pregnancies with early-onset FGR (<32 weeks) from the TRUFFLE trial, randomized to monitoring with cCTG-STV or DV Doppler. | Only one-third of deliveries followed the randomized assignment; the majority were triggered by safety-net criteria (recurrent decelerations, markedly reduced STV) or maternal indications. Intact survival at 2 years was higher in the DV groups (86%) compared with the cCTG group (77%, p = 0.049). When delivery was indicated directly by abnormal DV findings or reduced STV, intact survival rates were comparable (~80–88%). | Exclusive reliance on cCTG-STV, particularly when intervention was delayed until safety-net triggers occurred, was associated with poorer long-term outcomes. The best results were achieved with integrated monitoring (DV Doppler combined with cCTG), supporting a multiparametric approach to optimize delivery timing in early-onset FGR. |
| Wolf, H. 2017 [58] | 149 cases, early FGR from TRUFFLE study | <32 weeks: there was no association of STVariation regression coefficients, a last low STVariation or/and recurrent decelerations with short or long term infant outcomes. | Delivery is indicated in case of DV absent a wave associated with low ST variation or recurrent decelerations. |
| Graupner, O. 2018 [59] | 66 cases, late-onset SGA fetuses | No significant difference in STV median values between controls, SGA and FHR pregnancies | A higher proportion of late SGA fetuses had STV <5th percentile, despite nonsignificant median differences. |
| Baier, F. 2019 [60] | 41 fetuses with early-onset sever FGR | STV stable until delivery day; sharp fall only at birth. UA/MCA Doppler showed continuous deterioration 3 weeks before delivery; DV/UT remained stable. Poor STV–Doppler correlation. | STV is a late marker; UA/MCA Doppler changes precede and should guide timing. Daily combined monitoring recommended. |
| Strumpfe, F. M. 2019 [61] | 97 cases of SGA | Reduced STV on cCTG correlated with higher NICU admission risk. | Combination of abnormal Ductus venosus PI and low STV is more related to perinatal death compared to fetuses with a single abnormal parameter. |
| Esposito, G. 2021 [62] | 95 FGR cases, 154 were included in the control group | Delta Index, Short-Term Variability, Long-Term Variability, and both Acceleration and Deceleration Phase Rectified Slopes (APRS and DPRS)—were found to be reduced | Integrating advanced cCTG parameters with Doppler improved identification of fetuses at risk for NICU admission. |
| Bruin, C. 2022 [63] | 367 pregnancies with early-onset FGR (<32 wks), retrospective cohort (last 5 days before delivery/fetal death) | Compared PRSA (AAC/ADC) and STV, alone and in combination with recurrent decelerations. Both reduced PRSA and low STV (± decelerations) were associated with adverse perinatal condition; PRSA did not outperform STV. Combining either index with decelerations improved prediction (sensitivity between 80–90%, specificity 30–40%). | PRSA and STV are equivalent; strongest predictive value achieved when integrated with deceleration analysis. Single-parameter use is inadequate → supports multiparametric monitoring in early-onset FGR. |
3.2. Delivery Management Based on cCTG
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Average Acceleration Capacity |
| ACOG | American College of Obstetricians and Gynecologists |
| ADC | Average Deceleration Capacity |
| AED | Absent End- Diastolic |
| AFI | Amniotic Fluid Index |
| APRS | Acceleration phase-rectified slope |
| ARED | Absent or Reversed End-Diastolic flow |
| AUC | Area under the Curve |
| BPP | Biophysical Profile |
| cCTG | Computerized Cardiotocography |
| CPR | Cerebroplacental Ratio |
| CTG | Cardiotocography |
| DPRS | Deceleration phase-rectified slope |
| DV | Ductus Venosus |
| DVPI | Ductus Venosus Pulsatility Index |
| EFW | Estimated Fetal Weight |
| FIGO | International Federation of Gynecology and Obstetrics |
| FGR | Fetal Growth Restriction |
| FHR | Fetal Heart Rate |
| IGF-1 | Insulin-like Growth Factor I |
| IGF-II | Insulin-like Growth Factor II |
| IGFBP-2 | Insulin-like Growth Factor Binding Protein 2 |
| IGFBP-3 | Insulin-like Growth Factor Binding Protein 3 |
| IGFBP-4 | Insulin-like Growth Factor Binding Protein 4 |
| IUGR | Intrauterine Growth Restriction |
| ISUOG | International Society of Ultrasound in Obstetrics and Gynecology |
| LTV | Long-Term Variability |
| LZC | Lempel–Ziv Complexity (signal complexity index) |
| MCA | Middle Cerebral Artery |
| MSE | Multiscale Entropy (multi-scale signal entropy index) |
| NICU | Neonatal Intensive Care Unit |
| NST | Non-Stress Test |
| PAPP-A | Pregnancy-Associated Plasma Protein-A |
| PI | Pulsatility Index |
| PRSA | Phase-Rectified Signal Averaging |
| RCOG | Royal College of Obstetricians and Gynaecologists |
| SGA | Small for Gestational Age |
| SMFM | Society for Maternal-Fetal Medicine |
| SOGC | Society of Obstetricians and Gynaecologists of Canada |
| STV | Short-Term Variation |
| TRUFFLE | Trial of Randomized Umbilical and Fetal Flow in Europe |
| UA | Umbilical Artery |
| UtA/UT | Uterine Artery |
| VIP | Vasoactive Intestinal Peptide |
References
- Damhuis, S.E.; Ganzevoort, W.; Gordijn, S.J. Abnormal Fetal Growth: Small for Gestational Age, Fetal Growth Restriction, Large for Gestational Age: Definitions and Epidemiology. Obstet. Gynecol. Clin. N. Am. 2021, 48, 267–279. [Google Scholar] [CrossRef]
- Murray, E.; Fernandes, M.; Fazel, M.; Kennedy, S.H.; Villar, J.; Stein, A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1062–1072. [Google Scholar] [CrossRef]
- D’Agostin, M.; Di Sipio Morgia, C.; Vento, G.; Nobile, S. Long-term implications of fetal growth restriction. World J. Clin. Cases 2023, 11, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: A systematic review and meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef]
- Monier, I.; Ego, A.; Hocquette, A.; Benachi, A.; Goffinet, F.; Lelong, N.; Le Ray, C.; Zeitlin, J.; ENP2021 Study Group. Validity of a Delphi consensus definition of growth restriction in the newborn for identifying neonatal morbidity. Am. J. Obstet. Gynecol. 2025, 232, 224.e1–224.e13. [Google Scholar] [CrossRef]
- Marien, M.; Perron, S.; Bergeron, A.M.; Singbo, N.; Demers, S. Comparison of the accuracy of INTERGROWTH 21 and Hadlock ultrasound formulae for fetal weight prediction. J. Obstet. Gynaecol. Can. 2021, 43, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.R.; Prefumo, F.; Ferrazzi, E.; Unterscheider, J.; Khalil, A.; Ganzevoort, W.; et al. Clinical opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists (ACOG). Intrauterine Growth Restriction (IUGR); ACOG Practice Bulletin No. 227; ACOG: Washington, DC, USA, 2021; Available online: https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/01/intrauterine-growth-restriction (accessed on 10 June 2025).
- Fowden, A.L.; Forhead, A.J. Endocrine mechanisms of intrauterine programming. Reproduction 2004, 127, 515–526. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, A.J.; Dai, Z.; Xing, Y.; Boney, C.; Wilkie, M.B.; Lauder, J.M.; Han, V.K.; Clemmons, D.R. Brain growth retardation due to the expression of human insulin-like growth factor binding protein-1 in transgenic mice: An in vivo model for the analysis of IGF function in the brain. Brain Res. Dev. Brain Res. 1994, 82, 213–222. [Google Scholar]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine growth restriction: Antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Saki, F.; Dabbaghmanesh, M.H.; Ghaemi, S.Z.; Forouhari, S.; Ranjbar Omrani, G.; Bakhshayeshkaram, M. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int. J. Endocrinol. Metab. 2014, 12, e19378. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage based management protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152, 3–57. [Google Scholar] [CrossRef]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: Comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef]
- Besimoglu, B.; Uyan Hendem, D.; Atalay, A.; Göncü Ayhan, Ş.; Sınacı, S.; Tanaçan, A.; Şahin, D. Combination of Doppler measurements with amniotic fluid volume for the prediction of perinatal outcomes in fetal growth restriction. Int. J. Gynaecol. Obstet. 2023, 161, 190–197. [Google Scholar] [CrossRef] [PubMed]
- David, L.S.; Cherian, A.G.; Beck, M.M. Management of intrauterine growth restriction. Curr. Med. Issues 2017, 15, 271–277. [Google Scholar]
- Thompson, R.S.; Trudinger, B.J. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: An investigation using a mathematical model. Ultrasound Med. Biol. 1990, 16, 449–458. [Google Scholar] [CrossRef]
- Dixit, S.; Dixit, N.A.; Rawat, A.; Bajpai, A.; Alelyani, M.; Sabah, Z.U.; Raghuwanshi, S. Color Doppler ultrasound in high- and low-risk pregnancies and its relationship to fetal outcomes: A cross-sectional study. Front. Pediatr. 2024, 11, 1221766. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists (ACOG). Antepartum Fetal Surveillance; ACOG Practice Bulletin No. 229. Obstet. Gynecol. 2021, 137, e116–e127. [Google Scholar] [CrossRef]
- Khalil, A.A.; Morales-Rosello, J.; Morlando, M.; Hannan, H.; Bhide, A.; Papageorghiou, A.; Thilaganathan, B. Is fetal cerebroplacental ratio an independent predictor of intrapartum fetal compromise and neonatal unit admission? Am. J. Obstet. Gynecol. 2015, 213, 54.e1–54.e10. [Google Scholar] [CrossRef]
- Prior, T.; Mullins, E.; Bennett, P.; Kumar, S. Prediction of intrapartum fetal compromise using the cerebro-umbilical ratio: A prospective observational study. Am. J. Obstet. Gynecol. 2013, 208, 124.e1–124.e6. [Google Scholar] [CrossRef]
- Thompson, J.M.D.; Wilson, J.; Bradford, B.F.; Li, M.; Cronin, R.S.; Gordon, A.; Raynes-Greenow, C.H.; Stacey, T.; Culling, V.M.; Askie, L.M.; et al. A better understanding of the association between maternal perception of fetal movements and late stillbirth-findings from an individual participant data meta-analysis. BMC Med. 2021, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.J.L.; Dumville, J.C.; Walsh, T.; Higgins, L.E.; Fisher, M.; Akselsson, A.; Whitworth, M.; Heazell, A.E.P. Effect of encouraging awareness of reduced fetal movement and subsequent clinical management on pregnancy outcome: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100821. [Google Scholar] [CrossRef]
- Gardosi, J.; Madurasinghe, V.; Williams, M.; Malik, A.; Francis, A. The role of maternal per-ception of fetal movement in the management of pregnancies at risk of stillbirth. BMC Pregnancy Childbirth 2013, 24, 346. [Google Scholar]
- Beikou, A.; Gourounti, K. Reduced Fetal Movements and Perinatal Mortality. Master Sociomed. 2020, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.; Cronin, R.; McKinlay, C.; Thompson, J.; Mc Cowan, L. Maternally perceived fetal movement patterns: The influence of body mass index. Early Hum. Dev. 2020, 140, 104922. [Google Scholar] [CrossRef]
- Koshida, S.; Tokoro, S.; Katsura, D.; Tsuji, S.; Murakami, T.; Takahashi, K. Fetal movement counting is associated with the reduction of delayed maternal reaction after perceiving decreased fetal movements: A prospective study. Sci. Rep. 2021, 11, 10818. [Google Scholar] [CrossRef]
- Monari, F.; Menichini, D.; Salerno, C.; Gei, V.; Facchinetti, F.; Neri, I. Women’s perception of fetal movements and perinatal outcomes: Results of a prospective cohort study. J. Matern. Fetal Neonatal Med. 2023, 36, 2193664. [Google Scholar] [CrossRef]
- Grivell, R.M.; Alfirevic, Z.; Gyte, G.M.L.; Devane, D. Antenatal cardiotocography for fetal assessment. Cochrane Database Syst. Rev. 2015, 2015, CD007863. [Google Scholar] [CrossRef]
- Ogasawara, J.; Ikenoue, S.; Yamamoto, H.; Sato, M.; Kasuga, Y.; Mitsukura, Y.; Ikegaya, Y.; Yasui, M.; Tanaka, M.; Ochiai, D. Deep neural network-based classification of cardiotocograms outperformed conventional algorithms. Sci. Rep. 2021, 11, 13367. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Comert, Z.; Deng, Y. Computer-Aided Diagnosis System of Fetal Hypoxia Incorporating Recurrence Plot with Convolutional Neural Network. Front. Physiol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, Z.; Zhang, X.; Zhang, X.; Jiao, P.; Ye, X. Identifying fetal status with fetal heart rate: Deep learning approach based on long convolution. Comput. Biol. Med. 2023, 159, 106970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Deng, Y.; Zhang, Y.; Zhang, Y.; Zhang, X.; Shao, L. DeepFHR: Intelligent prediction of fetal acidemia using fetal heart rate signals based on convolutional neural network. BMC Med. Inform. Decis. Mak. 2019, 19, 286. [Google Scholar] [CrossRef]
- Mendis, L.; Palaniswami, M.; Keenan, E.; Brownfoot, F. Rapid detection of fetal compromise using input length invariant deep learning on fetal heart rate signals. Sci. Rep. 2024, 14, 12615. [Google Scholar] [CrossRef]
- Chandraharan, E. Updated NICE cardiotocograph (CTG) guideline: Is it suspicious or patho-logical. J. Clin. Med. Surg. 2023, 3, 1129. [Google Scholar] [CrossRef]
- Pardey, J.; Moulden, M.; Redman, C.W.G. A computer system for the numerical analysis of non stress tests. Am. J. Obstet. Gynecol. 2002, 186, 1095–1103. [Google Scholar] [CrossRef]
- Seliger, G.; Petroff, D.; Seeger, S.; Hoyer, D.; Tchirikov, M.; Schneider, U. Diurnal variations of short-term variation and the impact of multiple recordings on measurement accuracy. J. Perinatol. 2017, 37, 231–235. [Google Scholar] [CrossRef]
- Jones, G.D.; Cooke, W.R.; Vatish, M.; Redman, C.W.G. Computerized analysis of antepartum cardiotocography: A review. Matern. Fetal Med. 2022, 4, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Davis Jones, G.; Albert, B.; Cooke, W.; Vatish, M. Performance evaluation of computerized antepartum fetal heart rate monitoring: Dawes-Redman algorithm at term. Ultrasound Obstet. Gynecol. 2025, 65, 191–197. [Google Scholar] [CrossRef]
- Spairani, E.; Daniele, B.; Magenes, G.; Signorini, M.G. A novel large structured cardiotocographic database. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 1375–1378. [Google Scholar]
- Ribbert, L.S.; Snijders, R.J.; Nicolaides, K.H.; Visser, G.H. Relation of fetal blood gases and data from computer-assisted analysis of fetal heart rate patterns in small for gestational age fetuses. BJOG Int. J. Obstet. Gynaecol. 1991, 98, 820–823. [Google Scholar] [CrossRef]
- Waterman, E.J.; Magee, L.A.; Lim, K.I.; Skoll, A.; Rurak, D.; von Dadelszen, P. Do commonly used oral antihypertensives alter fetal or neonatal heart rate characteristics? A systematic review. Hypertens. Pregnancy 2004, 23, 155–169. [Google Scholar] [CrossRef]
- Thornton, C.E.; Makris, A.; Tooher, J.M.; Ogle, R.F.; Hennessy, A. Does the anti hypertensive drug clonidine affect the short term variation in CTG recordings? Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 456–459. [Google Scholar] [CrossRef]
- Hecher, K.; Bilardo, C.M.; Stigter, R.H.; Ville, Y.; Hackelöer, B.J.; Kok, H.J.; Senat, M.V.; Visser, G.H. Monitoring of fetuses with intrauterine growth restriction: A longitudinal study. Ultrasound Obstet. Gynecol. 2001, 18, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Anceschi, M.M.; Ruozi-Berretta, A.; Piazze, J.J.; Cosmi, E.; Cerekja, A.; Meloni, P.; Cosmi, E.V. Computerized cardiotocography in the management of intrauterine growth restriction associated with Doppler velocimetry alterations. Int. J. Gynaecol. Obstet. 2004, 86, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Soncini, E.; Ronzoni, E.; Macovei, D.; Grignaffini, A. Integrated monitoring of fetal growth restriction by computerized cardiotocography and Doppler flow velocimetry. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, M.; Signorini, M.G.; Magenes, G. Complexity analysis of the fetal heart rate variability: Early identification of severe intrauterine growth-restricted fetuses. Med. Biol. Eng. Comput. 2009, 47, 911–919. [Google Scholar] [CrossRef]
- Huhn, E.A.; Lobmaier, S.; Fischer, T.; Schneider, R.; Bauer, A.; Schneider, K.T.; Schmidt, G. New computerized fetal heart rate analysis for surveillance of intrauterine growth restriction. Prenat. Diagn. 2011, 31, 509–514. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Fanelli, A.; Esposito, G.; Esposito, F.G.; Magenes, G.; Signorini, M.G.; Campanile, M.; Martinelli, P. Evaluation of the Acceleration and Deceleration Phase-Rectified Slope to Detect and Improve IUGR Clinical Management. Comput. Math. Methods Med. 2015, 2015, 236896. [Google Scholar] [CrossRef] [PubMed]
- Lobmaier, S.M.; Mensing van Charante, N.; Ferrazzi, E.; Giussani, D.A.; Shaw, C.J.; Müller, A.; Ortiz, J.U.; Ostermayer, E.; Haller, B.; Prefumo, F.; et al. Phase-rectified signal averaging method to predict perinatal outcome in infants with very preterm fetal growth restriction—A secondary analysis of TRUFFLE trial. Am. J. Obstet. Gynecol. 2016, 215, 630.e1–630.e7. [Google Scholar] [CrossRef]
- Amorim Costa, C.; Ayres de Campos, D.; Bernardes, J. Cardiotocographic parameters in small for gestational age fetuses: How do they vary from normal at different gestational ages? BJOG Int. J. Obstet. Gynaecol. 2017, 124, 379–388. [Google Scholar]
- Stroux, L.; Redman, C.W.; Georgieva, A.; Payne, S.J.; Clifford, G.D. Doppler-based fetal heart rate analysis markers for the detection of early intrauterine growth restriction. Acta Obstet. Gynecol. Scand. 2017, 96, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Frusca, T.; Todros, T.; Lees, C.; Bilardo, C.M.; Hecher, K.; Visser, G.H.; Papageorghiou, A.T.; Marlow, N.; Thilaganathan, B.; van Wassenaer-Leemhuis, A.; et al. Outcome in early-onset fetal growth restriction is best combining computerized fetal heart rate analysis with ductus venosus Doppler: Insights from the TRUFFLE trial. Am. J. Obstet. Gynecol. 2018, 218, S783–S789. [Google Scholar] [CrossRef]
- Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; Ferrazzi, E.; Fratelli, N.; Frusca, T.; Ganzevoort, W.; Lees, C.C.; Napolitano, R.; Todros, T.; et al. Fetal monitoring indications for delivery and 2-year outcome in 310 infants with fetal growth restriction delivered before 32 weeks’ gestation in the TRUFFLE study. Ultrasound Obstet. Gynecol. 2017, 50, 347–352. [Google Scholar] [CrossRef]
- Wolf, H.; Arabin, B.; Lees, C.C.; Oepkes, D.; Prefumo, F.; Thilaganathan, B.; Todros, T.; Visser, G.H.; Bilardo, C.M.; Derks, J.B.; et al. Longitudinal study of computerized cardiotocography in early fetal growth restriction. Ultrasound Obstet. Gynecol. 2017, 50, 71–78. [Google Scholar] [CrossRef]
- Graupner, O.; Ortiz, J.U.; Haller, B.; Wacker-Gussmann, A.; Oberhoffer, R.; Kuschel, B.; Weyrich, J.; Lees, C.; Lobmaier, S.M. Performance of computerized cardiotocography-based short-term variation in late-onset small-for-gestational-age fetuses and reference ranges for the late third trimester. Arch. Gynecol. Obstet. 2019, 299, 353–360. [Google Scholar] [CrossRef]
- Baier, F.; Weinhold, L.; Stumpfe, F.M.; Kehl, S.; Pretscher, J.; Bayer, C.M.; Topal, N.; Pontones, C.; Mayr, A.; Schild, R.; et al. Longitudinal Course of Short-Term Variation and Doppler Parameters in Early Onset Growth Restricted Fetuses. Ultraschall Med. 2020, 41, e23–e32. [Google Scholar] [CrossRef]
- Stumpfe, F.M.; Faschingbauer, F.; Kehl, S.; Pretscher, J.; Stelzl, P.; Mayr, A.; Schild, R.L.; Schmid, M.; Beckmann, M.W.; Schneider, M.O. Correlation of short-term variation and Doppler parameters with adverse perinatal outcome in small-for-gestational-age fetuses at term. Arch. Gynecol. Obstet. 2019, 300, 575–581. [Google Scholar] [CrossRef]
- Esposito, G.; Pini, N.; Tagliaferri, S.; Campanile, M.; Zullo, F.; Magenes, G.; Maruotti, G.M.; Signorini, M.G. An integrated approach based on advanced CTG parameters and Doppler measurements for late growth restriction management. BMC Pregnancy Childbirth 2021, 21, 775. [Google Scholar] [CrossRef]
- Bruin, C.M.; Lobmaier, S.M.; Ganzevoort, W.; Müller, A.; Wolf, H. Comparison of phase rectified signal averaging and short term variation in predicting perinatal outcome in early onset fetal growth restriction. J. Perinat. Med. 2022, 51, 634–640. [Google Scholar] [CrossRef]
- Kiserud, T.; Kessler, J.; Ebbing, C.; Rasmussen, S. Ductus venosus shunting in growth-restricted fetuses and the effect of umbilical circulatory compromise. Ultrasound Obstet. Gynecol. 2006, 28, 143–149. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, L.; Boers, K.E.; Gordijn, S.J.; Ganzevoort, W.; Bremer, H.A.; Kwee, A.; Pajkrt, E.; van Huizen, M.E.; Scherjon, S.A.; van Loon, A.J.; et al. Perinatal death in a term fetal growth restriction randomized controlled trial: The paradox of prior risk and consent. Am. J. Obstet. Gynecol. MFM 2020, 2, 100239. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzi, E.; Lees, C.; Acharya, G. The controversial role of the ductus venosus in hypoxic human fetuses. Acta Obstet. Gynecol. Scand. 2019, 98, 823–829. [Google Scholar] [CrossRef]
- Manning, F.A.; Snijders, R.; Harman, C.R.; Nicolaides, K.; Menticoglou, S.; Morrison, I. Fetal biophysical profile score VI: Correlation with antepartum umbilical venous fetal pH. Am. J. Obstet. Gynecol. 1993, 169, 755–763. [Google Scholar] [CrossRef]
- Morris, R.K.; Johnstone, E.; Lees, C.; Morton, V.; Smith, G.; Royal College of Obstetricians and Gynaecologists. Investigation and Care of a Small-for-Gestational-Age Fetus and a Growth Restricted Fetus (Green-top Guideline No. 31). BJOG 2024, 131, e31–e80. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine. SMFM Consult Series #52: Diagnosis and management of fetal growth restriction. Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Performance of fetal biometry and growth assessment. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Society of Obstetricians and Gynaecologists of Canada. SOGC Clinical Practice Guideline No. 426: Fetal Surveillance in Pregnancies at Increased Risk of Uteroplacental Insufficiency. J. Obstet. Gynaecol. Can. 2020, 42, 579–590.e1. [Google Scholar]
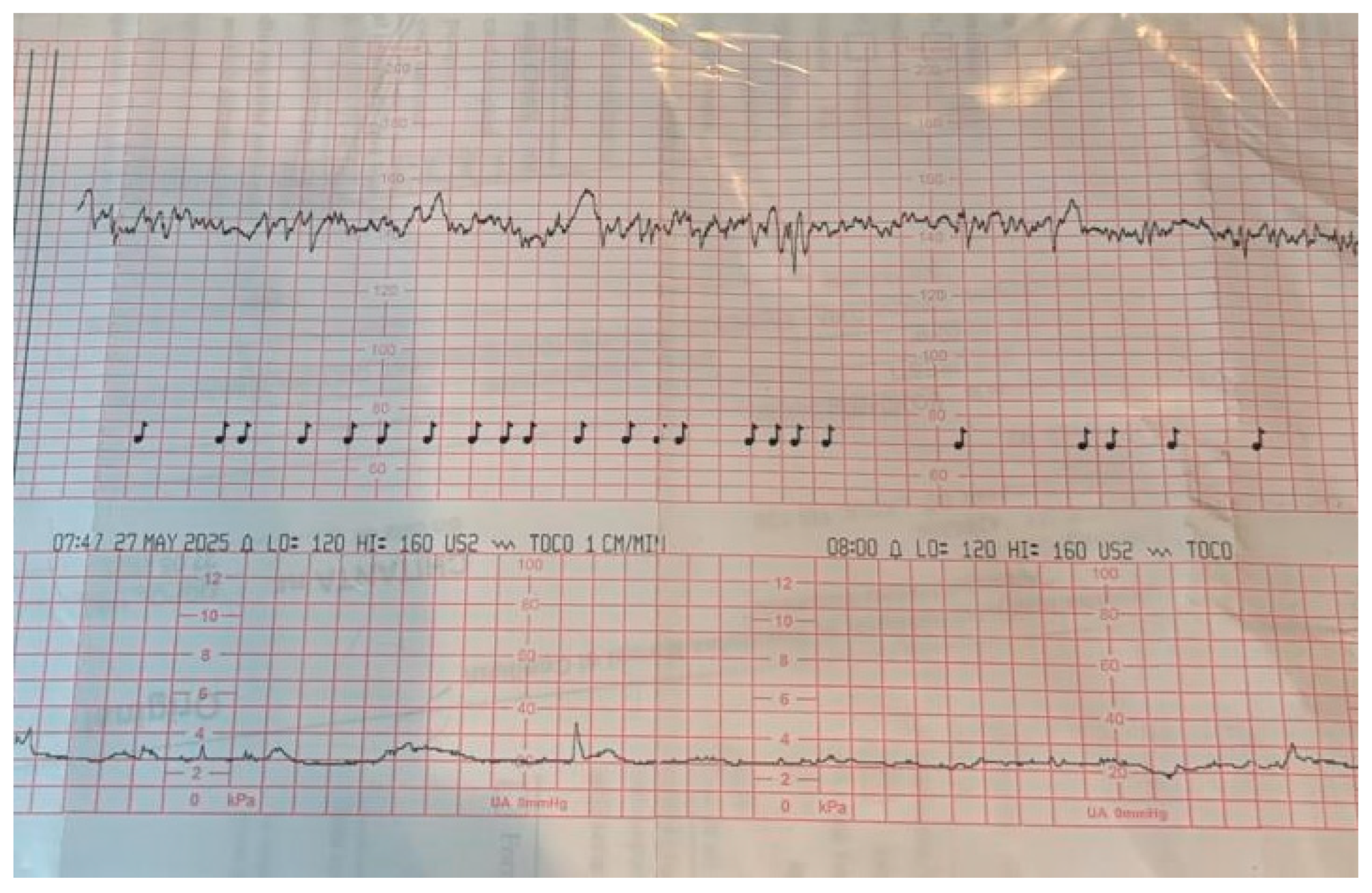
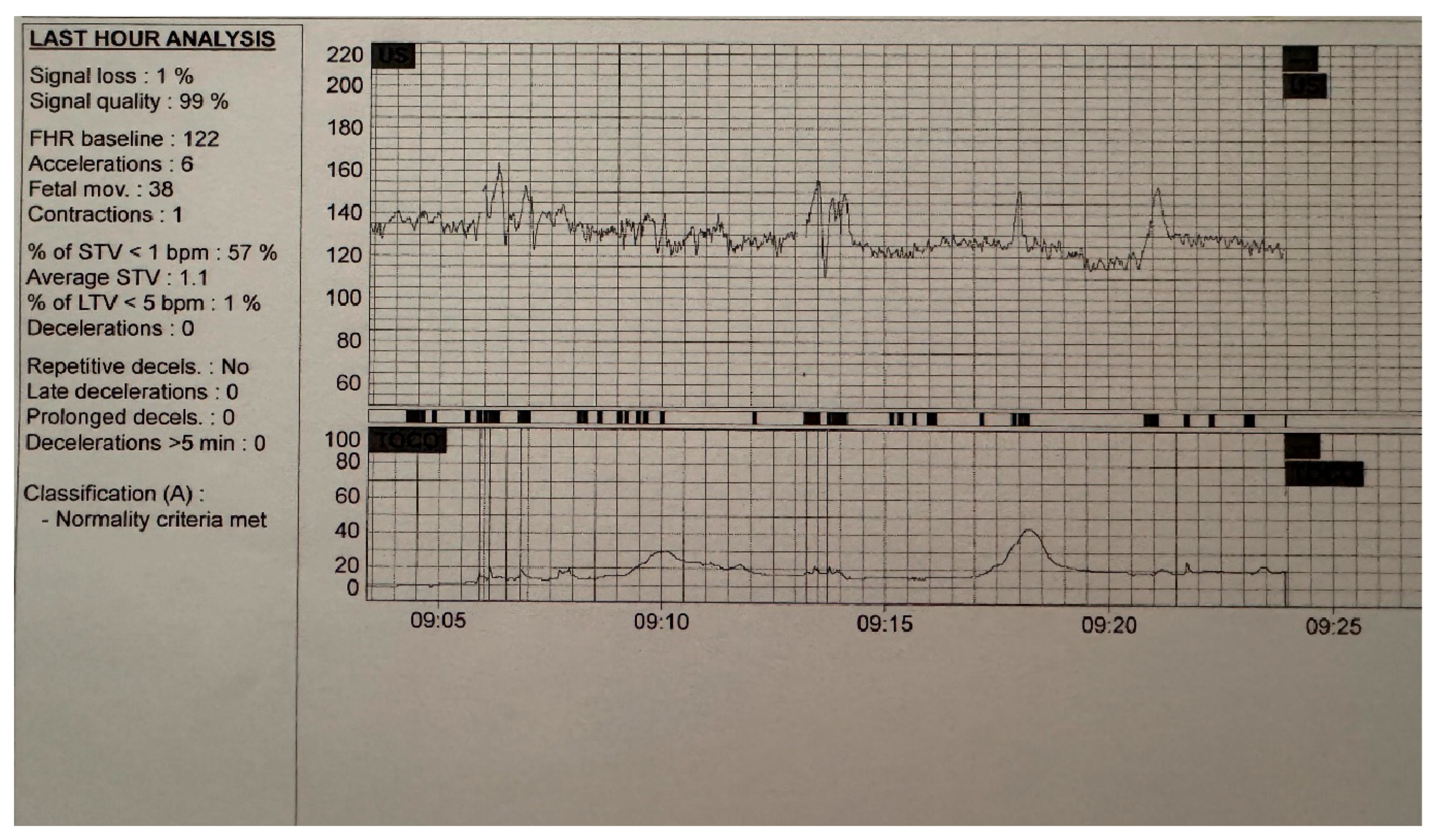
| Early-Onset FGR (<32 Weeks) | Late-Onset FGR (≥32 Weeks) |
|---|---|
| EFW or AC <3rd percentile | EFW or AC <3rd percentile |
| UA with AREDV | ≥2 of the following 3 criteria:
|
EFW or AC <10th percentile, combined with one or more of the following:
| growth failure (drop in EFW or AC centile) or abnormal fetal Dopplers |
| Gestational Age (weeks) | 24–27 (Periviability) | 28–30 (Early Viability) | 31–33 (Viability) | 34–36 (Late Preterm) | ≥37 (Term) |
|---|---|---|---|---|---|
| Absolute Indications for Delivery (regardless of GA) | Severe maternal condition (e.g., pre-eclampsia) BPP score < 4/10 Sinusoidal pattern Repetitive late decelerations Absent baseline variability cCTG STVariation < 2.6 ms | Severe maternal condition (e.g., pre-eclampsia) Sinusoidal pattern or repetitive late decelerations Absent baseline variability BPP score < 4/10 cCTG STVariation < 2.6 ms | Severe maternal condition (e.g., pre-eclampsia) Sinusoidal pattern or repetitive late decelerations Absent baseline variability BPP score < 4/10 cCTG STVariation < 2.6 ms | Severe maternal condition (e.g., pre-eclampsia) Sinusoidal pattern or repetitive late decelerations Absent baseline variability BPP score < 4/10 cCTG STVariation< 2.6 ms | Severe maternal condition (e.g., pre-eclampsia) Sinusoidal pattern or repetitive late decelerations Absent baseline variability BPP score < 4/10 cCTG STVariation < 2.6 ms |
| Relative Indications for Delivery (GA-adjusted) | - | Absent or reversed DV a-wave BPP score < 6/10 cCTG STVariation < 3.0 ms | Reversed umbilical artery end-diastolic flow cCTG STVariation < 3.5 ms | Absent umbilical artery end-diastolic flow cCTG STVariation < 4.5 ms | Elevated umbilical artery PI |
| Management Strategy | Individualized decision based on maternal status and fetal condition if EFW > 500 g | Maternal monitoring and Doppler of the umbilical artery and DV, with NST or cCTG and BPP | Maternal and fetal surveillance including Doppler of umbilical and middle cerebral arteries (± DV), NST/cCTG and BPP | Maternal and fetal surveillance including Doppler of umbilical and middle cerebral arteries (± DV), NST/cCTG and BPP | Delivery may be indicated if EFW < 10th percentile, regardless of Doppler results |
| Aspect | ACOG (2021) [9] | RCOG (2022) [68] | FIGO (2015) [16] | SMFM (2020) [69] | ISUOG (2019) [70] | SOGC (2020) [71] |
|---|---|---|---|---|---|---|
| Definition of FGR | EFW <10th percentile confirmed by ultrasound; differentiation between early and late onset | EFW or AC <10th percentile; severe if EFW <3rd percentile or abnormal Doppler | EFW <3rd percentile or EFW/AC <10th percentile + abnormal Doppler | EFW <10th percentile, reduced growth velocity, or Doppler abnormalities | EFW <10th percentile or declining growth trajectory with abnormal Doppler | EFW <10th percentile or AC <10th percentile with additional risk factors |
| Use of Doppler Ultrasound | Recommended for all suspected FGR cases: UA Doppler primary; consider MCA and DV | Mandatory: UA for all; MCA and CPR/UCR for late-onset; DV for early-onset | Central to diagnosis: UA, MCA, CPR and DV if <32 weeks | UA and MCA in all; DV for early-onset or abnormal UA | Recommended: UA first-line; MCA, CPR and DV for fetal compromise | Routine use of UA; other vessels based on clinical context |
| Role of cCTG/STV | Optional; used if BPP inconclusive or in high-risk cases | Not routine; used only with abnormal Doppler or clinical signs | Recommended adjunct in early-onset or high-risk pregnancies | Preferred in late-onset FGR with reduced variability or absent accelerations | Useful for assessing fetal well-being when other parameters borderline | Considered in tertiary settings with access to computerized systems |
| Biophysical Profile (BPP) | Used alongside NST or cCTG to evaluate fetal well-being | Accepted, especially in late preterm and term FGR | Complementary to Doppler and CTG in early-onset | Used to aid timing of delivery with cCTG and Doppler | Recommended only in selected cases; not primary tool | Supportive tool, especially when Doppler or CTG uncertain |
| Biomarkers (PlGF/sFlt-1) | Not routinely recommended | Considered if preeclampsia suspected | Under evaluation for clinical utility | Optional if preeclampsia or placental dysfunction suspected | Not used routinely; research use | Not standard but discussed in placental dysfunction |
| Timing of Delivery (Early-onset FGR) | <32 weeks if reversed end-diastolic flow or DV abnormalities; 32–34 weeks for absent EDF | Delivery at <32 weeks for absent/reversed EDF or DV changes | <32 weeks if reversed EDF or DV abnormal; steroids recommended | Individualized; typically <32 weeks for reversed EDF or abnormal DV | Generally deliver <32 weeks for DV abnormalities or reversed flow | Planned delivery at <32 weeks in presence of significant Doppler abnormalities |
| Timing of Delivery (Late-onset FGR) | Delivery from 37 weeks if isolated FGR; earlier if Doppler abnormal | 36–37 weeks if normal Doppler; earlier if CPR <5th percentile or abnormal CTG | From 37 weeks unless Doppler abnormal or STV reduced | 36–38 weeks depending on fetal condition and Doppler | At 37 weeks or earlier with abnormal MCA/CPR | Around 37 weeks unless compromise indicated earlier |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danciu, B.M.; Simionescu, A.A. Optimizing Fetal Surveillance in Fetal Growth Restriction: A Narrative Review of the Role of the Computerized Cardiotocographic Assessment. J. Clin. Med. 2025, 14, 7010. https://doi.org/10.3390/jcm14197010
Danciu BM, Simionescu AA. Optimizing Fetal Surveillance in Fetal Growth Restriction: A Narrative Review of the Role of the Computerized Cardiotocographic Assessment. Journal of Clinical Medicine. 2025; 14(19):7010. https://doi.org/10.3390/jcm14197010
Chicago/Turabian StyleDanciu, Bianca Mihaela, and Anca Angela Simionescu. 2025. "Optimizing Fetal Surveillance in Fetal Growth Restriction: A Narrative Review of the Role of the Computerized Cardiotocographic Assessment" Journal of Clinical Medicine 14, no. 19: 7010. https://doi.org/10.3390/jcm14197010
APA StyleDanciu, B. M., & Simionescu, A. A. (2025). Optimizing Fetal Surveillance in Fetal Growth Restriction: A Narrative Review of the Role of the Computerized Cardiotocographic Assessment. Journal of Clinical Medicine, 14(19), 7010. https://doi.org/10.3390/jcm14197010







