Assessment of Maternal–Fetal Redox Balance in Gestational Diabetes Mellitus: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
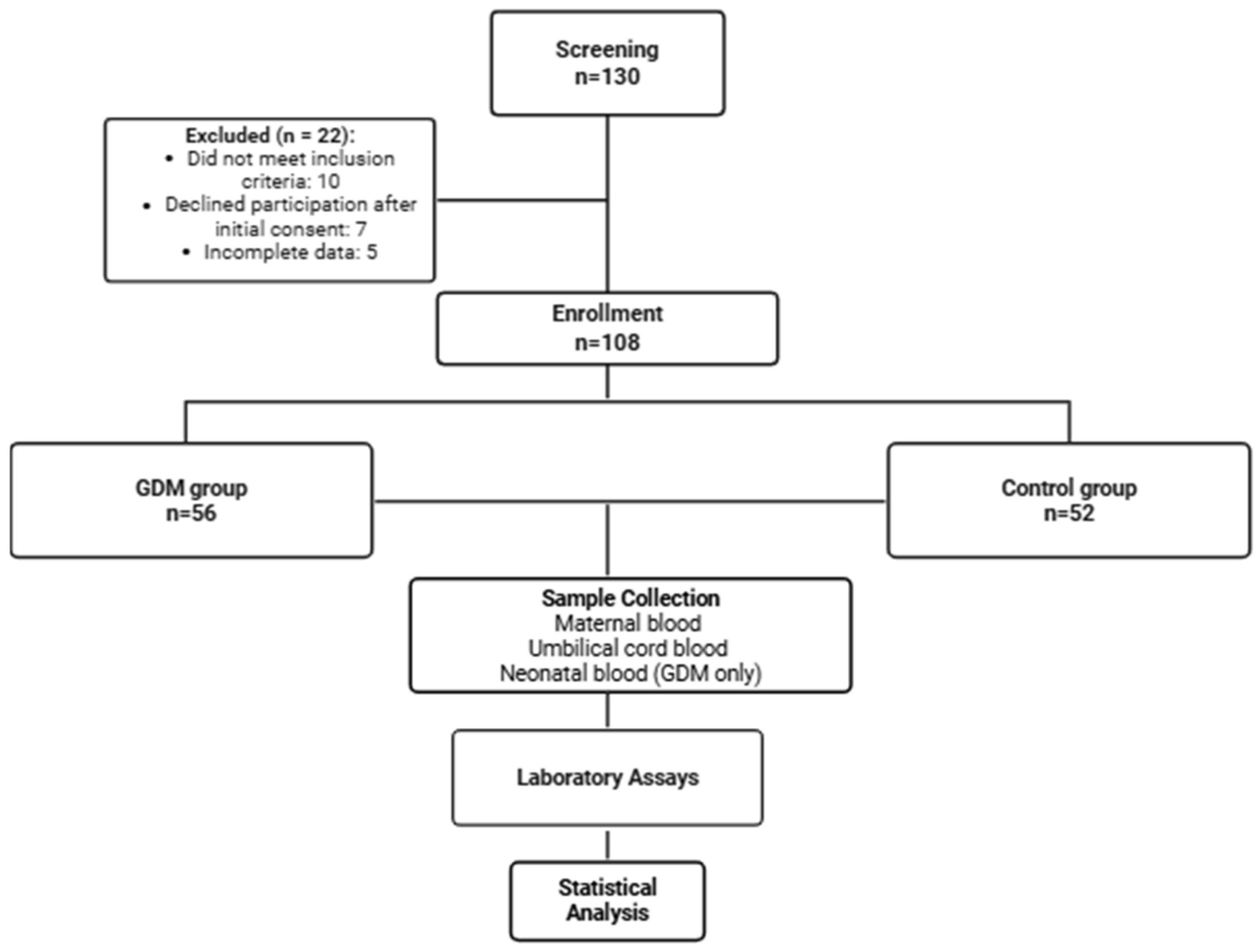
2.1. Study Design and Participants
2.2. Sample Collection
2.3. Oxidative-Stress and Antioxidant Assays
2.4. Patient Demographics and Laboratory Measures
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics and Laboratory Data
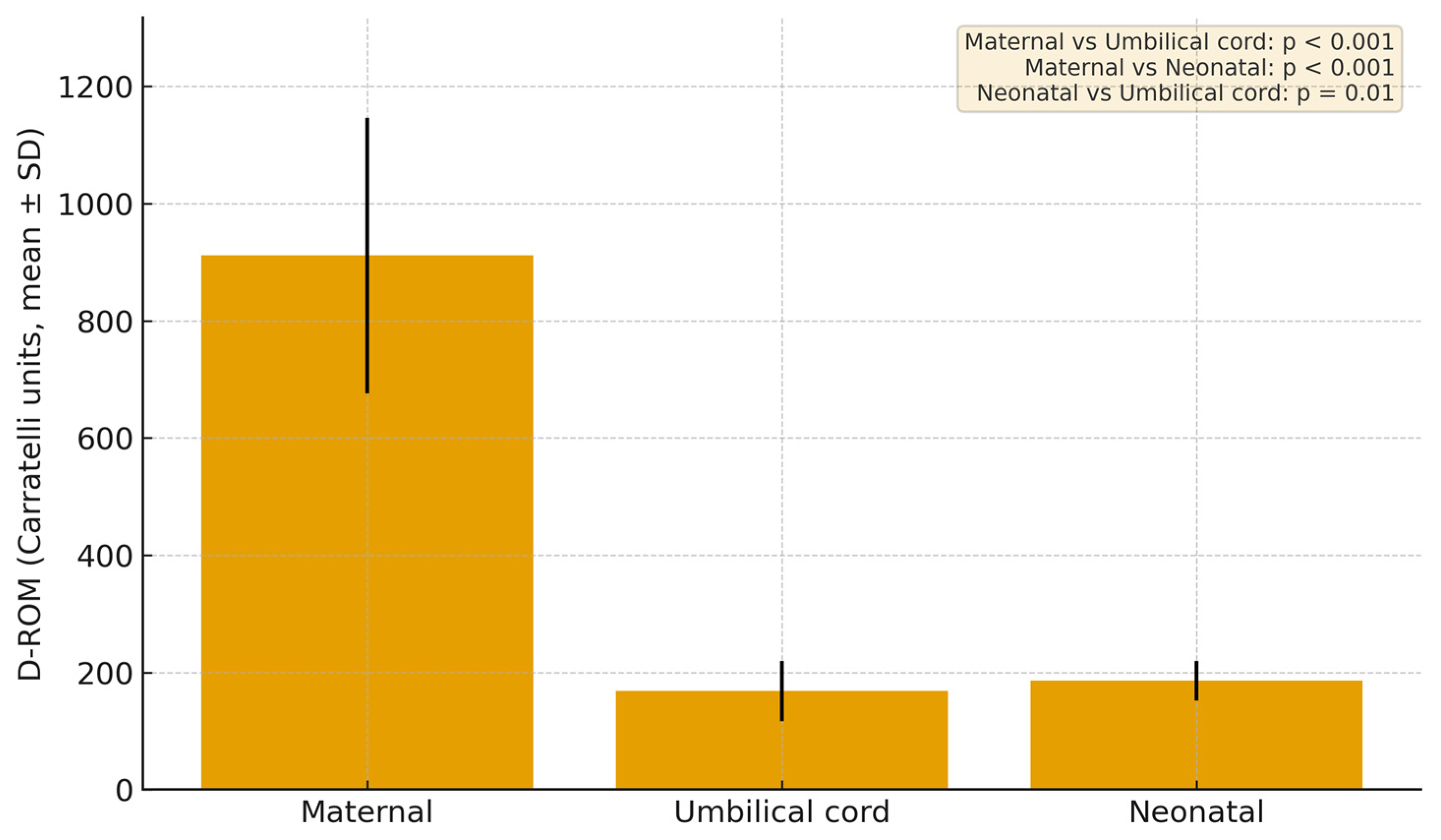
3.2. Results of d-ROM in Different Sample Compartments
3.3. Results of BAP in Different Sample Compartments
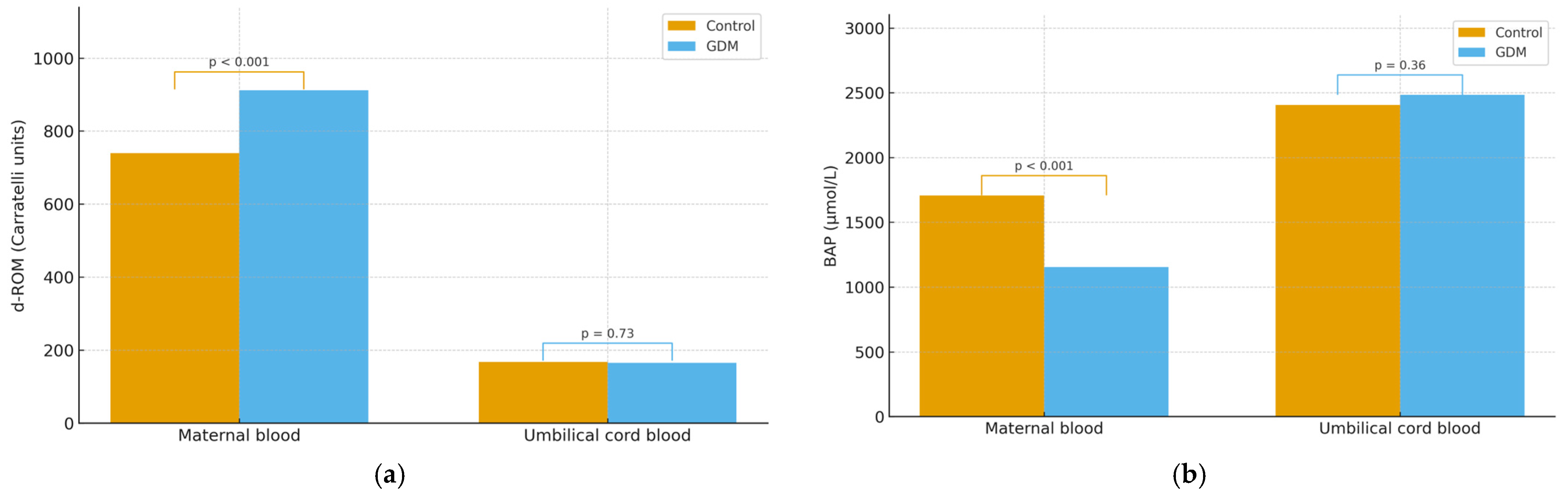
3.4. Comparison of d-ROM Between Groups
3.5. Comparison of BAP Between Groups
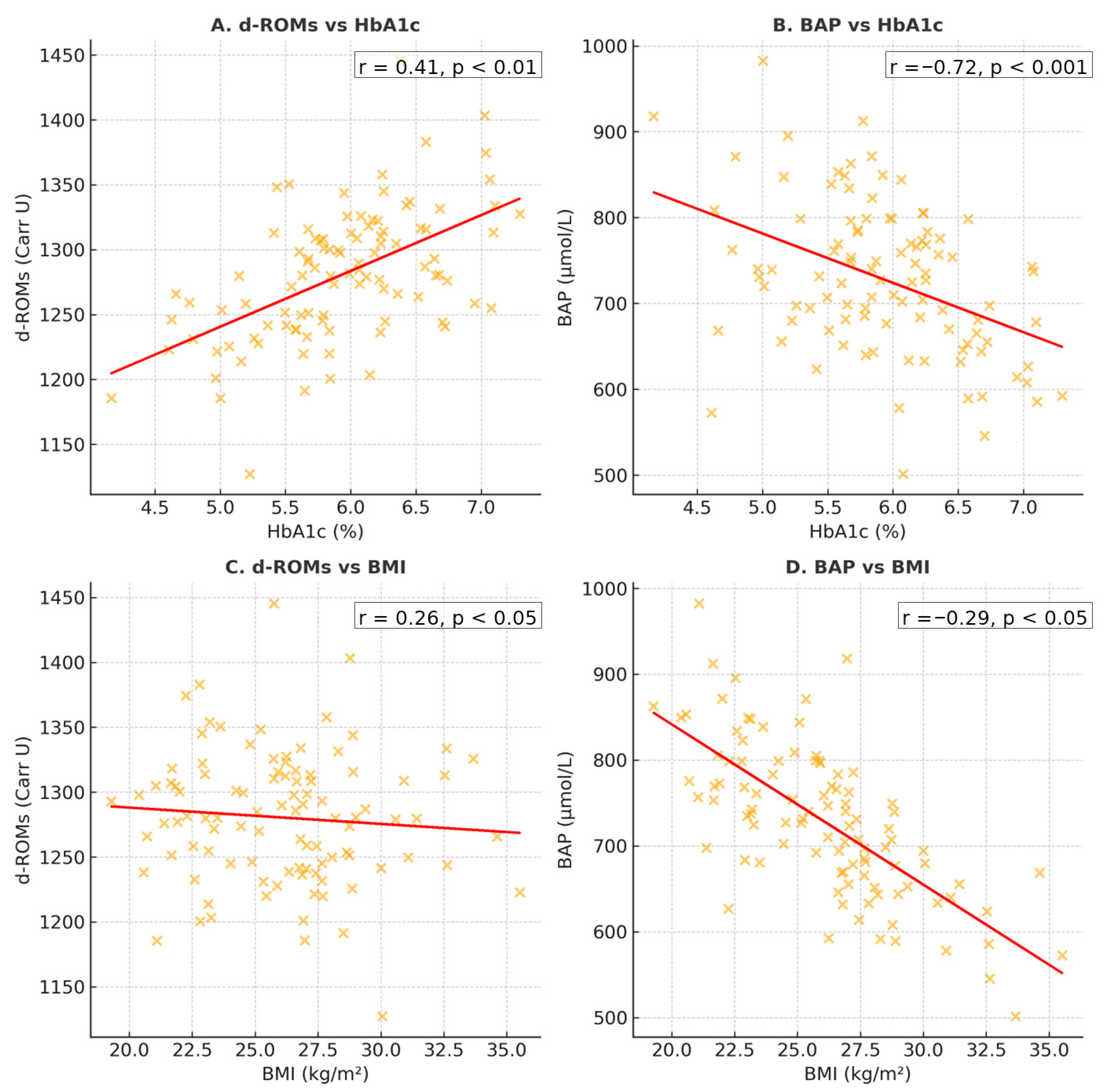
3.6. Correlations BAP/d-ROM
3.7. Regression Analyses
3.8. Glycemic Control and Maternal BMI
3.9. Clinical Correlations
3.9.1. Pregnancy-Related Complications
3.9.2. Fetal Outcomes
4. Discussion
5. Strengths, Limitations, and Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakshine, V.S.; Jogdand, S.D. A Comprehensive Review of Gestational Diabetes Mellitus: Impacts on Maternal Health, Fetal Development, Childhood Outcomes, and Long-Term Treatment Strategies. Cureus 2023, 15, e47500. [Google Scholar] [CrossRef]
- Wicklow, B.; Retnakaran, R. Gestational Diabetes Mellitus and Its Implications across the Life Span. Diabetes Metab. J. 2023, 47, 333–344. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Nilofer, A.; Raju, V.; Dakshayini, B.; Zaki, S. Screening in High-Risk Group of Gestational Diabetes Mellitus with Its Maternal and Fetal Outcomes. Indian J. Endocrinol. Metab. 2012, 16, 74. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Mohammad-Alizadeh, S.; Mirgafourvand, M.; Yaghoubi, S.; Shahrisa, E.; Farshbaf-Khalili, A. Effects of Fish Oil Supplementation on Gestational Diabetes Mellitus (GDM): A Systematic Review. Iran. Red Crescent Med. J. 2016, 18, e24690. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological Changes in Pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Vasam, G.; Green, A.E.; Bainbridge, S.A.; Menzies, K.J. Placental Mitochondrial Function and Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2023, 24, 4177. [Google Scholar] [CrossRef]
- Popkov, V.A.; Buyan, M.I.; Makievskaya, K.I.; Brezgunova, A.A.; Pevzner, I.B.; Zorova, L.D.; Zorov, D.B.; Plotnikov, E.Y.; Andrianova, N.V. Mitochondrial Function and Resistance to Oxidative Stress in the Kidney during Pregnancy. Bull. Exp. Biol. Med. 2024, 177, 442–448. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative Stress in Pregnancy and Reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Chełchowska, M.; Gajewska, J.; Ambroszkiewicz, J.; Mazur, J.; Ołtarzewski, M.; Maciejewski, T.M. Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants 2021, 10, 1866. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, J.; Shen, J.; Del Rosario, I.; Janzen, C.; Devaskar, S.U.; Lakey, P.S.J.; Shiraiwa, M.; Weichenthal, S.; Zhu, Y.; et al. Ambient Exposure to Fine Particulate Matter with Oxidative Potential Affects Oxidative Stress Biomarkers in Pregnancy. Am. J. Epidemiol. 2025, 194, 27–34. [Google Scholar] [CrossRef] [PubMed]
- El Sherbiny, S.; Squillacioti, G.; Colombi, N.; Ghelli, F.; Lenta, E.; Dalla Costa, C.; Bono, R. The Effect of Dietary Patterns and Nutrient Intake on Oxidative Stress Levels in Pregnant Women: A Systematic Review. Antioxidants 2023, 12, 1427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.W.; Candia, A.A.; Sferruzzi-Perri, A.N. Placental Inflammation, Oxidative Stress, and Fetal Outcomes in Maternal Obesity. Trends Endocrinol. Metab. 2024, 35, 638–647. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Oxidative Stress Triggered Damage to Cellular Biomolecules. In Reactive Oxygen Species in Plants; Springer Nature: Singapore, 2023; pp. 45–59. ISBN 978-981-19-9883-6. [Google Scholar]
- Cao, L.; Xi, Y.; Jing, Z.; Bao, Z.; Bai, B.; Lian, X.; Zhang, X.; Di, J.; Liu, F. Exploring Research Trends and Mechanisms: Maternal Diabetes and Neural Tube Defects (1991–2023). J. Multidiscip. Healthc. 2025, 18, 1107–1121. [Google Scholar] [CrossRef]
- Depla, A.L.; De Wit, L.; Steenhuis, T.J.; Slieker, M.G.; Voormolen, D.N.; Scheffer, P.G.; De Heus, R.; Van Rijn, B.B.; Bekker, M.N. Effect of Maternal Diabetes on Fetal Heart Function on Echocardiography: Systematic Review and Meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 539–550. [Google Scholar] [CrossRef]
- De Mendonça, E.L.S.S.; Fragoso, M.B.T.; De Oliveira, J.M.; Xavier, J.A.; Goulart, M.O.F.; De Oliveira, A.C.M. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants 2022, 11, 129. [Google Scholar] [CrossRef]
- Hansen, J.M.; Jones, D.P.; Harris, C. The Redox Theory of Development. Antioxid. Redox Signal. 2020, 32, 715–740. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khoo, M.I.; Ismail, E.H.E.; Hussain, N.H.N.; Zin, A.A.M.; Noordin, L.; Abdullah, S.; Mahdy, Z.A.; Lah, N.A.Z.N. Oxidative Stress Biomarkers in Pregnancy: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 93. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S306–S320. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Redman, L.M. Weight Gain in Pregnancy and Application of the 2009 IOM Guidelines: Toward a Uniform Approach. Obesity 2015, 23, 507–511. [Google Scholar] [CrossRef]
- Chaudhari, L.; Tandon, O.P.; Vaney, N.; Agarwal, N. Lipid Peroxidation and Antioxidant Enzymes in Gestational Diabetics. Indian J. Physiol. Pharmacol. 2003, 47, 441–446. [Google Scholar]
- Figen Gurdol, S.G. The Relation of Oxidative Stress Biomarkers with Proinflammatory Cytokines in Gestational Diabetes. Clin. Investig. 2017, 7, 43–48. [Google Scholar]
- Li, H.; Yin, Q.; Li, N.; Ouyang, Z.; Zhong, M. Plasma Markers of Oxidative Stress in Patients with Gestational Diabetes Mellitus in the Second and Third Trimester. Obstet. Gynecol. Int. 2016, 2016, 3865454. [Google Scholar] [CrossRef]
- Zhuang, W.; Lv, J.; Liang, Q.; Chen, W.; Zhang, S.; Sun, X. Adverse Effects of Gestational Diabetes-Related Risk Factors on Pregnancy Outcomes and Intervention Measures. Exp. Ther. Med. 2020, 20, 3361–3367. [Google Scholar] [CrossRef]
- Furukawa, S.; Nakajima, A.; Sameshima, H. The Longitudinal Change of Extracellular Antioxidant Status during Pregnancy Using an Electron Spin Resonance Method. J. Matern. Fetal Neonatal Med. 2016, 29, 2994–2999. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Cos, P.; Briedé, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative Stress in Healthy Pregnancy and Preeclampsia Is Linked to Chronic Inflammation, Iron Status and Vascular Function. PLoS ONE 2018, 13, e0202919. [Google Scholar] [CrossRef]
- Bînă, A.M.; Anechitei, A.I.; Lelcu, T.; Lința, A.V.; Chiriac, D.V.; Mocanu, A.G.; Bernad, E.; Popa, Z.L.; Craina, M.L.; Muntean, D.M.; et al. Assessment of the Systemic Oxidative Stress in Preeclampsia. Serbian J. Exp. Clin. Res. 2022, 23, 45–50. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial Dysfunction and Preeclampsia: Role of Oxidative Stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.; Lin, Y.; Xu, W. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-X.; Feng, X.; Zhuang, L. The Effect of Oxidative Stress and Antioxidants Treatment on Gestational Diabetes Mellitus Outcome: A Scoping Review. Cell Biochem. Biophys. 2024, 82, 3003–3013. [Google Scholar] [CrossRef]
- Kawashiro, Y.; Ishii, K.; Hosoyamada, Y.; Miyaso, H.; Matsuno, Y.; Kubonoya, K.; Mori, C.; Hanazato, M. Changes in Diacron-reactive Oxygen Metabolites and Biological Antioxidant Potential in Maternal Serum during Pregnancy (910.6). FASEB J. 2014, 28, 910.6. [Google Scholar] [CrossRef]
- Hryniewicka, J.; Buczyńska, A.; Siewko, K.; Anna, P.-K.; Adamska, A.; Krętowski, A.; Szelachowska, M. Assessment of Oxidative Stress Levels in Gestational Diabetes Mellitus (GDM) Patients: A Cross-Sectional Study. Endocr. Abstr. 2024, 99, EP537. [Google Scholar] [CrossRef]
- Rajdl, D.; Racek, J.; Steinerová, A.; Novotný, Z.; Stozický, F.; Trefil, L.; Siala, K. Markers of Oxidative Stress in Diabetic Mothers and Their Infants during Delivery. Physiol. Res. 2005, 54, 429–436. [Google Scholar] [CrossRef]
- Bogavac, M.; Lakic, N.; Simin, N.; Nikolic, A.; Sudji, J.; Bozin, B. Biomarkers of Oxidative Stress in Amniotic Fluid and Complications in Pregnancy. J. Matern. Fetal Neonatal Med. 2012, 25, 104–108. [Google Scholar] [CrossRef]
- Qiu, C.; Hevner, K.; Abetew, D.; Enquobahrie, D.A.; Williams, M.A. Oxidative DNA Damage in Early Pregnancy and Risk of Gestational Diabetes Mellitus: A Pilot Study. Clin. Biochem. 2011, 44, 804–808. [Google Scholar] [CrossRef]
- Gerszi, D.; Orosz, G.; Török, M.; Szalay, B.; Karvaly, G.; Orosz, L.; Hetthéssy, J.; Vásárhelyi, B.; Török, O.; Horváth, E.M.; et al. Risk Estimation of Gestational Diabetes Mellitus in the First Trimester. J. Clin. Endocrinol. Metab. 2023, 108, e1214–e1223. [Google Scholar] [CrossRef]
- Sudharshana Murthy, K.; Bhandiwada, A.; Chandan, S.; Gowda, S.; Sindhusree, G. Evaluation of Oxidative Stress and Proinflammatory Cytokines in Gestational Diabetes Mellitus and Their Correlation with Pregnancy Outcome. Indian J. Endocrinol. Metab. 2018, 22, 79. [Google Scholar] [CrossRef]
- Turpin, C.A.; Sakyi, S.A.; Owiredu, W.K.; Ephraim, R.K.; Anto, E.O. Association between Adverse Pregnancy Outcome and Imbalance in Angiogenic Regulators and Oxidative Stress Biomarkers in Gestational Hypertension and Preeclampsia. BMC Pregnancy Childbirth 2015, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Oğlak, S.C.; Aşır, F.; Yılmaz, E.Z.; Bolluk, G.; Korak, T.; Ağaçayak, E. The Immunohistochemical and Bioinformatics Analysis of the Placental Expressions of Vascular Cell Adhesion Protein 1 (VCAM-1) and High Mobility Group Box 1 (HMGB1) Proteins in Gestational Diabetic Mothers. Z. Für Geburtshilfe Neonatol. 2025, 229, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Alwan, N.A.; West, J.; Brown, S.; McKinlay, C.J.; Farrar, D.; Crowther, C.A. Lifestyle Interventions for the Treatment of Women with Gestational Diabetes. Cochrane Database Syst. Rev. 2017, 5, CD011970. [Google Scholar] [CrossRef] [PubMed]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Lawlor, D.A. Treatments for Gestational Diabetes: A Systematic Review and Meta-Analysis. BMJ Open 2017, 7, e015557. [Google Scholar] [CrossRef]
- Ryu, R.J.; Hays, K.E.; Hebert, M.F. Gestational Diabetes Mellitus Management with Oral Hypoglycemic Agents. Semin. Perinatol. 2014, 38, 508–515. [Google Scholar] [CrossRef]
- Jamilian, M.; Hashemi Dizaji, S.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Karamali, M.; Akbari, M.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Biomarkers of Oxidative Stress, Inflammation and Pregnancy Outcomes in Gestational Diabetes. Can. J. Diabetes 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Maged, A.M.; Torky, H.; Fouad, M.A.; GadAllah, S.H.; Waked, N.M.; Gayed, A.S.; Salem, A.K. Role of Antioxidants in Gestational Diabetes Mellitus and Relation to Fetal Outcome: A Randomized Controlled Trial. J. Matern. Fetal Neonatal Med. 2016, 29, 4049–4054. [Google Scholar] [CrossRef]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of Probiotic Supplements in Women with Gestational Diabetes Mellitus on Inflammation and Oxidative Stress Biomarkers: A Randomized Clinical Trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Vatanparast, H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Ran-domized Control Trials. Nutrients 2017, 9, 20. [Google Scholar] [CrossRef]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The Effects of Magnesium-Zinc-Calcium-Vitamin D Co-Supplementation on Biomarkers of Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef]


| Parameter | Gestational Diabetes Group | Control Group | p-Value |
|---|---|---|---|
| Age (years) mean ± SD | 31.16 ± 5.42 | 30.4 ± 4.38 | 0.42 |
| BMI (kg/m2) mean ± SD | 28.7 ± 4.5 | 25.1 ± 3.8 | <0.001 |
| Gestational age at birth (weeks) mean ± SD | 38.23 ±2.47 | 38.67 ±0.63 | 0.20 |
| Gestational age at maternal sampling (weeks) mean ± SD | 33.21 ±0.70 | 34.1 ±0.35 | <0.001 |
| Glucose (mmol/L) mean ± SD | 6.53 ± 1.85 | 4.23 ± 0.79 | <0.001 |
| HbA1c (%) median (IQR) | 5.44 ± 0.73 | 4.67 ± 0.15 | <0.001 |
| ESR (mm/h) median (IQR) | 42 (28–58) | 30 (18–46) | <0.001 |
| CRP (mg/dL) median (IQR) | 2.3 (0.9–5.6) | 0.6 (0.3–1.2) | <0.001 |
| Total Bilirubin (µmol/L) median (IQR) | 9.0 (7.5–11.2) | 8.7 (6.8–10.1) | 0.39 |
| Direct Bilirubin (µmol/L) median (IQR) | 3.9 (2.5–6.0) | 4.1 (3.2–5.0) | 0.98 |
| Complication | Number of Patients | d-ROMs (Mean and SD) | p-Value | BAP (Mean and SD) | p-Value |
|---|---|---|---|---|---|
| Anemia | Yes (n = 24) | 748.7± 123.7 | 0.91 | 1 586 ± 3 558.2 | 0.90 |
| No (n = 28) | 743.9 ± 192.4 | 1 679.3 ± 281.4 | |||
| HTAIS | Yes (n = 9) | 858.5 ± 142.9 | 0.03 | 1 748.7 ± 139.2 | 0.46 |
| No (n = 43) | 725.7 ± 147.9 | 1 683.9 ± 489.8 | |||
| Infection | Yes (n = 8) | 675.5 ± 261.3 | 0.38 | 1 915.3 ± 317.0 | 0.06 |
| No (n = 44) | 763.5 ± 139.9 | 1 648.7 ± 465.0 |
| Complication | Number of Patients | d-ROMs (Mean and SD) | p-Value | BAP (Mean and SD) | p-Value |
|---|---|---|---|---|---|
| Anemia | Yes (n = 29) | 912.3 ± 232.7 | 0.09 | 1076.6 ± 43.5 | 0.07 |
| No (n = 27) | 832.1 ± 95.2 | 1265.7 ± 525.5 | |||
| HTAIS | Yes (n = 16) | 854.9 ± 164.9 | 0.23 | 906.1 ± 274.7 | <0.001 |
| No (n = 40) | 928.9 ± 281.2 | 1243.6 ± 411.5 | |||
| Infection | Yes (n = 13) | 883.6 ± 147.7 | 0.84 | 1234.1 ± 339.7 | 0.34 |
| No (n = 43) | 895.8 ± 278.8 | 1122.8 ± 428.3 | |||
| Excessive gestational weight gain | Yes (n = 8) | 977.4 ± 175.6 | 0.18 | 1030.6 ± 197.6 | 0.20 |
| No (n = 48) | 874.3 ± 258.1 | 1153.8 ± 419.7 | |||
| Oligohydramnios | Yes (n = 3) | 733.17 ± 96.27 | 0.03 | 812.5 ± 163.05 | 0.01 |
| No (n = 53) | 946.24 ± 251.90 | 1324.27 ± 449.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelu, S.C.; Chiriac, V.D.; Andrei, D.; Stoicescu, E.R.; Borza, C. Assessment of Maternal–Fetal Redox Balance in Gestational Diabetes Mellitus: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 7003. https://doi.org/10.3390/jcm14197003
Chelu SC, Chiriac VD, Andrei D, Stoicescu ER, Borza C. Assessment of Maternal–Fetal Redox Balance in Gestational Diabetes Mellitus: A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):7003. https://doi.org/10.3390/jcm14197003
Chicago/Turabian StyleChelu, Sorina Cristina, Veronica Daniela Chiriac, Diana Andrei, Emil Robert Stoicescu, and Claudia Borza. 2025. "Assessment of Maternal–Fetal Redox Balance in Gestational Diabetes Mellitus: A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 7003. https://doi.org/10.3390/jcm14197003
APA StyleChelu, S. C., Chiriac, V. D., Andrei, D., Stoicescu, E. R., & Borza, C. (2025). Assessment of Maternal–Fetal Redox Balance in Gestational Diabetes Mellitus: A Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 7003. https://doi.org/10.3390/jcm14197003







