Systematic Review and Meta-Analysis of Myocarditis Prevalence and Diagnostics in COVID-19:Acute, Post-COVID, and MIS-C (2020–2025)
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias
2.5. Statistical Analysis
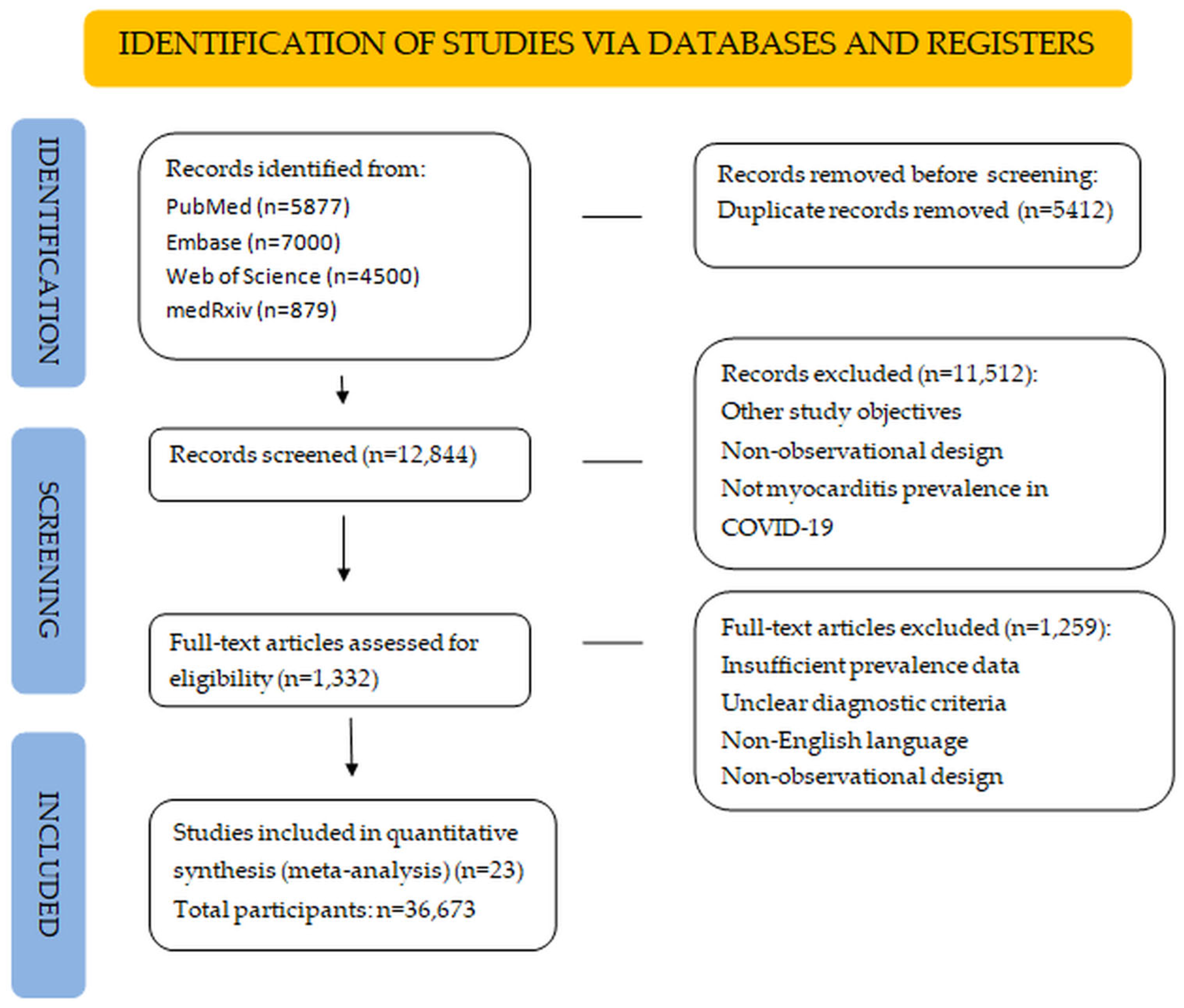
3. Results
3.1. Overview of Included Studies
3.2. Risk of Bias Assessment
3.3. Pooled Prevalence of Myocarditis
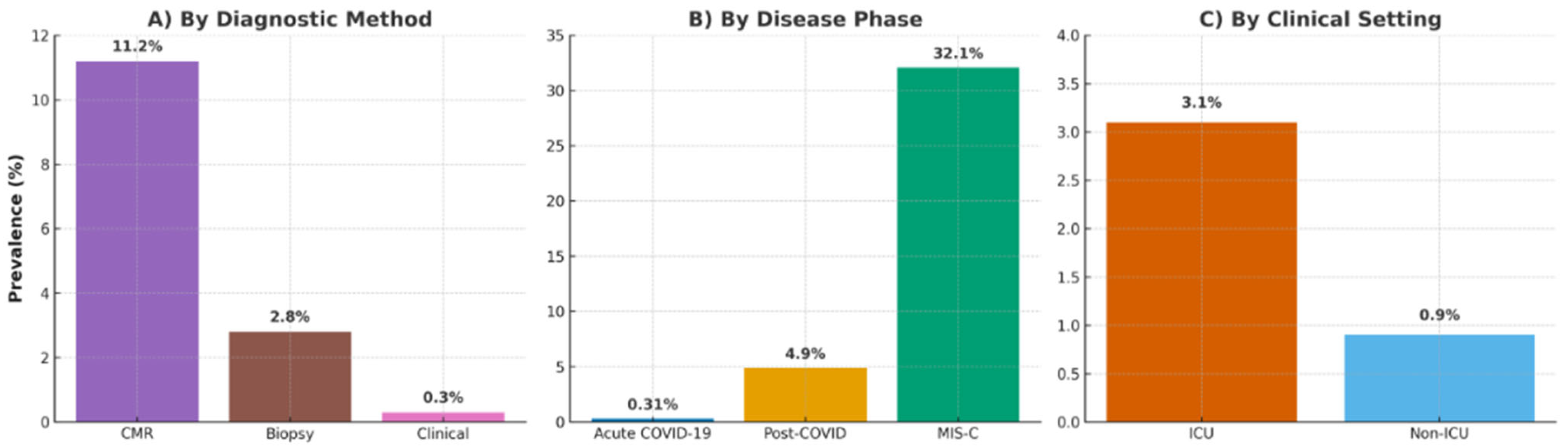
3.4. Subgroup Analyses
- Disease Phase: Prevalence was highest in MIS-C (32.1%, 95% CI: 28.4–36.0%; e.g., Blondiaux [26], Benvenuto [33], Karas [34], Scarduelli [35], and Patel [37]), followed by post-COVID (4.9%, 95% CI: 3.8–6.2%; e.g., Puntmann [12], Huang [13], Rajpal [16], Starekova [17], Vago [18], Daniels [29], Kim [22], and Martinez [28]), and lowest in acute COVID-19 (0.31%, 95% CI: 0.22–0.44%; e.g., Esposito [11], Ammirati [21], and Artico [32]) (p < 0.001), as in Figure 3.
- Diagnostic Method: CMR detected a prevalence of 11.2% (95% CI: 8.4–14.5%; e.g., Esposito [11], Puntmann [12], Huang [13], Rajpal [16], Starekova [17], Vago [18], Doeblin [31], and Artico [32]), biopsy 2.8% (95% CI: 1.6–4.3%; e.g., Ammirati [21]), and clinical criteria 0.3% (95% CI: 0.2–0.5%; e.g., Kim [22], Martinez [28], Moulson [19], and Petek [20]) (p < 0.001), indicating CMR’s utility for subclinical cases, as in Figure 3.
- Vaccination Status: Vaccinated cohorts (14 studies, n = 15,672; e.g., Gröschel [24], Tugade [25], andDoeblin [31]) had a prevalence of 1.1% (95% CI: 0.7–1.6%) vs. 2.7% (95% CI: 1.9–3.7%; e.g., Ammirati [21], and Patel [37]) in unvaccinated cohorts (p = 0.03), supporting vaccination’s protective role.
3.5. Cardiac Outcomes
3.6. Meta-Regression and Heterogeneity
3.7. Sensitivity Analyses
3.8. Publication Bias
4. Discussion
4.1. Diagnostic Variability and Prevalence
4.2. Disease Phase and Clinical Setting
4.3. Impact of Vaccination and Variant Era
4.4. Interpretation of Cardiac Outcomes
4.5. Sources of Heterogeneity
4.6. Clinical Implications
4.7. Strengths and Limitations
4.8. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| CMR | Cardiac Magnetic Resonance |
| MIS-C | Multisystem Inflammatory Syndrome in Children |
| LVEF | Left Ventricular Ejection Fraction |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| NOS | Newcastle–Ottawa Scale |
| JBI | Joanna Briggs Institute |
| ESC/AHA | European Society of Cardiology/American Heart Association |
| LL2018 | Lake Louise 2018 Criteria |
| ICU | Intensive Care Unit |
| LMIC | Low- and Middle-Income Country |
| ECG | Electrocardiogram |
| CK-MB | Creatine Kinase-Myocardial Band |
References
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A.; Rana, B.S.; Albert, C.M. Recognizing COVID-19–related myocarditis: Pathophysiology, diagnosis, and treatment. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Mehta, J.J.; Weyandt, J.; Bhatt, G.; Schneider, J.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020, 72, 1791–1805. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; VanderHeide, R.S. Pulmonary and cardiac pathology in COVID-19: The first autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Natale, L.; Ligabue, G.; Peretto, G.; Lovati, C.; Rizzo, S.; Basso, C.; De Luca, G.; Bucciarelli-Ducci, C.; et al. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2462–2465. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from COVID-19. JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac involvement in patients recovered from COVID-19 identified using magnetic resonance imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Mehta, J.J.; Weyandt, J.; Bhatt, G.; Schneider, J.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Dashboard; WHO: Geneva, Switzerland, 2025; Available online: https://covid19.who.int/ (accessed on 13 August 2025).
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Eckhardt, L.L.; Grist, T.M.; Kusmirek, J.E.; Schiebler, M.L.; Reeder, S.B. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021, 6, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Vago, H.; Szabo, L.; Dohy, Z.; Merkely, B. Cardiac magnetic resonance findings in patients recovered from COVID-19: Initial experiences in elite athletes. JACC Cardiovasc. Imaging 2021, 14, 1279–1281. [Google Scholar] [CrossRef]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kovan, J.S.; Baggish, A.L.; Patel, A.; Daniels, C.J.; Kim, J.H.; Martinez, M.W.; et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef]
- Petek, B.J.; Moulson, N.; Baggish, A.L.; Kovan, J.S.; Drezner, J.A.; Harmon, K.G.; Martinez, M.W.; Kim, J.H.; Daniels, C.J.; Emery, M.S.; et al. SARS-CoV-2 cardiac involvement in collegiate athletes: Findings from the ORCCA registry. Circulation 2022, 145, 934–944. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Gorga, E.; Adler, E.D.; Hsich, E.; Mastroianni, C.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Kim, J.H.; Levine, B.D.; Phelan, D.; Emery, M.S.; Martinez, M.W.; Chung, E.H.; Thompson, P.D.; Baggish, A.L. Coronavirus disease 2019 and the athletic heart: Emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021, 6, 219–227. [Google Scholar] [CrossRef]
- Kravchenko, D.; Isaak, A.; Mesropyan, N.; Brenner, I.; Zimmer, S.; Maintz, D.; Stöbe, S.; Merkel, A.; Doeblin, P.; Kelle, S.; et al. Cardiac magnetic resonance follow-up of COVID-19 vaccine-associated acute myocarditis. Front. Cardiovasc. Med. 2022, 9, 1049256. [Google Scholar] [CrossRef]
- Gröschel, J.; Grassow, L.; van Dijck, P.; Bhoyroo, Y.; Blaszczyk, E.; Schulz-Menger, J. Trajectories of functional and structural myocardial parameters in post-COVID-19 syndrome—Insights from mid-term follow-up by cardiovascular magnetic resonance. Front. Cardiovasc. Med. 2024, 11, 1357349. [Google Scholar] [CrossRef]
- Tugade, R.E.R.; Palileo, N.E.; Leycano, D.A.; Correa, M.; Santiago, S.A.; David, B.J.; De Castro, A.L.; Evangelista, L.; Manalo, J.; Dimagiba, C.; et al. Incidence of Myocarditis among Patients Recovered from COVID-19 Identified using Cardiac Magnetic Resonance: A 1-year Single-centre Retrospective Study. J. Asian Pac. Soc. Cardiol. 2024, 3, e36. [Google Scholar] [CrossRef]
- Blondiaux, E.; Parisot, P.; Redheuil, A.; Tzaroukian, L.; Levy, Y.; Sileo, C.; Blanchard, E.; Chassagnon, G.; Bréhin, A.; Boudjemline, Y. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19. Radiology 2020, 297, E283–E288. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Baggish, A.L. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Vidula, M.K.; Ambale-Venkatesh, B.; Zghaib, T.; Raman, S.V.; Schelbert, E.B.; Farhad, H.; Gonzalez, J.A.; Lima, J.A.C.; Bluemke, D.A.; Kwong, R.Y.; et al. Myocardial injury on CMR in patients with COVID-19 and suspected cardiac involvement. JACC Cardiovasc. Imaging 2023, 16, 609–624. [Google Scholar] [CrossRef]
- Doeblin, P.; Isaak, A.; Zimmer, S.; Mesropyan, N.; Brenner, I.; Maintz, D.; Stöbe, S.; Merkel, A.; Kravchenko, D.; Skurk, C.; et al. CMR findings after COVID-19 and after vaccination. Int. J. Cardiovasc. Imaging 2022, 38, 2057–2071. [Google Scholar] [CrossRef]
- Artico, J.; Shanmuganathan, M.; Mitchell, T.; Levelt, E.; Shanmuganathan, M.; McCann, G.P.; Neubauer, S.; Piechnik, S.K.; Ferreira, V.M.; Rider, O.J.; et al. Myocardial involvement after hospitalization for COVID-19: A prospective multicenter cardiac MRI study. Circulation 2023, 147, 628–639. [Google Scholar] [CrossRef]
- Benvenuto, S.; Simonini, G.; Della Paolera, S.; Abu Rumeileh, S.; Mastrolia, M.V.; Manerba, A.; Ferraro, A.; Giorgetti, A.; Mariani, F.; Ciliberti, P.; et al. Cardiac MRI in midterm follow-up of MIS-C: A multicenter study. Eur. J. Pediatr. 2023, 182, 845–854. [Google Scholar] [CrossRef]
- Karas, J.; Scala, A.; Biebl, A.; Steiner, J.; Fellner, F.; Tulzer, G.; Tulzer, A. Cardiac MRI six months after the onset of multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19: A retrospective follow-up study. Cardiol. Young 2024, 34, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Scarduelli, G.; De Guillebon De Resnes, J.-M.; Ducreux, D.; Bernardor, J.; Afanetti, M.; Dupont, A.; Barthélemy, S.; Gondon, E.; Leporati, J.; Giovannini-Chami, L.; et al. Cardiac manifestations of MIS-C: Cardiac magnetic resonance and speckle-tracking data. Front. Cardiovasc. Med. 2023, 10, 1288176. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.Y.; Özdemir, İ.H.; Gülleroğlu, K.; Akgün, Ö.; Gökdemir, Y.; Çetin, İ.İ.; Karadeniz, C.; Kaya, E.; Kılıç, M.; Yıldırım, A. Cardiac assessment in children with MIS-C: Late magnetic resonance imaging findings. Pediatr. Cardiol. 2023, 44, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Kelleman, M.; West, Z.; Peter, A.; Dove, M.L.; Butto, A.; Oster, M.E. Comparison of MIS-C Related Myocarditis, Classic Viral Myocarditis, and COVID-19 Vaccine-Related Myocarditis in Children. J. Am. Heart Assoc. 2022, 11, e024393. [Google Scholar] [CrossRef]
- Pelliccia, A.; Solberg, E.E.; Papadakis, M.; Adami, P.E.; Biffi, A.; Caselli, S.; Caselli, G.; Sharma, S.; Corrado, D.; Thiene, G.; et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2019, 40, 19–33. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. [Google Scholar]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, SA, Australia, 2020. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Freeman, M.F.; Tukey, J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. The Global Economic Burden of Non-Communicable Diseases; WEF: Geneva, Switzerland, 2011; Available online: https://www3.weforum.org/docs/WEF_Harvard_HE_GlobalEconomicBurdenNonCommunicableDiseases_2011.pdf (accessed on 13 August 2025).
- Casella, M.; Gasperetti, A.; Gaetano, F.; Busana, M.; Sommariva, E.; Catto, V.; Conte, G.; Molon, G.; Anselmino, M.; Compagnucci, P.; et al. Different phases of disease in lymphocytic myocarditis: Insights from clinical and cardiac magnetic resonance follow-up. JACC Clin. Electrophysiol. 2023, 9, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G.; Choong, A.M.T.L.; Sharma, V.K.; Wong, J.J.L.; et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Casella, M.; Conti, S.; Compagnucci, P.; Ribatti, V.; Narducci, M.L.; Marcon, L.; Cavaliere, F.; Sticchi, A.; Brugada, R.; Russo, A.D.; et al. Incidence of ventricular arrhythmias related to COVID infection and vaccination in patients with Brugada syndrome: Insights from a large Italian multicenter registry. J. Cardiovasc. Electrophysiol. 2023, 34, 1386–1394. [Google Scholar] [CrossRef]
- Rey, J.R.; Caro-Codón, J.; Rosillo, S.O.; Iniesta, Á.M.; Castrejón-Castrejón, S.; Marco-Clement, I.; Martín-Polo, L.; Merino-Argos, C.; Rodríguez-Sotelo, J.L.; García-Veas, J.M.; et al. Heart failure in COVID-19 patients: Prevalence, incidence and outcomes. Eur. J. Heart Fail. 2020, 22, 2205–2215. [Google Scholar] [CrossRef]
- Compagnucci, P.; Volpato, G.; Falanga, U.; Cipolletta, L.; Conti, M.A.; Grifoni, G.; Biagini, E.; Buttiglieri, S.; Santangeli, P.; Crea, F. Myocardial inflammation, sports practice, and sudden cardiac death: 2021 update. Medicina 2021, 57, 277. [Google Scholar] [CrossRef]
- Compagnucci, P.; Volpato, G.; Pascucci, R.; Falanga, U.; Misiani, A.; Molini, S.; Paci, C.; Galli, A.; Bertini, M.; Boriani, G. Impact of the COVID-19 pandemic on a tertiary-level electrophysiology laboratory in Italy. Circ. Arrhythm. Electrophysiol. 2020, 13, e008774. [Google Scholar] [CrossRef]
- Arantes, M.A.F., Jr.; Conegundes, A.F.; Miranda, B.C.B.; Campos, A.S.R.; Vieira, A.L.F.; Faleiro, M.D.; Campos, M.A.; Kroon, E.G.; Bentes, A.A. Cardiac manifestations in children with the multisystem inflammatory syndrome (MIS-C) associated with SARS-CoV-2 infection: Systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2432. [Google Scholar] [CrossRef]


| First Author (Ref) | Year | Country | Design | Setting | Phase | Population | Sample Size | Diagnostic Criteria | Myocarditis Cases (%) |
|---|---|---|---|---|---|---|---|---|---|
| Esposito [11] | 2020 | Italy | Case series | Hospitalized, acute | Acute | Adults | 4 | CMR (LLC 2018) | 2 (50%) |
| Puntmann [12] | 2020 | Germany | Prospective cohort | Recovered outpatients | Post-COVID | Adults | 100 | CMR (LLC 2018) | 60 (60%) |
| Huang [13] | 2020 | China | Prospective | Recovered inpatients | Post-COVID | Adults | 26 | CMR (LLC 2018) | 15 (58%) |
| Rajpal [16] | 2020 | USA | Prospective | Athletes | Post-COVID | Young adults | 26 | CMR (LLC 2018) | 4 (15%) |
| Starekova [17] | 2021 | USA | Cross-sectional | Student athletes | Post-COVID | Young adults | 145 | CMR | 2 (1.4%) |
| Vago [18] | 2021 | Hungary | Case series | Elite athletes | Post-COVID | Young adults | 12 | CMR | 2 (17%) |
| Daniels [29] | 2021 | USA | Prospective registry | Athletes (Big Ten registry) | Post-COVID | Young adults | 1420 | CMR + clinical | 37 (2.6%) |
| Kim [22] | 2021 | USA | Review & registry | Athletes | Post-COVID | Athletes | 789 | Clinical, CMR if indicated | 5 (0.6%) |
| Martinez [28] | 2021 | USA | Prospective registry | Professional athletes | Post-COVID | Athletes | 789 | Clinical + CMR if indicated | 5 (0.6%) |
| Moulson [19] | 2021 | USA | Prospective registry | Athletes (ORCCA) | Post-COVID | Young adults | 3018 | Clinical + CMR subset | 21 (0.7%) |
| Petek [20] | 2022 | USA | Registry | Collegiate athletes | Post-COVID | Young adults | 3694 | Clinical + CMR | 21 (0.6%) |
| Ammirati [21] | 2022 | Italy/US multicenter | Prospective cohort | Hospitalized | Acute | Adults | 54 | Biopsy + CMR | 22 (41%) |
| Vidula [30] | 2023 | USA multicenter | Retrospective cohort | Hospitalized/ambulatory | Acute + post-acute | Adults | 980 | CMR + clinical | 60 (6.1%) |
| Doeblin [31] | 2022 | Germany | Cohort | CMR referred | Acute/Post-acute | Adults | 104 | CMR (LLC 2018) | 7 (6–7%) |
| Artico [32] | 2023 | UK multicenter | Prospective cohort | Hospitalized, troponin↑ | Acute | Adults | ~200 | CMR (LLC 2018) | 20 (≈10%) |
| Gröschel [24] | 2024 | Germany | Longitudinal | Post-COVID syndrome | Post-COVID | Adults | 120 | CMR follow-up | 8 (7%) |
| Tugade [25] | 2024 | Philippines | Retrospective | Hospitalized, recovered | Post-COVID | Adults | 100 | CMR | 6 (6%) |
| Blondiaux [26] | 2020 | France | Case series | MIS-C | Acute | Children | 4 | CMR | 4 (100%) |
| Benvenuto [33] | 2023 | Italy multicenter | Cohort | MIS-C | Post-COVID | Children | 67 | CMR | 8 (12%) |
| Karas [34] | 2024 | Lithuania | Cohort | MIS-C follow-up | Post-COVID | Children/adolescents | 28 | CMR | 3 (11%) |
| Scarduelli [35] | 2023 | Italy | Observational | MIS-C | Acute | Children | 32 | CMR + speckle-tracking | 5 (16%) |
| Arslan [36] | 2023 | Turkey | Observational | MIS-C late follow-up | Post-COVID | Children | 30 | CMR | 3 (10%) |
| Patel [37] | 2022 | USA | Comparative | MIS-C, viral myocarditis, vaccine myocarditis | Acute | Children | 111 | Clinical + CMR | 21 (19%) |
| Study ID (Ref) | Definition | N Myocarditis | Age Mean (SD) | Male % | LVEF Mean (SD) | Ventricular Arrhythmias (%) | Troponin Elevation (%) | Mortality % |
|---|---|---|---|---|---|---|---|---|
| Esposito_2020_JACC [11] | CMR (LLC 2018) | 2/4 (50%) | 52 (±12) | 75 | 55 (±8) | 0 | 50 | 0 |
| Puntmann_2020_JAMA [12] | CMR (LLC 2018) | 60/100 (60%) | 49 (±13) | 53 | 56 (±9) | – | 71 | 0 |
| Huang_2020_JACC [13] | CMR (LLC 2018) | 15/26 (58%) | 38 (±12) | 50 | 60 (±7) | – | 58 | 0 |
| Rajpal_2020_JAMA [16] | CMR (LLC 2018) | 4/26 (15%) | 19 (±1) | 85 | 62 (±4) | 0 | 8 | 0 |
| Starekova_2021_JAMA [17] | CMR | 2/145 (1.4%) | 20 (±2) | 78 | 61 (±5) | 0 | 2 | 0 |
| Vago_2021_JACC [18] | CMR | 2/12 (17%) | 21 (±2) | 92 | 60 (±4) | 0 | 0 | 0 |
| Daniels_2021_JAMA [29] | CMR + clinical | 37/1420 (2.6%) | 19 (±1) | 90 | 62 (±5) | <1 | 3 | 0 |
| Kim_2021_JAMA [22] | Clinical ± CMR | 5/789 (0.6%) | 20 (±2) | 85 | Preserved | <1 | <1 | 0 |
| Martinez_2021_JAMA [28] | Clinical + CMR if indicated | 5/789 (0.6%) | 25 (±3) | 90 | 60 (±5) | <1 | <1 | 0 |
| Moulson_2021_Circ [19] | Clinical ± CMR subset | 21/3018 (0.7%) | 20 (±2) | 88 | Preserved | Rare PVCs | <1 | 0 |
| Petek_2022_Circ [20] | Clinical ± CMR | 21/3694 (0.6%) | 20 (±2) | 87 | Preserved | – | <1 | 0 |
| Ammirati_2022_Circ [21] | Biopsy + CMR | 22/54 (41%) | 52 (±15) | 72 | 48 (±10) | 6 | 100 | 11 |
| Vidula_2023_JACC [30] | CMR + clinical | 60/980 (6.1%) | 50 (±14) | 60 | 57 (±7) | 2 | 18 | 3 |
| Doeblin_2022_IJCVI [31] | CMR (LLC 2018) | 7/104 (6–7%) | 46 (±12) | 60 | 58 (±6) | 3 | 15–20 | 0 |
| Artico_2023_Circ [32] | CMR (LLC 2018) | 20/200 (≈10%) | 58 (±15) | 70 | 55 (±8) | 6 | 100 | 8–10 |
| Gröschel_2024_FCVM [24] | CMR follow-up | 8/120 (7%) | 47 (±13) | 58 | 59 (±6) | – | 12 | 0 |
| Tugade_2024_JAPSC [25] | CMR | 6/100 (6%) | 52 (±10) | 55 | 58 (±7) | – | 10 | 2 |
| Blondiaux_2020_Radiology [26] | CMR | 4/4 (100%) | 11 (±2) | 50 | 53 (±9) | – | 100 | 0 |
| Benvenuto_2023_EurJPeds [33] | CMR | 8/67 (12%) | 10 (±3) | 60 | 55 (±6) | – | 18 | 0 |
| Karas_2024_CardiolYoung [34] | CMR | 3/28 (11%) | 12 (±4) | 57 | 56 (±7) | – | 10 | 0 |
| Scarduelli_2023_FCVM [35] | CMR + speckle | 5/32 (16%) | 11 (±3) | 55 | 57 (±6) | – | 15 | 0 |
| Arslan_2023_PediatrCardiol [36] | CMR | 3/30 (10%) | 11 (±3) | 60 | 61 (±4) | 0 | <5 | 0 |
| Patel_2022_JAHA [37] | Clinical + CMR | 21/111 (19%) | 13 (±3) | 55 | 55 (±7) | – | 30 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotet, I.-G.; Mateescu, D.-M.; Ilie, A.-C.; Guse, C.; Pah, A.-M.; Badalica-Petrescu, M.; Iurciuc, S.; Craciun, M.-L.; Buleu, F.; Tudoran, C. Systematic Review and Meta-Analysis of Myocarditis Prevalence and Diagnostics in COVID-19:Acute, Post-COVID, and MIS-C (2020–2025). J. Clin. Med. 2025, 14, 7008. https://doi.org/10.3390/jcm14197008
Cotet I-G, Mateescu D-M, Ilie A-C, Guse C, Pah A-M, Badalica-Petrescu M, Iurciuc S, Craciun M-L, Buleu F, Tudoran C. Systematic Review and Meta-Analysis of Myocarditis Prevalence and Diagnostics in COVID-19:Acute, Post-COVID, and MIS-C (2020–2025). Journal of Clinical Medicine. 2025; 14(19):7008. https://doi.org/10.3390/jcm14197008
Chicago/Turabian StyleCotet, Ioana-Georgiana, Diana-Maria Mateescu, Adrian-Cosmin Ilie, Cristina Guse, Ana-Maria Pah, Marius Badalica-Petrescu, Stela Iurciuc, Maria-Laura Craciun, Florina Buleu, and Cristina Tudoran. 2025. "Systematic Review and Meta-Analysis of Myocarditis Prevalence and Diagnostics in COVID-19:Acute, Post-COVID, and MIS-C (2020–2025)" Journal of Clinical Medicine 14, no. 19: 7008. https://doi.org/10.3390/jcm14197008
APA StyleCotet, I.-G., Mateescu, D.-M., Ilie, A.-C., Guse, C., Pah, A.-M., Badalica-Petrescu, M., Iurciuc, S., Craciun, M.-L., Buleu, F., & Tudoran, C. (2025). Systematic Review and Meta-Analysis of Myocarditis Prevalence and Diagnostics in COVID-19:Acute, Post-COVID, and MIS-C (2020–2025). Journal of Clinical Medicine, 14(19), 7008. https://doi.org/10.3390/jcm14197008






