Effect of Transcatheter Aortic Valve Implantation on Non-Invasive Myocardial Work Parameters: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Eligibility
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
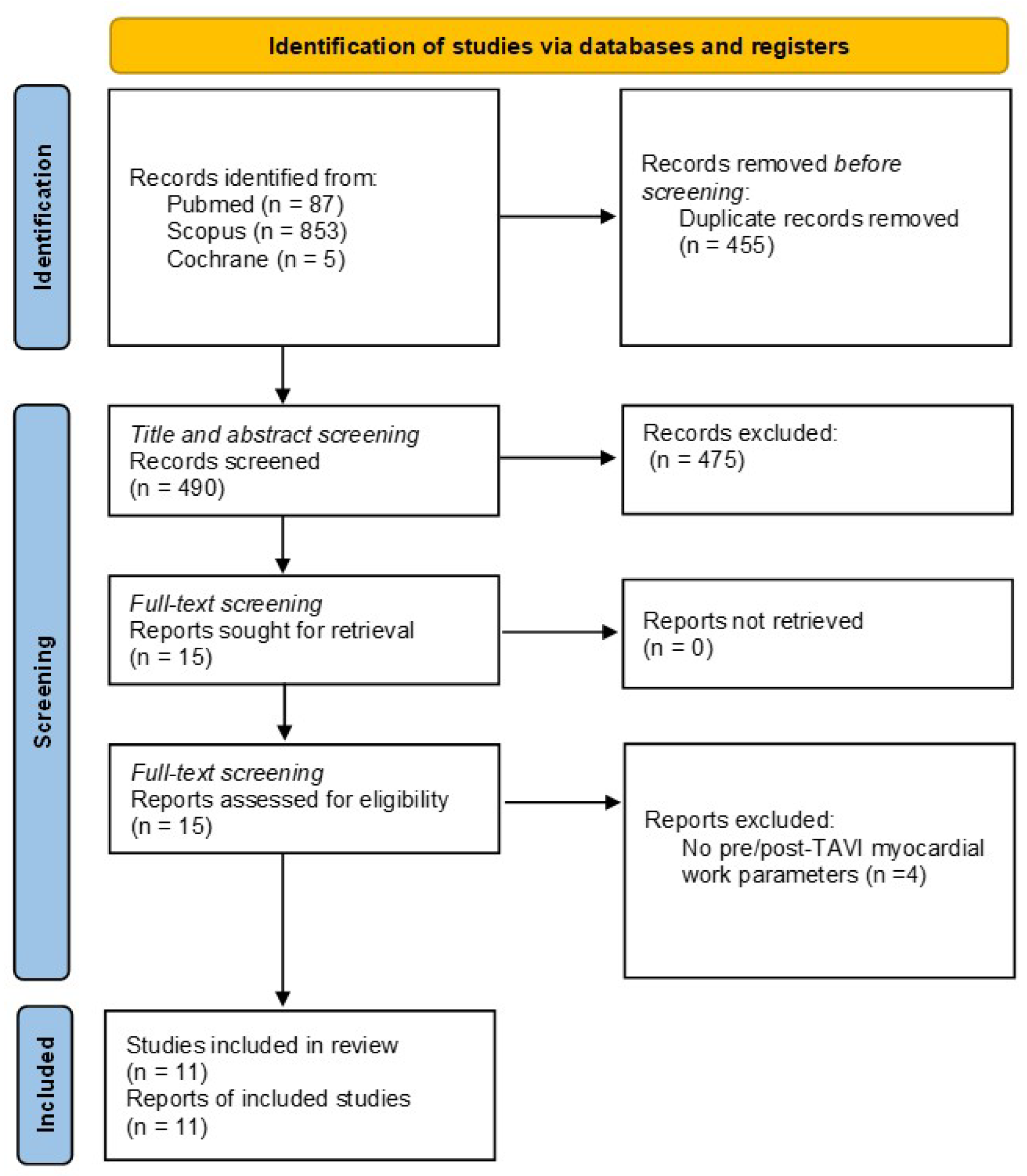
3.1. Literature Search
3.2. Study Characteristics
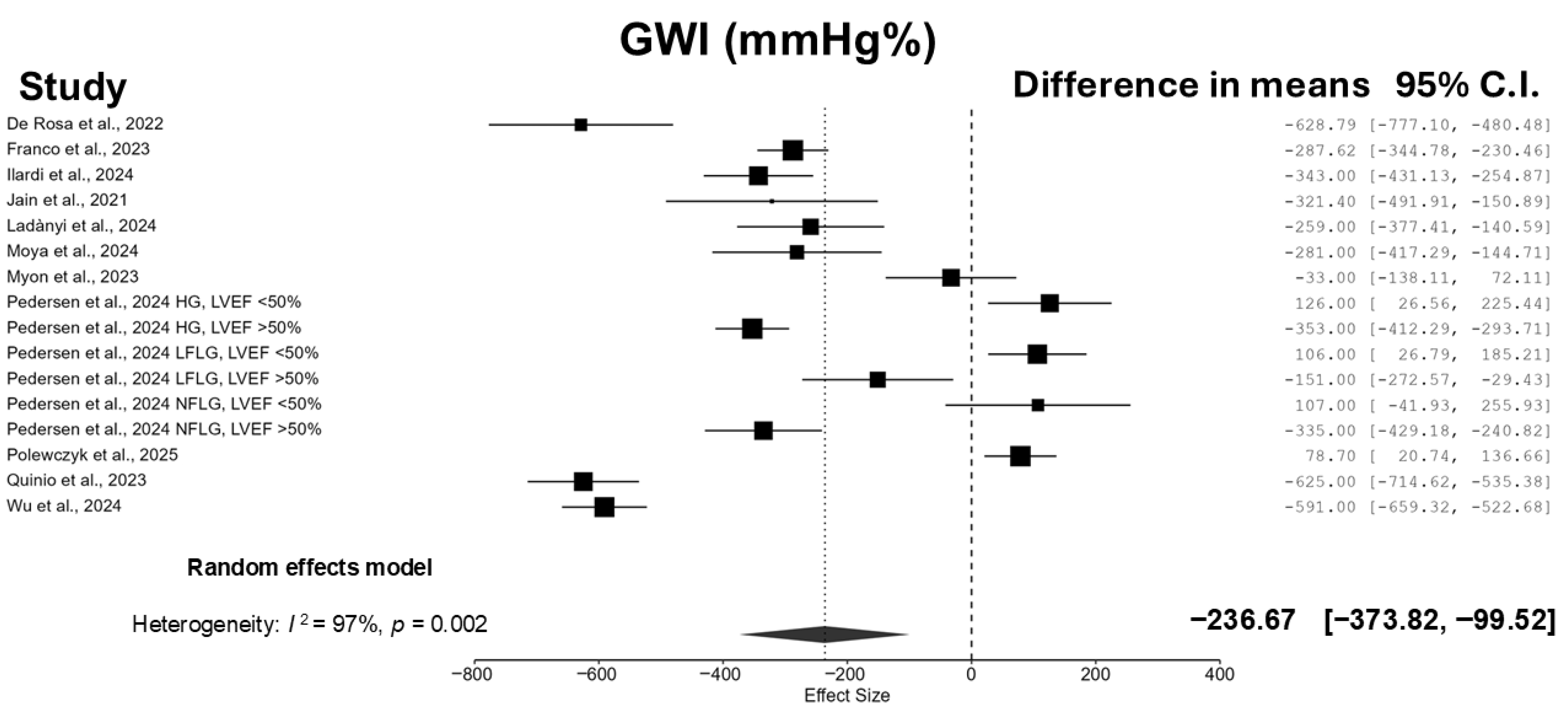
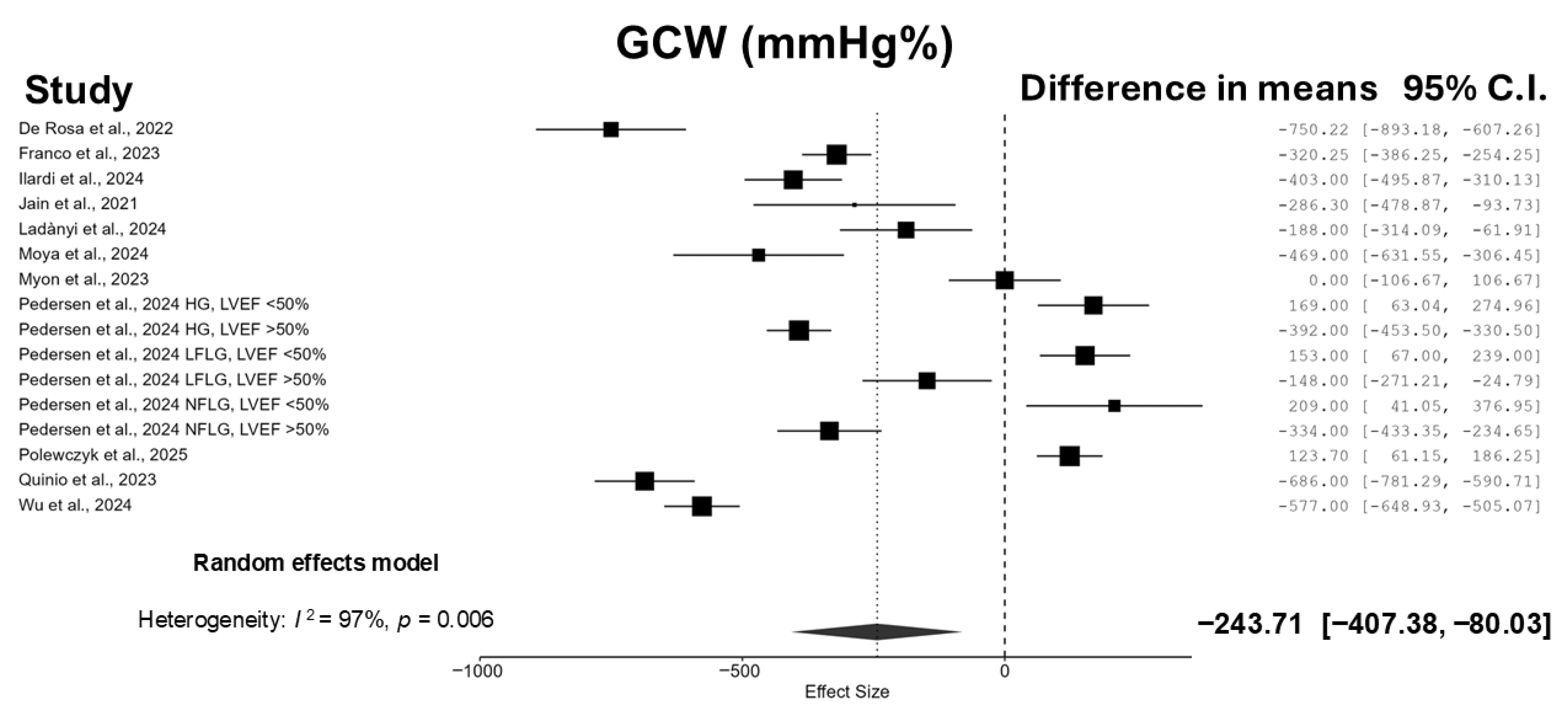
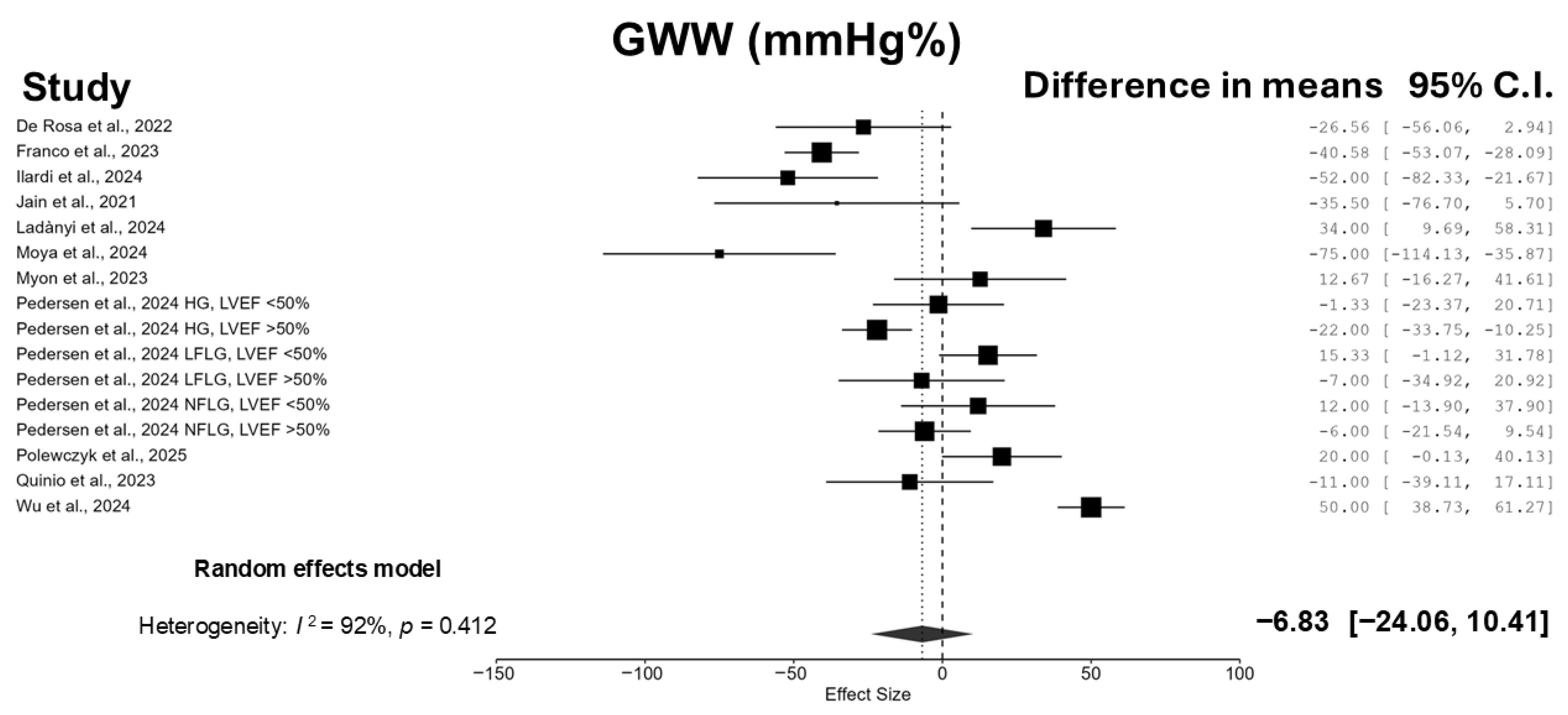
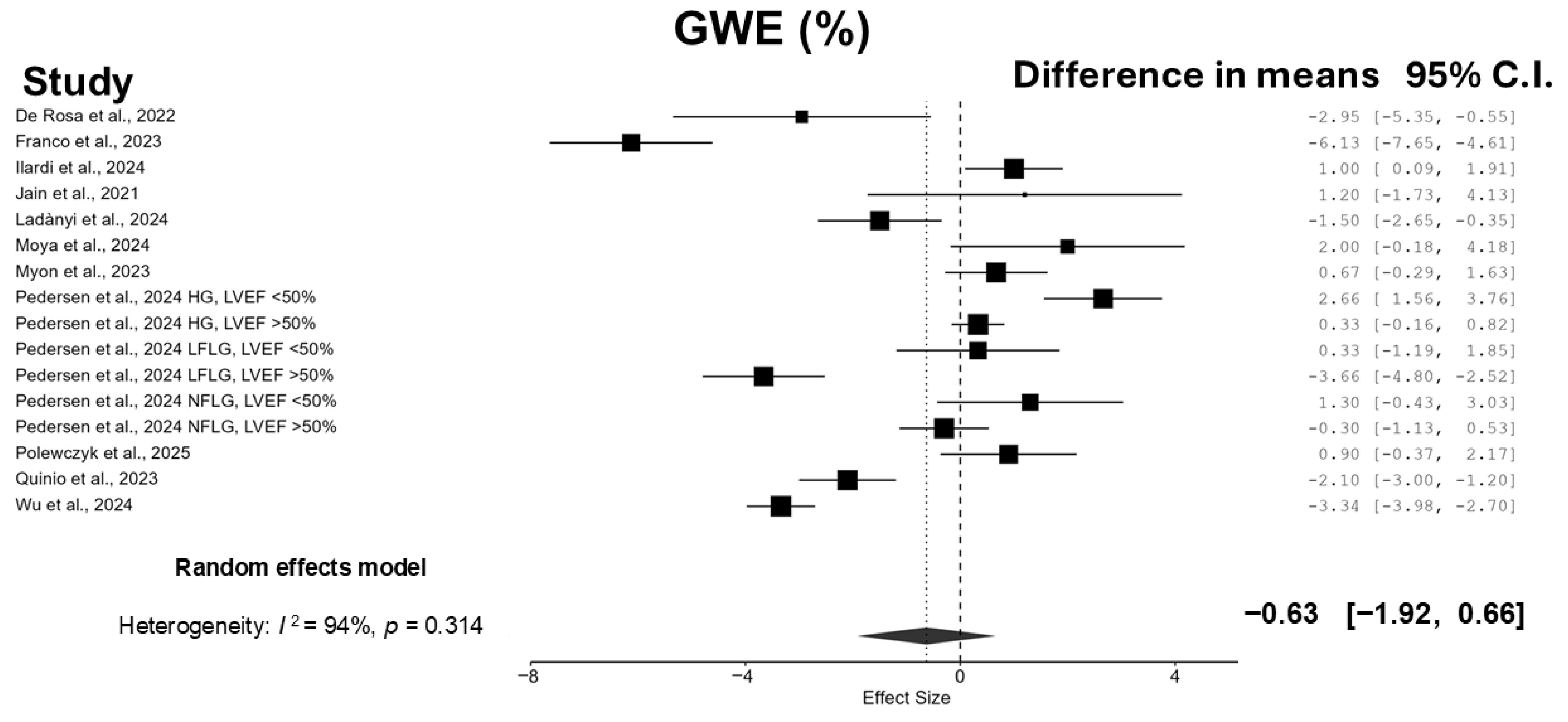
3.3. Meta-Analyses
3.4. Sensitivity Analysis
3.5. Meta-Regression Analyses
3.6. Publication Bias and Grading of Evidence
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| CI | Confidence interval |
| GCW | Global constructive work |
| GWE | Global work efficiency |
| GWI | Global work index |
| GWW | Global wasted work |
| HF | Heart failure |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| MW | Myocardial work |
| SD | Standard deviation |
| TAVI | Transcatheter aortic valve implantation |
References
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Rader, F.; Sachdev, E.; Arsanjani, R.; Siegel, R.J. Left Ventricular Hypertrophy in Valvular Aortic Stenosis: Mechanisms and Clinical Implications. Am. J. Med. 2015, 128, 344–352. [Google Scholar] [CrossRef]
- Masson, J.B.; Kovac, J.; Schuler, G.; Ye, J.; Cheung, A.; Kapadia, S.; Tuzcu, M.E.; Kodali, S.; Leon, M.B.; Webb, J.G. Transcatheter aortic valve implantation: Review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc. Interv. 2009, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Leo, I.; Strangio, A.; La Bella, S.; Sorrentino, S.; Mongiardo, A.; Spaccarotella, C.; Polimeni, A.; Indolfi, C. Early reduction of left atrial function predicts adverse clinical outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Open Heart 2021, 8, e001685. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Leo, I.; Strangio, A.; Spaccarotella, C.; Polimeni, A.; Sorrentino, S.; Di Salvo, G.; Indolfi, C. Prediction of Significant Coronary Artery Disease Through Advanced Echocardiography: Role of Non-invasive Myocardial Work. Front. Cardiovasc. Med. 2021, 8, 719603. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef]
- Higgins, J.P.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 143–176. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- De Rosa, S.; Sabatino, J.; Strangio, A.; Leo, I.; Romano, L.R.; Spaccarotella, C.A.; Mongiardo, A.; Polimeni, A.; Sorrentino, S.; Indolfi, C. Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis. J. Clin. Med. 2022, 11, 747. [Google Scholar] [CrossRef]
- Franco, D.; Santoro, A.; Gioia, G.D.; Ferrone, M.; Tramonte, S.; Salemme, L.; Cioppa, A.; Popusoi, G.; Pucciarelli, A.; Verdoliva, S.; et al. Assessing the impact of transcatheter aortic valve replacement on myocardial work indices and left ventricular diastolic function in aortic valve stenosis patients. Echocardiography 2023, 40, 768–774. [Google Scholar] [CrossRef]
- Ilardi, F.; Franzone, A.; Iapicca, C.; Manzo, R.; Angellotti, D.; Nappa, D.; Castiello, D.S.; Mariani, A.; Santoro, C.; Avvedimento, M.; et al. Changes and prognostic impact of noninvasive myocardial work indices in patients undergoing transcatheter aortic valve implantation. J. Cardiovasc. Med. 2024, 25, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Bajwa, T.; Roemer, S.; Huisheree, H.; Allaqaband, S.Q.; Kroboth, S.; Perez Moreno, A.C.; Tajik, A.J.; Khandheria, B.K. Myocardial work assessment in severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Ladányi, Z.; Bálint, T.; Fábián, A.; Ujvári, A.; Turschl, T.K.; Nagy, D.; Straub, É.; Fejér, C.; Zima, E.; Apor, A.; et al. Non-invasive myocardial work as an independent predictor of postprocedural NT-proBNP in elderly patients undergoing transcatheter aortic valve replacement. GeroScience 2024, 47, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; de Oliveira, E.K.; Delrue, L.; Beles, M.; Buytaert, D.; Goethals, M.; Verstreken, S.; Dierckx, R.; Bartunek, J.; Heggermont, W.; et al. Myocardial work and risk stratification in patients with severe aortic valve stenosis referred for transcatheter aortic valve replacement. Int. J. Cardiol. Heart Vasc. 2024, 53, 101474. [Google Scholar] [CrossRef]
- Myon, F.; Marut, B.; Kosmala, W.; Auffret, V.; Leurent, G.; L’Official, G.; Curtis, E.; Le Breton, H.; Oger, E.; Donal, E. Transcatheter aortic valve implantation in severe aortic stenosis does not necessarily reverse left ventricular myocardial damage: Data of long-term follow-up. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 821–828. [Google Scholar] [CrossRef]
- Pedersen, A.L.D.; Frederiksen, C.A.; Povlsen, J.A.; Ladefoged, B.T.; Mejren, A.H.J.; Terkelsen, C.J.; Poulsen, S.H. Changes and Prognostic Implications of Myocardial Work in Aortic Stenosis Subtypes Undergoing Transcatheter Valve Implantation. JACC Adv. 2024, 3, 101124. [Google Scholar] [CrossRef]
- Quinio, L.; Taconne, M.; Le Rolle, V.; Curtis, L.; Auffret, V.; Boulmier, D.; Leurent, G.; Le Breton, H.; Galli, E.; Oger, E.; et al. Evolution of non-invasive myocardial work variables after transcatheter aortic valve implantation in patients with severe aortic stenosis. Arch. Cardiovasc. Dis. 2023, 116, 192–201. [Google Scholar] [CrossRef]
- Wu, H.W.; Fortuni, F.; Muzafarova, T.; Sarrazyn, C.; Lopez Santi, M.P.; Chua, A.P.A.; Butcher, S.C.; Van Der Kley, F.; De Weger, A.; Jukema, J.W.; et al. Evolution and prognostic impact of left ventricular myocardial work indices after transcatheter aortic valve replacement in patients with severe aortic stenosis. Eur. Heart J. 2024, 45, e70006. [Google Scholar] [CrossRef]
- Polewczyk, A.; Pietrzyk, E.; Polewczyk, M.; Jarek, D.; Dudek, D. Usefulness of Echocardiographic Parameters of Myocardial Work in Patients with Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2025, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, A.; Albanese, M.; Salerno, N.; Aquila, I.; Sabatino, J.; Sorrentino, S.; Leo, I.; Cacia, M.; Signorile, V.; Mongiardo, A.; et al. Predictors of outcomes in patients with mitral regurgitation undergoing percutaneous valve repair. Sci. Rep. 2020, 10, 17144. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
- Hirt, M.N.; Sörensen, N.A.; Bartholdt, L.M.; Boeddinghaus, J.; Schaaf, S.; Eder, A.; Vollert, I.; Stöhr, A.; Schulze, T.; Witten, A.; et al. Increased afterload induces pathological cardiac hypertrophy: A new in vitro model. Basic. Res. Cardiol. 2012, 107, 307. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Ajmone Marsan, N.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2025, ehaf194. [Google Scholar] [CrossRef]
- Magne, J.; Cosyns, B.; Popescu, B.A.; Carstensen, H.G.; Dahl, J.; Desai, M.Y.; Kearney, L.; Lancellotti, P.; Marwick, T.H.; Sato, K.; et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis: An Individual Participant Data Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 84–92. [Google Scholar] [CrossRef]
- Angelillis, M.; Giannini, C.; De Carlo, M.; Adamo, M.; Nardi, M.; Colombo, A.; Chieffo, A.; Bedogni, F.; Brambilla, N.; Tamburino, C.; et al. Prognostic Significance of Change in the Left Ventricular Ejection Fraction After Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis and Left Ventricular Dysfunction. Am. J. Cardiol. 2017, 120, 1639–1647. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Nakase, M.; Tomii, D.; Heg, D.; Praz, F.; Stortecky, S.; Reineke, D.; Samim, D.; Lanz, J.; Windecker, S.; Pilgrim, T. Long-Term Impact of Cardiac Damage Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2024, 17, 992–1003. [Google Scholar] [CrossRef]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef]
- Fortuni, F.; Butcher, S.C.; van der Kley, F.; Lustosa, R.P.; Karalis, I.; de Weger, A.; Priori, S.G.; van der Bijl, P.; Bax, J.J.; Delgado, V.; et al. Left Ventricular Myocardial Work in Patients with Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 257–266. [Google Scholar] [CrossRef]
- Ilardi, F.; Postolache, A.; Dulgheru, R.; Trung, M.N.; de Marneffe, N.; Sugimoto, T.; Go, Y.Y.; Oury, C.; Esposito, G.; Lancellotti, P. Prognostic Value of Non-Invasive Global Myocardial Work in Asymptomatic Aortic Stenosis. J. Clin. Med. 2022, 11, 1555. [Google Scholar] [CrossRef]
- Sabatino, J.; De Rosa, S.; Leo, I.; Spaccarotella, C.; Mongiardo, A.; Polimeni, A.; Sorrentino, S.; Di Salvo, G.; Indolfi, C. Non-invasive myocardial work is reduced during transient acute coronary occlusion. PLoS ONE 2020, 15, e0244397. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Nussbaum, S.; Winkler, N.E.; Benz, D.C.; Zuercher, D.; Donati, T.G.; Tsiourantani, G.; Wilzeck, V.; Michel, J.M.; Kasel, A.M.; et al. Left ventricular global work index and prediction of cardiovascular mortality after transcatheter aortic valve implantation. Int. J. Cardiol. 2024, 399, 131660. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Bax, J.J.; Zhao, Y.; Makkar, R.R.; Kapadia, S.; Thourani, V.H.; Mack, M.J.; Nazif, T.M.; et al. Evolution and Prognostic Impact of Cardiac Damage After Aortic Valve Replacement. J. Am. Coll. Cardiol. 2022, 80, 783–800. [Google Scholar] [CrossRef]
- Paolisso, P.; Belmonte, M.; Gallinoro, E.; Scarsini, R.; Bergamaschi, L.; Portolan, L.; Armillotta, M.; Esposito, G.; Moscarella, E.; Benfari, G.; et al. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc. Diabetol. 2024, 23, 420. [Google Scholar] [CrossRef]

| Study | Sample Size | Age (y) | Male n (%) | Hypertension n (%) | Diabetes n (%) | Dyslipidemia n (%) | eGFR at Baseline (mL/min/1.73 m2) | Clinically Relevant CAD n (%) | Euro-Score II | Mean Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| De Rosa et al. [12], 2022 | 73 | 80.8 ± 6 | 39 (54%) | 69 (94.5%) | 17 (23%) | 45(62%) | N/A | 14 (19%) | N/A | 24 months |
| Franco et al. [13], 2023 | 53 | 80.15 ± 2.8 | 21 (40%) | 53 (100%) | 26 (49%) | 53 (100%) | N/A | 30 (57%) | N/A | 6 days |
| Ilardi et al. [14], 2024 | 95 | 79.9 ± 6.4 | 35 (40%) | 80 (90.9%) | 23 (26%) | 51 (58%) | N/A | 29 (33%) | 5.3 ± 6.0 | 12 months |
| Jain et al. [15], 2021 | 35 | 81 ± 7.6 | 18 (51%) | 27 (77%) | 10 (29%) | 24 (69%) | N/A | 24 (69%) | N/A | N/A |
| Ladányi et al. [16], 2024 | 90 | 80 [75–84] | 50 (56%) | 87 (97%) | 34 (38%) | N/A | N/A | 43 (48%) | 11.5 [7.2–18.1] | 12 months |
| Moya et al. [17], 2024 | 110 | 83 ± 6 | 51 (46%) | 67 (62%) | 33 (31%) | 49 (45%) | N/A | N/A | N/A | 521 days |
| Myon et al. [18], 2023 | 102 | 84.3 ± 6.2 | 56 (55%) | 69 (68%) | 12 (12%) | 53 (52%) | 83.0 [35.8] | N/A | N/A | 22 months |
| Pedersen et al. [19], 2024 HG, LVEF > 50% | 169 | 80 ± 6 | N/A | N/A | N/A | N/A | 68 [51–82] | N/A | 2.2 [1.6–3.3] | 60 months |
| Pedersen et al. [19], 2024 HG, LVEF < 50% | 60 | 80 ± 4 | N/A | N/A | N/A | N/A | 60 [42–75] | N/A | 3.2 [2.1–6.0] | 60 months |
| Pedersen et al. [19], 2024 LFLG, LVEF < 50% | 82 | 79 ± 8 | N/A | N/A | N/A | N/A | 56 [46–67] | N/A | 4.6 [2.2–8.2] | 60 months |
| Pedersen et al. [19], 2024 LFLG, LVEF > 50% | 45 | 80 ± 7 | N/A | N/A | N/A | N/A | 62 [51–75] | N/A | 2.2 [1.6–3.8] | 60 months |
| Pedersen et al. [19], 2024 NFLG, LVEF < 50% | 34 | 80 ± 7 | N/A | N/A | N/A | N/A | 64 [46–74] | N/A | 3.7 [2.4–8.3] | 60 months |
| Pedersen et al. [19], 2024 NFLG, LVEF > 50% | 83 | 78 ± 9 | N/A | N/A | N/A | N/A | 72 [60–85] | N/A | 2.2 [1.7–3.9] | 60 months |
| Polewczyk et al. [22], 2025 | 135 | 79 ± 7 | 69 (51%) | 86 (64%) | 49 (36%) | N/A | N/A | 86 (64%) | N/A | 6 days |
| Quinio et al. [20], 2023 | 125 | N/A | 40 (59%) | N/A | N/A | N/A | N/A | 66 (53%) | N/A | 7 days |
| Wu et al. [21], 2024 | 255 | 82 [77–85] | 130 (51%) | 177 (69%) | 70 (28%) | 133 (52%) | 61 ± 22 | 141(55%) | N/A | 59 months |
| Study | LVEDVi (mL/m2) | LVEF (%) | LVMi (g/m2) | LAVi (mL/m2) | AV Mean Gradient (mmHg) | AVA (cm2) | LVGLS (%) | GWI (mmHg%) | GCW (mmHg%) | GWW (mmHg%) | GWE (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Rosa et al. [12], 2022 | N/A | 52.8 ±9.3 | N/A | 43.8 ± 8.4 | 46.7± 15.8 | 0.78 ± 0.16 | −16.8 ± 5.0 | 2288.5 ± 905.2 | 2713.3 ± 114.9 | 205.0 ± 179.8 | 90.5 ± 6.7 |
| Franco et al. [13], 2023 | N/A | 47.7 ± 9.1 | N/A | N/A | 57.14 ± 12.63 | 0.38 ± 0.9 * | N/A | 2139.7 ± 278.5 | 2367.17 ± 318.57 | 286.47 ± 60.79 | 100.5 ± 14.7 |
| Ilardi et al. [14], 2024 | 37.9 ± 17.9 | 58.2 ± 8.5 | 55.4 ± 15.9 | 47 ± 14.3 | 49.3 ± 12.5 | 0.7 ± 0.2 | 18.4 ± 4.2 | 2406 ± 567 | 2783 ± 616 | 238 ± 203 | 90 ± 6 |
| Jain et al. [15], 2021 | N/A | 58.2 ± 15.7 | 115.4 ± 37.0 | 50 ± 17.9 | 38.4 ± 12.4 | 0.8 ± 0.2 | −14.2 ± 4.3 | 1425.7 ± 595.8 | 1586.2 ± 705.1 | 133.5 ± 109.3 | 88.9 ± 11.9 |
| Ladányi et al. [16], 2024 | 60.1 ± 21.9 | 52.6 ± 13.1 | 124.91 ± 37.34 | 45.1 ± 17.2 | 45.6 ± 19.9 | 0.71 ± 0.25 | −13.5 ± 4.6 | 1913 ± 799 | 2365 ± 851 | 260 ± 158 | 88.2 ± 7.1 |
| Moya et al. [17], 2024 | N/A | 51 ± 10 | N/A | 45 ± 17 | 50 ± 15 | 0.6 ± 0.3 | −15.7 ± 5.8 | 2381 ± 785 | 2940 ± 942 | 239 ± 191 | 91± 6 |
| Myon et al. [18], 2023 | 58.47 ± 23.42 | 63 [54–70] | 111.5 [92.2–139.0] | 46.0 [40.2–60.0] | 52.29 ± 16.72 | 0.72 [0.57–0.83] | −14.11 ± 3.88 | 2099 ± 692 | 2463 ± 736 | 214 [149–357] | 87.0 ± 5.66 |
| Pedersen et al. [19], 2024 HG, LVEF > 50% | N/A | 59 ± 6 | N/A | 41 [32–50] | 51 [45–61] | 0.4 ± 0.1 * | −13.6 ± 3.9 | 2396 ± 521 | 2723 ± 550 | 167 [110–254] | 91 [89–94] |
| Pedersen et al. [19], 2024 HG, LVEF < 50% | N/A | 48 ± 8 | N/A | 42 [33–53] | 50 [44–64] | 0.3 ± 0.1 * | −9.2 ± 3.5 | 1577 ± 494 | 1853 ± 536 | 168 [118–252] | 87 [84–92] |
| Pedersen et al. [19], 2024 LFLG, LVEF < 50% | N/A | 43 ± 10 | N/A | 47 [36–60] | 28 [21–32] | 0.4 ± 0.1 * | −10.6 ± 3.3 | 1171 ± 503 | 1407 ± 533 | 155 [108–207] | 87 [81–90] |
| Pedersen et al. [19], 2024 LFLG, LVEF > 50% | N/A | 57 ± 6 | N/A | 45 [30–59] | 30 [25–36] | 0.3 ± 0.1 * | −13.6 ± 3.9 | 1850 ± 581 | 2190 ± 590 | 188 [113–263] | 90 [87–93] |
| Pedersen et al. [19], 2024 NFLG, LVEF > 50% | N/A | 59 ± 6 | N/A | 34 [27–48] | 33 [27–37] | 0.5 ± 0.1 * | −15.8 ± 3.3 | 2275 ± 576 | 2555 ± 590 | 143 [98–216] | 92 [89–95] |
| Pedersen et al. [19], 2024 LFLG, LVEF < 50% | N/A | 40 ± 7 | N/A | 36 [28–47] | 33 [25–36] | 0.4 ± 0.1 * | −10.6 ± 3.3 | 1468 ± 572 | 1707 ± 648 | 155 [114–237] | 88 [84–93] |
| Polewczyk et al. [22], 2025 | N/A | 49.5 ± 12.8 | N/A | N/A | N/A | N/A | −10.5 ± 3.8 | 966.5 ± 456.1 | 1315.5 ± 487.5 | 238.8 ± 160.7 | 81.6 ± 10.5 |
| Quinio et al. [20], 2023 | N/A | 59.8 ± 13.3 | 111 [IQR 46.0] | 46.0 [IQR 19.9] | 52.5 ± 16.1 | N/A | −14.0 ± 3.90 | 2014 ± 714 | 2379 ± 761 | 282 ± 175 | 87.1 ± 5.98 |
| Wu et al. [21], 2024 | N/A | 56 [45–63] | N/A | N/A | 43 [33–53] | 0.7 [0.6–0.8] | −13.6 [−11.0–16.1] | 1882 ± 767 | 2248 ± 809 | 247 [128–300] | 89 [84–93] |
| Certainty Assessment | Certainty | ||||||
|---|---|---|---|---|---|---|---|
| Number of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | |
| GWI | |||||||
| 11 | Retrospective and prospective cohort studies | Serious concern | Serious concern | Serious concern | Some concern | No publication bias | Very low |
| GWE | |||||||
| 11 | Retrospective and prospective cohort studies | Serious concern | Serious concern | Serious concern | Some concern | No publication bias | Very low |
| GCW | |||||||
| 11 | Retrospective and prospective cohort studies | Serious concern | Serious concern | Serious concern | Some concern | No publication bias | Very low |
| GWW | |||||||
| 11 | Retrospective and prospective cohort studies | Serious concern | Serious concern | Serious concern | Some concern | No publication bias | Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, I.; Sicilia, F.; Sabatino, J.; Cersosimo, A.; Carabetta, N.; Strangio, A.; Panuccio, G.; Canino, G.; Ielapi, J.; Salerno, N.; et al. Effect of Transcatheter Aortic Valve Implantation on Non-Invasive Myocardial Work Parameters: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6997. https://doi.org/10.3390/jcm14196997
Leo I, Sicilia F, Sabatino J, Cersosimo A, Carabetta N, Strangio A, Panuccio G, Canino G, Ielapi J, Salerno N, et al. Effect of Transcatheter Aortic Valve Implantation on Non-Invasive Myocardial Work Parameters: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(19):6997. https://doi.org/10.3390/jcm14196997
Chicago/Turabian StyleLeo, Isabella, Federico Sicilia, Jolanda Sabatino, Angelica Cersosimo, Nicole Carabetta, Antonio Strangio, Giuseppe Panuccio, Giovanni Canino, Jessica Ielapi, Nadia Salerno, and et al. 2025. "Effect of Transcatheter Aortic Valve Implantation on Non-Invasive Myocardial Work Parameters: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 19: 6997. https://doi.org/10.3390/jcm14196997
APA StyleLeo, I., Sicilia, F., Sabatino, J., Cersosimo, A., Carabetta, N., Strangio, A., Panuccio, G., Canino, G., Ielapi, J., Salerno, N., Sorrentino, S., Torella, D., & De Rosa, S. (2025). Effect of Transcatheter Aortic Valve Implantation on Non-Invasive Myocardial Work Parameters: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(19), 6997. https://doi.org/10.3390/jcm14196997







