Assessment of Intervertebral Lumbar Disk Herniation: Accuracy of Dual-Energy CT Compared to MRI
Abstract
1. Introduction
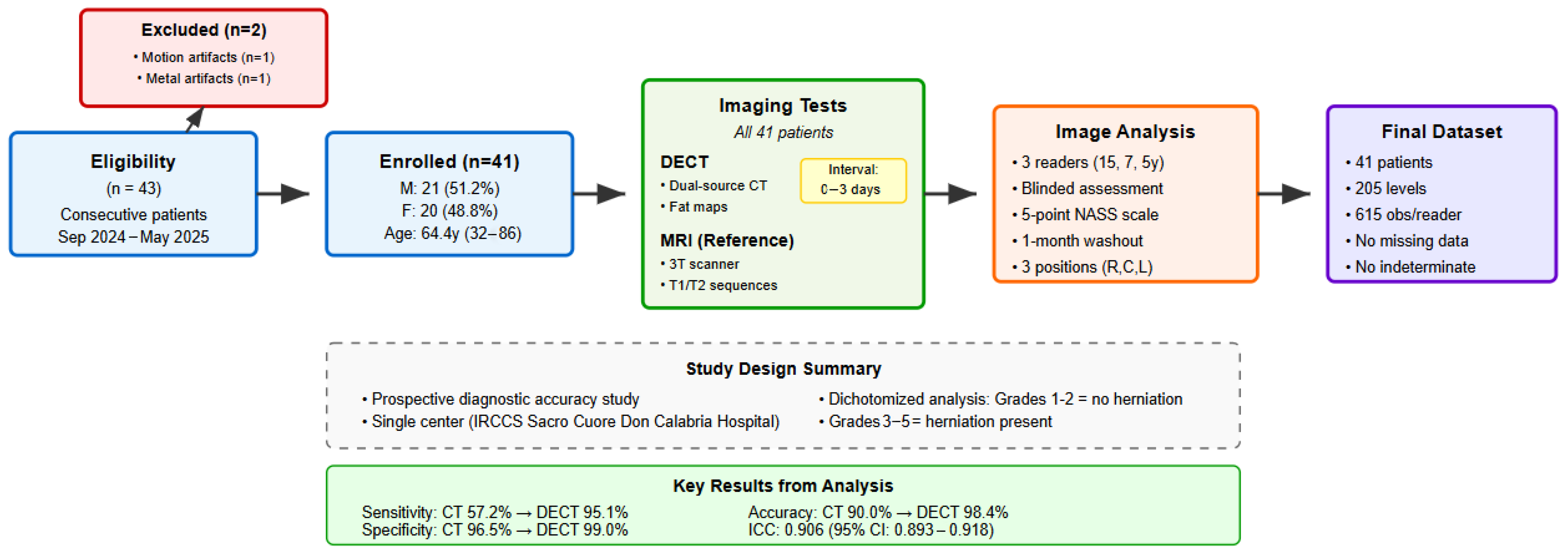
2. Materials and Methods
3. Statistical Analysis
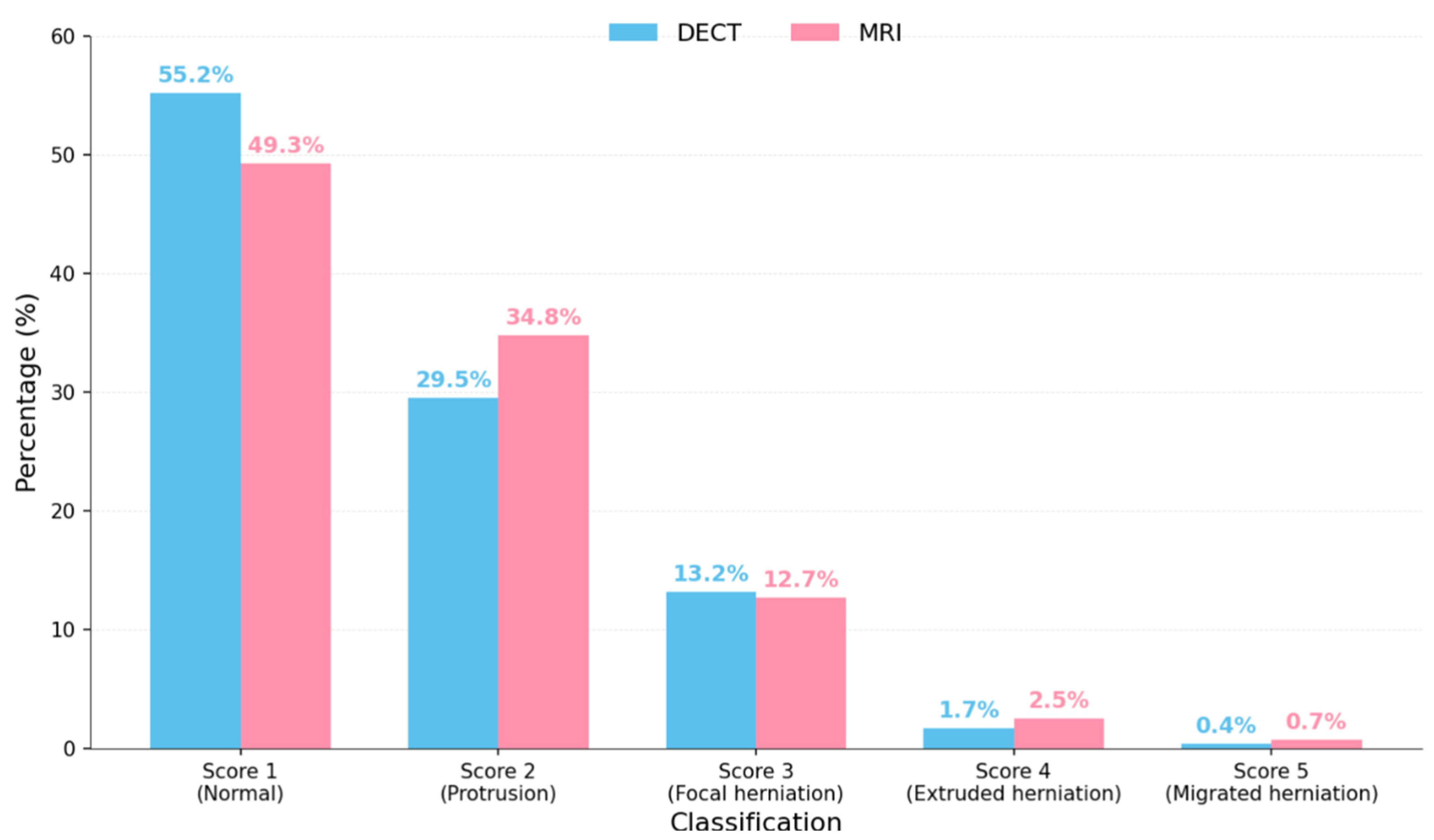
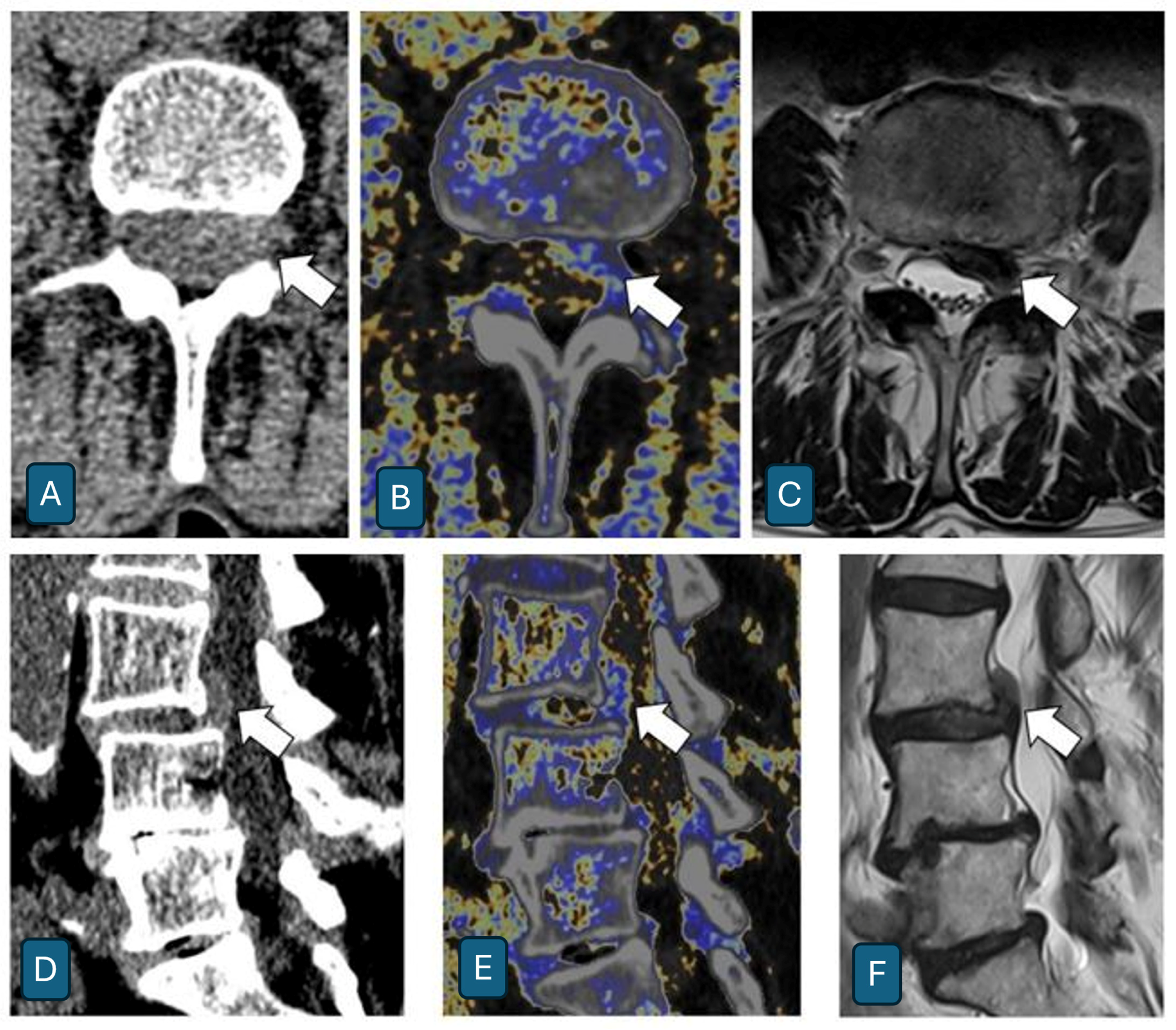
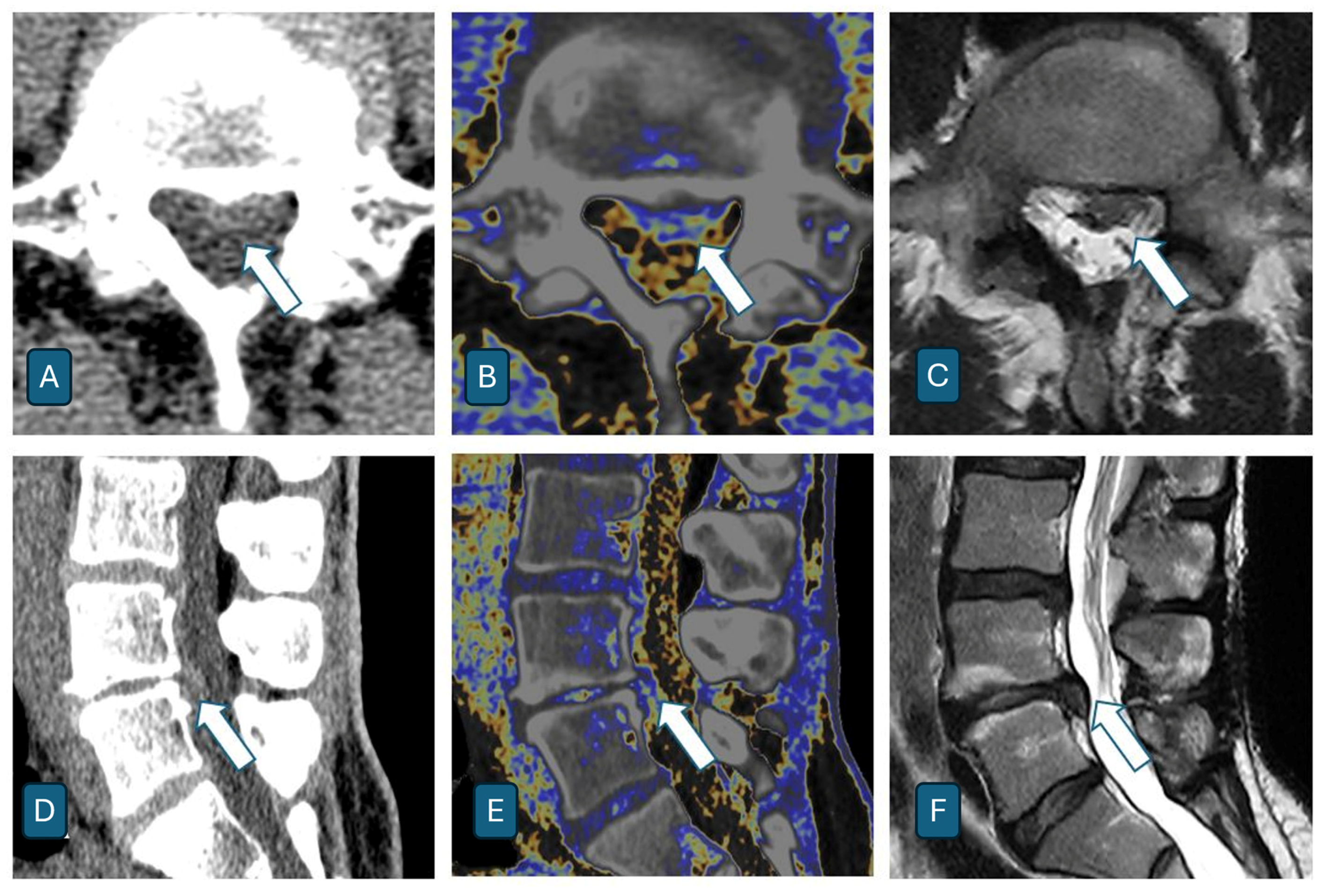
4. Results
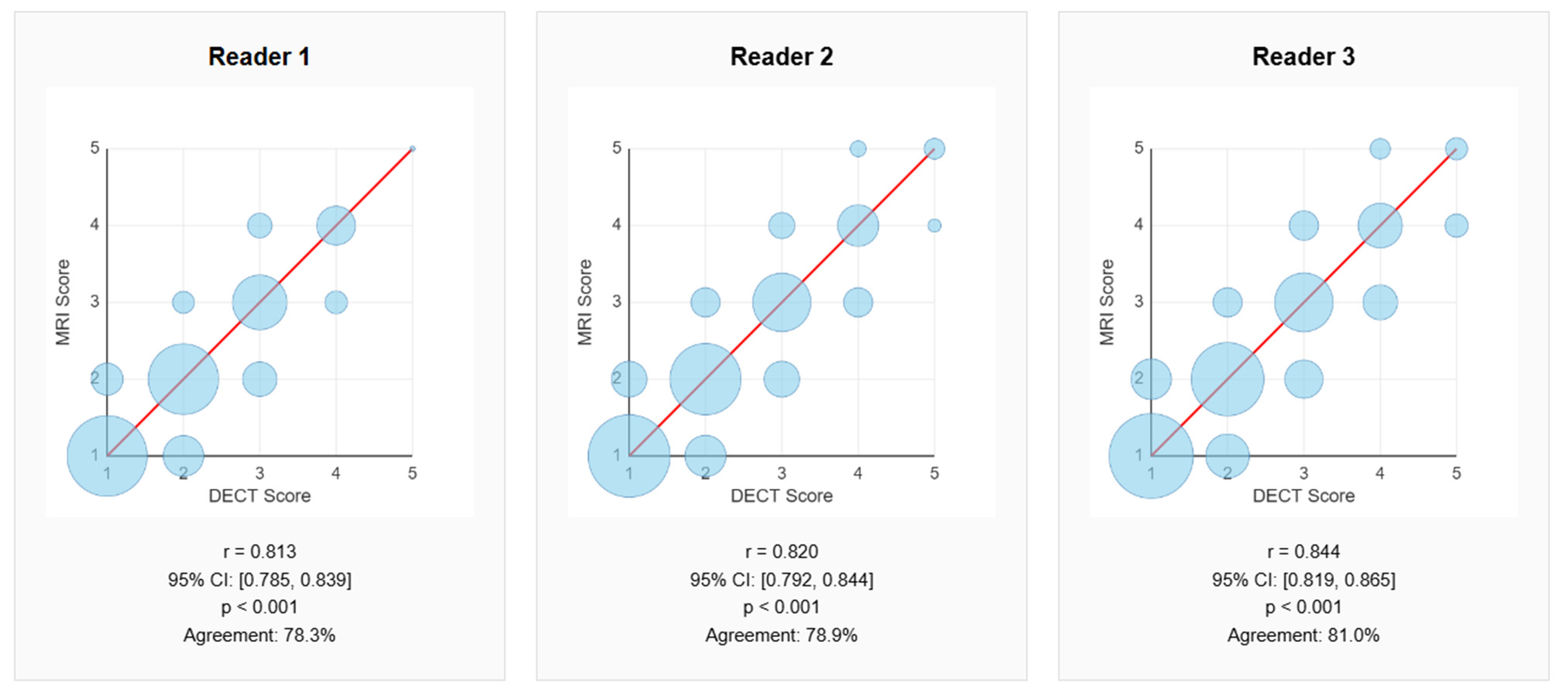
- Correlation between Dual-Energy CT and MRI
- Inter-rater Reliability
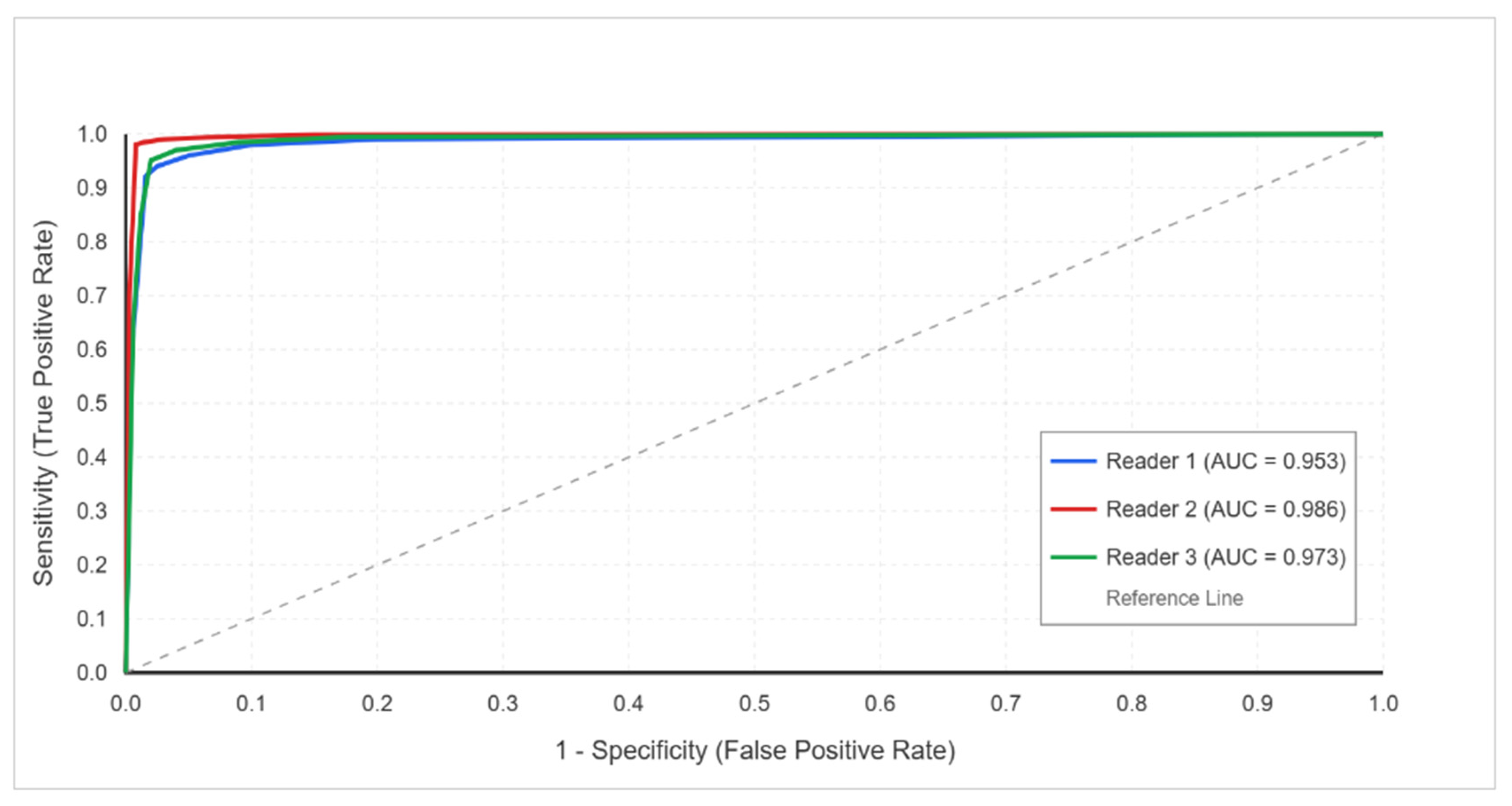
- ROC Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiri, R.; Falah-Hassani, K.; Heliövaara, M.; Solovieva, S.; Amiri, S.; Lallukka, T.; Burdorf, A.; Husgafvel-Pursiainen, K.; ViikariJuntura, E. Risk Factors for Low Back Pain: A Population-Based Longitudinal Study. Arthritis Care Res. 2019, 71, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hassett, G.; Hart, D.J.; Manek, N.J.; Doyle, D.V.; Spector, T.D. Risk factors for progression of lumbar spine disc degeneration: The Chingford Study. Arthritis Rheum. 2003, 48, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.K.; Kongsted, A.; Kjaer, P.; Koes, B. Diagnosis and treatment of sciatica. BMJ 2019, 367, l6273. [Google Scholar] [CrossRef] [PubMed]
- Jarvik, J.G.; Deyo, R.A. Diagnostic evaluation of low back pain with emphasis on imaging. Ann. Intern. Med. 2002, 137, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M.S.; Al-Edanni, M.S.; Al-Ameri, L.T. Correlation between clinical and MRI findings in disc herniation in the lumbosacral region. Ir. J. Med. Sci. 2024, 193, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.S.; Xu, A.; Ansari, K.; Hardacker, K.; Anderson, G.; Alsoof, D.; Daniels, A.H. Lumbar Disc Herniation: Diagnosis and Management. Am. J. Med. 2023, 136, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, P.; Zhang, C.; Wu, J.; Liu, R. Value of imaging examinations in diagnosing lumbar disc herniation: A systematic review and meta-analysis. Front. Surg. 2023, 9, 1020766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fardon, D.F.; Williams, A.L.; Dohring, E.J.; Murtagh, F.R.; Gabriel Rothman, S.L.; Sze, G.K. Lumbar disc nomenclature: Version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014, 14, 2525–2545. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.C.W.; Yan, Y.Y.; Sheikh, A.; Ouellette, H.A.; Munk, P.L.; Murray, N.; Mallinson, P.I. Dual-energy CT applications in musculoskeletal disorders. Br. J. Radiol. 2024, 97, 705–715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foti, G.; Bortoli, L.; Tronu, M.; Montefusco, S.; Serra, G.; Filippini, R.; Iacono, V. Identification of Achille’s Tendon Tears: Diagnostic Accuracy of Dual-Energy CT with Respect to MRI. J. Clin. Med. 2024, 13, 4426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reschke, P.; Koch, V.; Sommer, C.M.; Gotta, J.; Höhne, E.; Booz, C.; D’Angelo, T.; Eichler, K.; Vogl, T.J.; Gruenewald, L.D. The Association between opportunistic DECT-derived bone values and vertebral fracture status. Eur. J. Radiol. 2025, 188, 112159. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, H.C.; Wu, C.H.; Perka, C.; Panics, G. Dual-Energy Computed Tomography in Spine Fractures: A Systematic Review and Meta-Analysis. Int. J. Spine Surg. 2021, 15, 525–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foti, G.; Longo, C.; Oliboni, E.; Faccioli, N.; Sanfilippo, L.; Guerriero, M.; Augelli, R.; Motta, L.; Marocco, S. Spondylodiscitis of the thoraco-lumbar spine: Diagnostic performance of dual-energy CT vs MRI. Eur. Radiol. 2025, 35, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, S.M.; Seo, J.S.; Kim, T.W.; Rhee, S.J.; Jeong, H.S. Metal Artifact Reduction Dual-Energy CT as an Accurate and Reliable Method for Measuring Total Knee Arthroplasty Femoral Component Rotation Compared to Conventional CT. J. Knee Surg. 2023, 36, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Zhao, W.; Gao, W.; Zhu, J.; Xi, Y.; Chen, Y.; Zhu, W. A Dual-Energy Metal Artifact Redcution Method for DECT Image Reconstruction. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Copenhagen, Denmark, 14–17 July 2023. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.L.; Xia, P.; Klahr, P.; Djemil, T. Dosimetric impact of orthopedic metal artifact reduction (O-MAR) on Spine SBRT patients. J. Appl. Clin. Med. Phys. 2015, 16, 106–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foti, G.; Tripodi, G.; Ocello, G.; Manenti, G.; Merci, G.; Mignolli, T.; Sanfilippo, L.; Guerriero, M.; Serra, G. Endoscopic Foraminotomy for the Treatment of Lumbar Neuro-Foramen Stenosis: Role of CT in Treatment Planning and Post-Operative Assessment. Life 2025, 15, 615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shim, E.; Kim, B.H.; Kang, W.Y.; Hong, S.J.; Kang, C.H.; Ahn, K.S.; Lee, H.; Kwack, T.J. Diagnostic performance of electron-density dual-energy CT in detection of cervical disc herniation in comparison with standard gray-scale CT and virtual non-calcium images. Eur. Radiol. 2022, 32, 2209–2220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernatz, S.; Hoppe, A.T.; Gruenewald, L.D.; Koch, V.; Martin, S.S.; Engelskirchen, L.; Radic, I.; Bucolo, G.; Gotta, J.; Reschke, P.; et al. Assessment of thoracic disc degeneration using dual-energy CT-based collagen maps. Eur. Radiol. Exp. 2024, 8, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Booz, C.; Nöske, J.; Martin, S.S.; Albrecht, M.H.; Yel, I.; Lenga, L.; Gruber-Rouh, T.; Eichler, K.; D’Angelo, T.; Vogl, T.J.; et al. Virtual Noncalcium Dual-Energy CT: Detection of Lumbar Disk Herniation in Comparison with Standard Gray-scale CT. Radiology 2019, 290, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Rosskopf, A.B.; Marth, A.A.; Feuerriegel, G.C.; Berli, M.C.; Fritz, B.; Sutter, R. Accuracy of Dual-Energy CT-derived Fat Maps and Bone Marrow Edema Maps in Pedal Osteomyelitis Diagnosis. Radiology 2025, 315, e232900. [Google Scholar] [CrossRef] [PubMed]
- Ditges, A.K.; Diekhoff, T.; Engelhard, N.; Muellner, M.; Pumberger, M.; Schömig, F. Neuroforamen stenosis remains a challenge in conventional computed tomography and new dual-energy techniques. Sci. Rep. 2022, 12, 6678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zi, Y.; Zhang, B.; Liu, L.; Cao, X.; Zeng, W.; Li, X.; Zhang, G.; Wan, J.; Shi, L.; Wu, H. Fat content in lumbar paravertebral muscles: Quantitative and qualitative analysis using dual-energy CT in correlation to MR imaging. Eur. J. Radiol. 2022, 148, 110150. [Google Scholar] [CrossRef] [PubMed]
- Muthalaly, R.G.; Nerlekar, N.; Ge, Y.; Kwong, R.Y.; Nasis, A. MRI in Patients with Cardiac Implantable Electronic Devices. Radiology 2018, 289, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Aran, S.; Shaqdan, K.W.; Abujudeh, H.H. Dual-energy computed tomography (DECT) in emergency radiology: Basic principles, techniques, and limitations. Emerg. Radiol. 2014, 21, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, I.; Verde, F.; Palumbo, L.; Di Pietto, F.; Puglia, M.; Scaglione, M.; Ragozzino, A.; Romano, S. Dual energy computed tomography evaluation of skeletal traumas. Eur. J. Radiol. 2021, 134, 109456. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.M. Current guidelines for MRI safety in patients with cardiovascular implantable electronic devices. Nursing 2020, 50, 24–29. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | 41 |
|---|---|
| Age (years) | |
| - Mean ± DS | 64.4 ± 13.2 |
| - Range | 32–86 |
| Sex, n (%) | |
| - Male | 21 (51.2%) |
| - Female | 20 (48.8%) |
| Intervertebral Levels Examined | |
| - Total Levels | 205 |
| - Levels Per Patient | 5 |
| - Levels Distribution | All examined |
| Examined Levels | L1–L2, L2–L3, L3–L4, L4–L5, L5–S1 |
| Reader | Pearson Correlation (r) | IC 95% | p-Value | Agreement (%) | IC 95% Agreement |
|---|---|---|---|---|---|
| 1 | 0.813 | 0.785–0.839 | <0.001 | 78.3 | 74.9–81.4 |
| 2 | 0.820 | 0.792–0.844 | <0.001 | 78.9 | 75.5–81.9 |
| 3 | 0.844 | 0.819–0.865 | <0.001 | 81.0 | 77.7–83.9 |
| Average | 0.826 | -- | -- | 79.4 | -- |
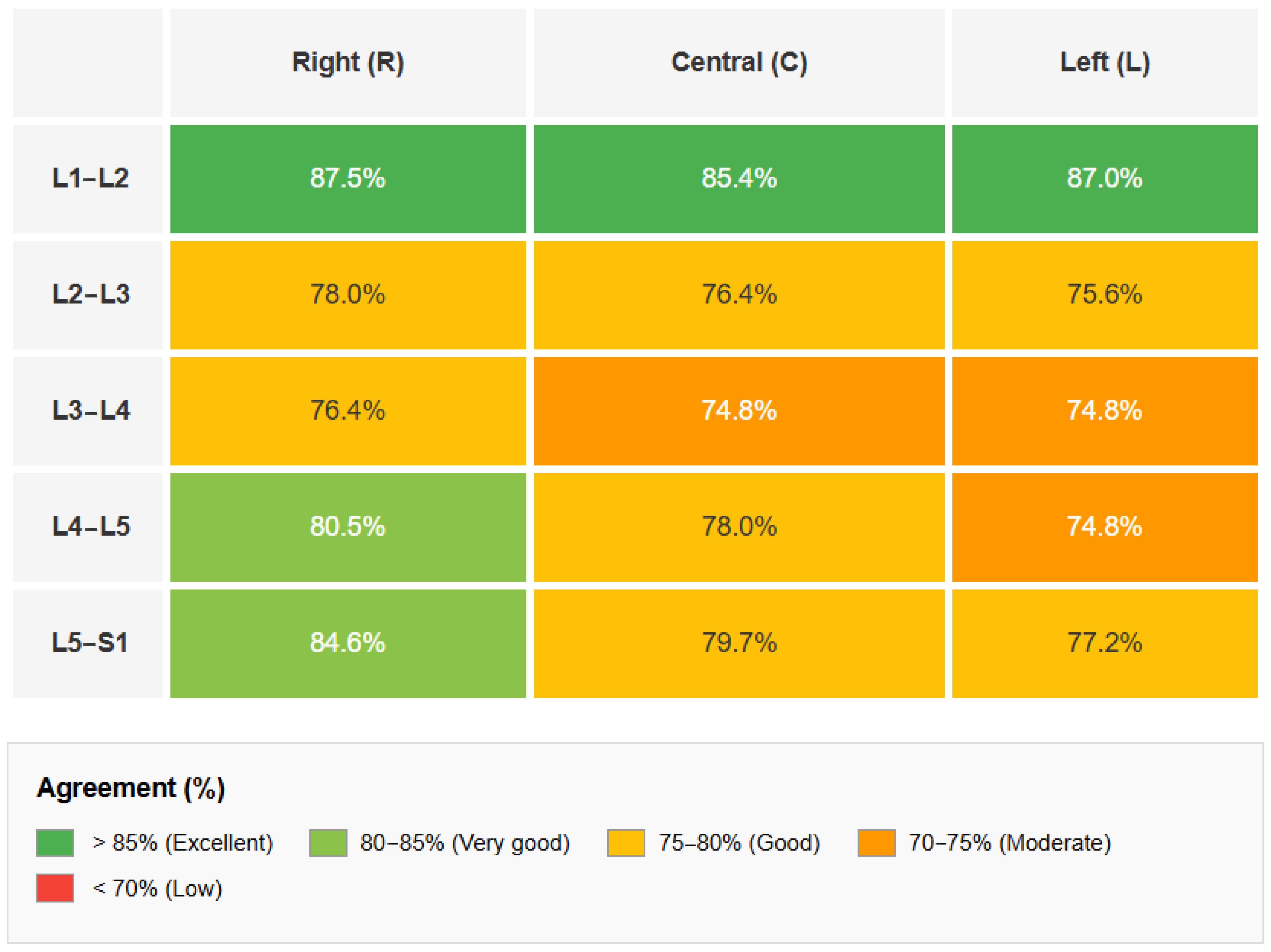
| Level | Average Agreement (%) | Reader 1 (%) | Reader 2 (%) | Reader 3 (%) |
|---|---|---|---|---|
| L1–L2 | 86.7 | 87.0 | 84.6 | 88.6 |
| L2–L3 | 76.7 | 74.8 | 77.2 | 78.0 |
| L3–L4 | 75.3 | 75.6 | 75.6 | 74.8 |
| L4–L5 | 77.8 | 74.8 | 78.0 | 80.5 |
| L5–S1 | 80.4 | 79.5 | 78.9 | 82.9 |
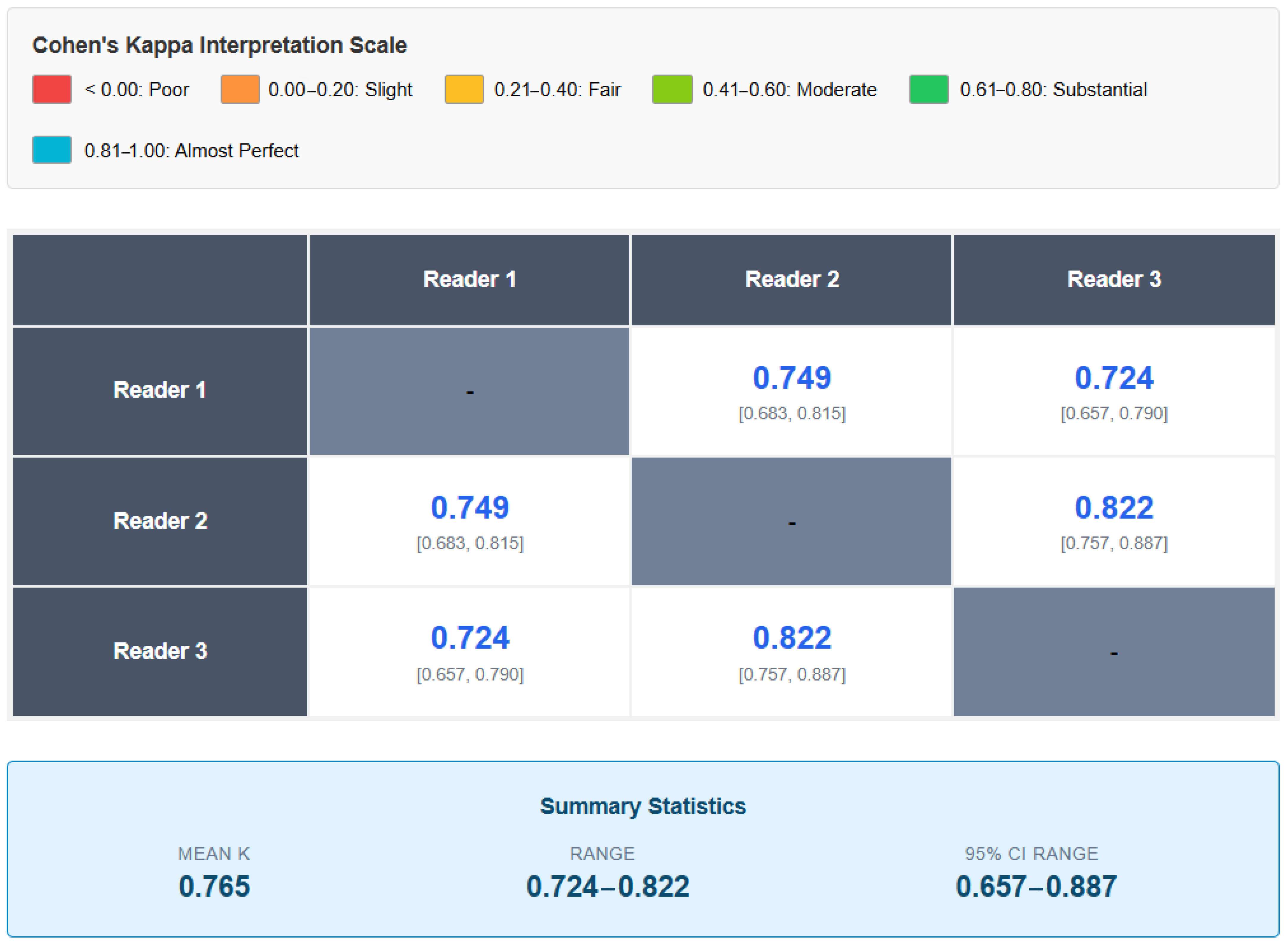
| Comparison | Kappa | IC 95% | Interpretation |
|---|---|---|---|
| Reader 1 vs. 2 | 0.749 | 0.683–0.815 | Substantial |
| Reader 1 vs. 3 | 0.724 | 0.657–0.790 | Substantial |
| Reader 2 vs. 3 | 0.822 | 0.757–0.887 | Almost Perfect |
| Average | 0.765 | -- | Substantial |
| Parameter | CT | DECT | Delta (Δ) |
|---|---|---|---|
| Reader 1 | |||
| Sensitivity (%) | 51.0 (41.4–60.5) | 92.2 (85.3–96.0) | +41.2 |
| Specificity (%) | 96.1 (94.1–97.5) | 98.4 (97.0–99.2) | +2.3 |
| Accuracy (%) | 88.6 (85.9–90.9) | 97.4 (95.8–98.4) | +8.8 |
| Reader 2 | |||
| Sensitivity (%) | 57.8 (48.1–67.0) | 98.0 (93.1–99.5) | +40.2 |
| Specificity (%) | 96.3 (94.3–97.6) | 99.2 (98.0–99.7) | +2.9 |
| Accuracy (%) | 89.9 (87.3–92.1) | 99.0 (97.9–99.6) | +9.1 |
| Reader 3 | |||
| Sensitivity (%) | 62.7 (53.1–71.5) | 95.1 (89.0–97.9) | +32.4 |
| Specificity (%) | 97.1 (95.2–98.2) | 99.4 (98.3–99.8) | +2.3 |
| Accuracy (%) | 91.4 (88.9–93.3) | 98.7 (97.5–99.3) | +7.3 |
| Average | |||
| Sensitivity (%) | 57.2 | 95.1 | +37.9 |
| Specificity (%) | 96.5 | 99.0 | +2.5 |
| Accuracy (%) | 90.0 | 98.4 | +8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocello, G.; Tripodi, G.; Spoto, F.; Monterubbiano, L.; Serra, G.; Merci, G.; Foti, G. Assessment of Intervertebral Lumbar Disk Herniation: Accuracy of Dual-Energy CT Compared to MRI. J. Clin. Med. 2025, 14, 7000. https://doi.org/10.3390/jcm14197000
Ocello G, Tripodi G, Spoto F, Monterubbiano L, Serra G, Merci G, Foti G. Assessment of Intervertebral Lumbar Disk Herniation: Accuracy of Dual-Energy CT Compared to MRI. Journal of Clinical Medicine. 2025; 14(19):7000. https://doi.org/10.3390/jcm14197000
Chicago/Turabian StyleOcello, Giuseppe, Gianluca Tripodi, Flavio Spoto, Leonardo Monterubbiano, Gerardo Serra, Giorgio Merci, and Giovanni Foti. 2025. "Assessment of Intervertebral Lumbar Disk Herniation: Accuracy of Dual-Energy CT Compared to MRI" Journal of Clinical Medicine 14, no. 19: 7000. https://doi.org/10.3390/jcm14197000
APA StyleOcello, G., Tripodi, G., Spoto, F., Monterubbiano, L., Serra, G., Merci, G., & Foti, G. (2025). Assessment of Intervertebral Lumbar Disk Herniation: Accuracy of Dual-Energy CT Compared to MRI. Journal of Clinical Medicine, 14(19), 7000. https://doi.org/10.3390/jcm14197000






