Comparative Impact of Coronary Imaging Strategies in CTO-PCI: A Retrospective Single-Center Analysis
Abstract
1. Introduction
2. Methods
2.1. Imaging Guidance Definitions
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brilakis, E.S.; Sandoval, Y.; Azzalini, L.; Leibundgut, G.; Garbo, R.; Hall, A.B.; Davies, R.E.; Mashayekhi, K.; Yamane, M.; Avran, A.; et al. Chronic Total Occlusion Percutaneous Coronary Intervention: Present and Future. Circ. Cardiovasc. Interv. 2025, 18, e014801. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Abi Rafeh, N.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019, 140, 420–433. [Google Scholar] [CrossRef]
- Campo, G.; Guiducci, V.; Escaned, J.; Moreno, R.; Casella, G.; Cavazza, C.; Cerrato, E.; Contarini, M.; Arena, M.; Iniguez Romo, A.; et al. Health-Status Outcomes in Older Patients with Myocardial Infarction: Physiology-Guided Complete Revascularization Versus Culprit-Only Strategy. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e010490. [Google Scholar] [CrossRef] [PubMed]
- Erriquez, A.; Campo, G.; Guiducci, V.; Escaned, J.; Moreno, R.; Casella, G.; Menozzi, M.; Cerrato, E.; Sacchetta, G.; Menozzi, A.; et al. Complete vs Culprit-Only Revascularization in Older Patients with Myocardial Infarction and High Bleeding Risk: A Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; Salerno, N.; De Rosa, S.; Torella, D. Timing of Complete Revascularization in Patients with STEMI and Multivessel Disease: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2023, 24, 58. [Google Scholar] [CrossRef]
- Panuccio, G.; Carabetta, N.; Torella, D.; De Rosa, S. Clinical impact of coronary revascularization over medical treatment in chronic coronary syndromes: A systematic review and meta-analysis. Hell. J. Cardiol. HJC Hell. Kardiologike Epitheorese 2024, 78, 60–71. [Google Scholar] [CrossRef]
- Panuccio, G.; Carabetta, N.; Torella, D.; De Rosa, S. Percutaneous coronary revascularization versus medical therapy in chronic coronary syndromes: An updated meta-analysis of randomized controlled trials. Eur. J. Clin. Investig. 2024, 54, e14303. [Google Scholar] [CrossRef]
- Gondi, K.T.; Goyal, A.; Kane, J.; Allana, S.S. Preprocedural Planning for Chronic Total Occlusion Percutaneous Coronary Intervention. Am. J. Cardiol. 2024, 233, 83–95. [Google Scholar] [CrossRef]
- Kovacic, M.; Cocoi, M.; Leibundgut, G. Equipment for Chronic Total Occlusions Percutaneous Coronary Intervention: Present and Future. Am. J. Cardiol. 2024, 232, 89–104. [Google Scholar] [CrossRef]
- Vadalà, G.; Galassi, A.R.; Werner, G.S.; Sianos, G.; Boudou, N.; Garbo, R.; Maniscalco, L.; Bufe, A.; Avran, A.; Gasparini, G.L.; et al. Contemporary outcomes of chronic total occlusion percutaneous coronary intervention in Europe: The ERCTO registry. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2024, 20, e185–e197. [Google Scholar] [CrossRef]
- Lefèvre, T.; Pan, M.; Stankovic, G.; Ojeda, S.; Boudou, N.; Brilakis, E.S.; Sianos, G.; Vadalà, G.; Galassi, A.R.; Garbo, R.; et al. CTO and Bifurcation Lesions: An Expert Consensus from the European Bifurcation Club and EuroCTO Club. JACC Cardiovasc. Interv. 2023, 16, 2065–2082. [Google Scholar] [CrossRef]
- Gomes, W.F.; Zerlotto, D.S.; Viana, P.; Lucena, L.A.; Carvalho, P.E.P.; Nicz, P.F.G.; Nercolini, D.C.; Ribeiro, M.H.; Quadros, A.S.; Bueno, R.R.L.; et al. Intravascular Imaging Improves Clinical Outcomes of Percutaneous Coronary Intervention for Chronic Total Occlusions: A Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiol. 2025, 245, 62–70. [Google Scholar] [CrossRef]
- Panuccio, G.; Abdelwahed, Y.S.; Carabetta, N.; Salerno, N.; Leistner, D.M.; Landmesser, U.; De Rosa, S.; Torella, D.; Werner, G.S. Clinical and Procedural Outcomes of IVUS-Guided vs. Angiography-Guided CTO-PCI: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4947. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Pavlidis, A.N.; Kaier, T.E.; Rigopoulos, A.G.; Karamasis, G.V.; Triantafyllis, A.S.; Vardas, P.; Brilakis, E.S.; Kalogeropoulos, A.S. The role of intravascular imaging in chronic total occlusion percutaneous coronary intervention. Front. Cardiovasc. Med. 2023, 10, 1199067. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; Abdelwahed, Y.S.; Carabetta, N.; Landmesser, U.; De Rosa, S.; Torella, D. The Role of Coronary Imaging in Chronic Total Occlusions: Applications and Future Possibilities. J. Cardiovasc. Dev. Dis. 2024, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S. Use of Coronary Computed Tomographic Angiography to Facilitate Percutaneous Coronary Intervention of Chronic Total Occlusions. Circ. Cardiovasc. Interv. 2019, 12, e007387. [Google Scholar] [CrossRef]
- Panuccio, G.; Werner, G.S.; De Rosa, S.; Torella, D.; Leistner, D.M.; Siegrist, P.T.; Haghikia, A.; Skurk, C.; Mashayekhi, K.; Landmesser, U.; et al. Full-Moon Coronary Calcification as Detected with Computed Tomography Angiography in Chronic Total Occlusion Percutaneous Coronary Intervention. Am. J. Cardiol. 2024, 222, 149–156. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef]
- Galassi, A.R.; Werner, G.S.; Boukhris, M.; Azzalini, L.; Mashayekhi, K.; Carlino, M.; Avran, A.; Konstantinidis, N.V.; Grancini, L.; Bryniarski, L.; et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2019, 15, 198–208. [Google Scholar] [CrossRef]
- Ybarra, L.F.; Rinfret, S.; Brilakis, E.S.; Karmpaliotis, D.; Azzalini, L.; Grantham, J.A.; Kandzari, D.E.; Mashayekhi, K.; Spratt, J.C.; Wijeysundera, H.C.; et al. Definitions and Clinical Trial Design Principles for Coronary Artery Chronic Total Occlusion Therapies: CTO-ARC Consensus Recommendations. Circulation 2021, 143, 479–500. [Google Scholar] [CrossRef]
- Vadalà, G.; Mashayekhi, K.; Behnes, M.; Ayoub, M.; Gorgulu, S.; Werner, G.S.; Kalay, N.; Avran, A.; Goktekin, O.; Garbo, R.; et al. Procedural Impact of Advanced Calcific Plaque Modification Devices Within Percutaneous Revascularization of Chronic Total Occlusions. JACC Cardiovasc. Interv. 2025, 18, 1376–1390. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, S.Y.; Kwon, W.; Lee, S.-J.; Lee, J.-Y.; Lee, S.H.; Shin, D.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; et al. Intravascular Imaging Predictors Associated with Cardiovascular Events After Complex PCIs. Circ. Cardiovasc. Interv. 2025, 18, e014920. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, K.H.; Kim, C.J.; Lee, J.M.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; et al. Impact of Intravascular Imaging-Guided Stent Optimization According to Clinical Presentation in Patients Undergoing Complex PCI. JACC Cardiovasc. Interv. 2024, 17, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, K.; Okutsu, M. Cardiac Computed Tomography for Success in Percutaneous Coronary Intervention for Chronic Total Occlusion. JACC Cardiovasc. Imaging 2022, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Danenberg, H. Preprocedural Coronary CT Angiography Effect on the Likelihood to Restore Flow in Chronic Total Occlusion. JACC Cardiovasc. Imaging 2021, 14, 2005–2007. [Google Scholar] [CrossRef]
- Duchnowski, P.; Śmigielski, W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Kardiol. Pol. 2024, 82, 423–426. [Google Scholar] [CrossRef]
- Werner, G.S.; Avran, A.; Boudou, N.; Galassi, A.R.; Garbo, R.; Bufe, A.; Bryniarski, L.; Christiansen, E.H.; Kalnins, A.; Lismanis, A.; et al. Improvement of Radiation Management in Percutaneous Interventions of Chronic Total Occlusions in a Multicenter Registry. JACC Cardiovasc. Interv. 2025, 18, 425–435. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, B.-K.; Shin, D.-H.; Nam, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; Kang, T.-S.; Kang, W.-C.; Her, A.-Y.; et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JAMA 2015, 314, 2155–2163. [Google Scholar] [CrossRef]
- Kim, B.-K.; Shin, D.-H.; Hong, M.-K.; Park, H.S.; Rha, S.-W.; Mintz, G.S.; Kim, J.-S.; Kim, J.S.; Lee, S.-J.; Kim, H.-Y.; et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention with Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ. Cardiovasc. Interv. 2015, 8, e002592. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, B.-K.; Cho, I.; Kim, H.-Y.; Rha, S.-W.; Lee, S.-H.; Park, S.M.; Kim, Y.H.; Chang, H.-J.; Ahn, C.-M.; et al. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention: A Randomized Trial. JACC Cardiovasc. Imaging 2021, 14, 1993–2004. [Google Scholar] [CrossRef]


| Baseline Characteristics | All N = 171 | Angiography-Guided N = 48 | IVUS-Guided N = 42 | CCTA-Guided N = 40 | CT+ IVUS-Guided N = 41 | p Value |
|---|---|---|---|---|---|---|
| Age | 68 [60–76] | 70.5 [61.2–78] | 71.5 [63.7–79] | 67 (59–74) | 67.0 [59.5–73] | 0.13 |
| Male sex | 130 (76) | 33 (68.7) | 33 (78.5) | 33 (82.5) | 31 (75.6) | 0.48 |
| Hypertension | 118 (69)) | 33 (68.7) | 29 (69.0) | 27 (67.5) | 29 (70.7) | 0.54 |
| Dyslipidemia | 122 (71.3) | 35 (72.9) | 31 (73.8) | 26 (65) | 30 (73.1) | 0.63 |
| Diabetes | 60 (35) | 11 (22.9) | 19 (45.2) | 16 (40) | 14 (34.1) | 0.16 |
| Smoking | 48 (28) | 13 (27.0) | 8 (19.0) | 14 (35) | 13 (31.7) | 0.35 |
| Peripheral artery disease (PAD) | 14 (8) | 1 (2.0) | 4 (9.5) | 5 (12.5) | 4 (9.7) | 0.24 |
| COPD | 12 (7.0) | 3 (6.2) | 3 (7.1) | 5 (12.5) | 1 (2.4) | 0.59 |
| Chronic kidney disease (CKD) | 22 (12.9) | 6 (12.5) | 6 (14.2) | 6 (15) | 4 (9.7) | 0.52 |
| Prior stroke | 7 (4) | 1 (2.0) | 3 (7.1) | 2 (5) | 1 (2.4) | 0.39 |
| Previous MI | 59 (34.5) | 19 (39.5) | 16 (38.0) | 12 (30) | 12 (29.2) | 0.50 |
| Previous PCI | 66 (38.5) | 16 (33.3) | 22 (52.3) | 14 (35) | 14 (34.1) | 0.08 |
| LVEF | 51.50 [40–60] | 55 [43.75–60] | 53 [36.25–63] | 52 (43–60) | 47 [40–55] | 0.56 |
| Prior CABG | 38 (22.2) | 4 (8.3) | 6 (14.2) | 14 (35) | 14 (34.1) | 0.009 |
| Syntax score | 24 [18–31] | 20 [15–26] | 23 [18–29] | 27 [21–33] | 29 [23–26] | 0.006 |
| CTO Artery | ||||||
| Right coronary artery | 92 (53.8) | 25 (52.0) | 17 (40.4) | 22 (55) | 28 (68.2) | 0.18 |
| Left anterior descending artery | 39 (22.8) | 10 (20.8) | 15 (35.7) | 8 (20) | 6 (14.6) | 0.89 |
| Left circumflex | 37 (21.6) | 12 (25.0) | 8 (19.0) | 10 (25) | 7 (17.0) | 0.13 |
| CTO location | ||||||
| Ostial | 23 (13.4) | 7 (14.5) | 6 (14.2) | 7 (17.5) | 3 (7.3) | 0.05 |
| Proximal | 91 (53.21) | 28 (58.3) | 16 (38.0) | 21 (52.5) | 26 (63.4) | 0.16 |
| Mid | 45 (26.3) | 10 (20.8) | 16 (38.1) | 10 (25) | 9 (22) | 0.23 |
| Distal | 12 (7) | 3 (6.2) | 4 (9.5) | 2 (5) | 3 (7.3) | 0.45 |
| In-stent CTO | 35 (20.4) | 7 (14.5) | 14 (33.3) | 9 (22.5) | 5 (12.1) | 0.06 |
| Bifurcation involvement | 38 (22.2) | 7 (14.5) | 13 (30.9) | 12 (30) | 6 (14.6) | 0.56 |
| Stump morphology | 66 (38.5) | 15 (31.2) | 15 (35.7) | 14 (35) | 22 (53.6) | 0.08 |
| Calcification | 99 (57.8) | 24 (50.0) | 24 (57.1) | 24 (60) | 27 (65.8) | 0.13 |
| Previous attempts | 13 (7.6) | 1(2) | 3 (7) | 4 (10) | 5 (12.1) | 0.56 |
| Radial access | 161 (94.1) | 46 (95.8) | 39 (92.8) | 37 (92.5) | 39 (95.1) | 0.25 |
| Contralateral injection | 50 (29.2) | 11 (22.9) | 10 (23.8) | 13 (32.5) | 16 (39.0) | 0.29 |
| Dual antiplatelet therapy | 171 (100) | 48 (100) | 42 (100) | 40 (100) | 41 (100) | 1.0 |
| IVUS | 83 (48.53) | 0 | 42 (100) | 0 | 41 (100) | 0.42 |
| Antegrade recanalization | 166 (97) | 46 (95.8) | 42 (100) | 38 (95) | 40 (97.6) | 0.40 |
| Antegrade dissection and re-entry | 17 (9.9) | 5 (10.4) | 1 (2.3) | 2 (5) | 9 (21.9) | 0.01 |
| Parallel wire | 10 (5.8) | 3 (6.2) | 1 (2.3) | 5 (12.5) | 1 (2.4) | 0.15 |
| Retrograde recanalization | 6 (3.5) | 0 | 1 (2.3) | 3 (7.5) | 2 (4.9) | 0.30 |
| J-CTO score | 1.7 ± 1.1 | 1.38 ± 0.9 | 1.64 ± 1.1 | 1.98 ± 1.1 | 1.95 ± 1.3 | 0.03 |
| EURO-CTO score | 2.2 ± 1.2 | 1.95 ± 1.0 | 2.12 ± 1.3 | 2.33 ± 1.1 | 2.31 ± 1.2 | 0.65 |
| KCCT score | 3.05 ± 1.2 | 2.93 ± 1.1 | 3.20 ± 1.4 | 0.35 | ||
| CT-RECTOR score | 1.87 ± 0.8 | 1.70 ± 0.7 | 2.06 ± 0.9 | 0.06 |
| Outcome | All N = 171 | Angiography-Guided | IVUS-Guided | CCTA-Guided | CCTA + IVUS-Guided | p Value |
|---|---|---|---|---|---|---|
| Procedural time | 115 [90–150] | 90 [78.25–123.75] | 115 [90–141.25] | 120 [90–160] | 131 [105–173] | <0.001 |
| Fluoroscopic time | 30 [20.0–44.0] | 22.5 [16–39] | 31.5 [20.75–51] | 33 [22–60] | 36 [25–50.5] | 0.007 |
| Contrast volume | 200 [169–244.25] | 190 [141–210] | 215 [180–249.25] | 200 [160–249] | 205 [160–249] | 0.054 |
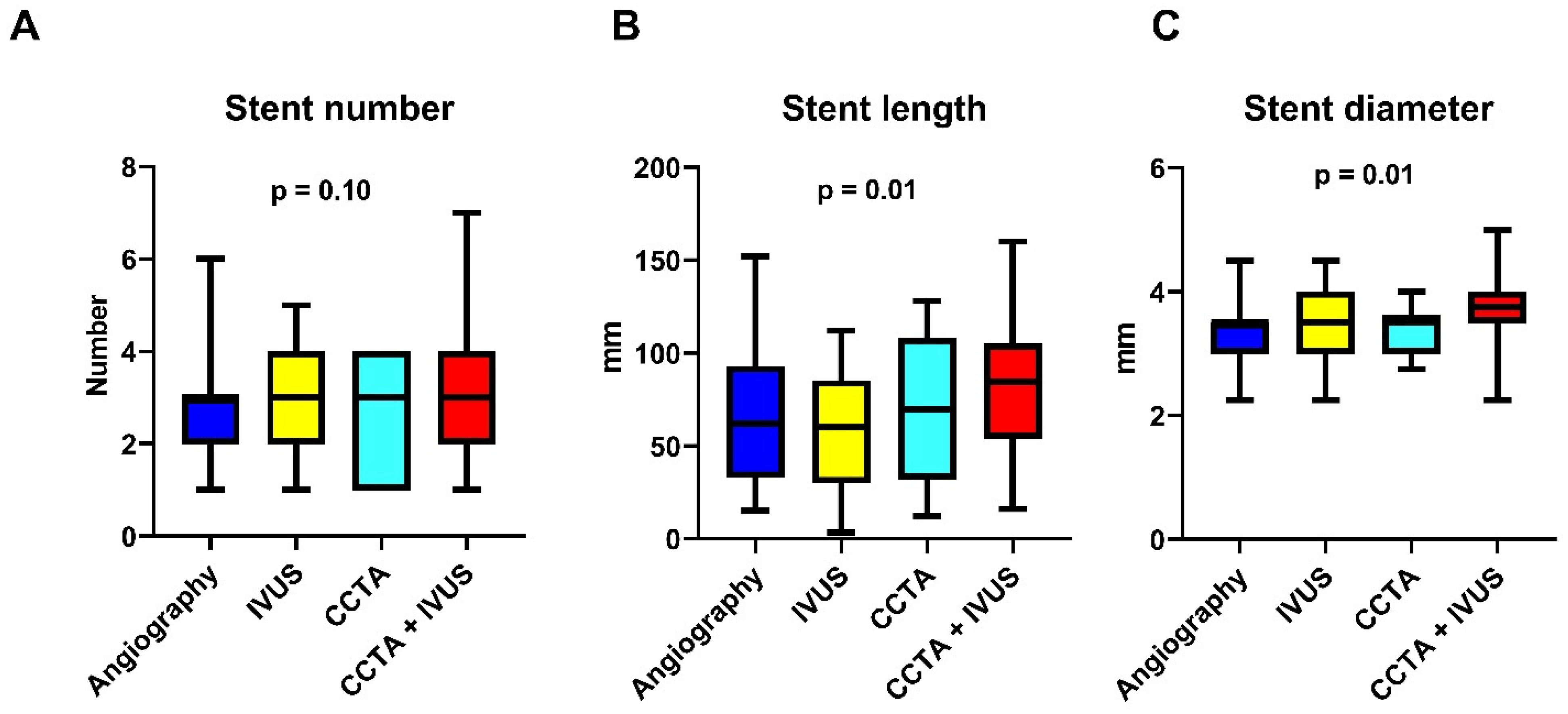
| Stent number | 3 [2–4] | 3 [2–3] | 3 [1.75–4] | 3 [1–4] | 3 [2–4] | 0.10 |
| Stent length | 70.5 [38.25–98.0] | 60 [32–91.5] | 60 [30–85] | 69.5 [32–108] | 84.5 [54–104.75] | 0.01 |
| Stent diameter | 3.5 [3–4] | 3.5 [3–3.5] | 3.5 [3–4] | 3.5 [3–3.625] | 3.75 [3.5–4] | 0.01 |
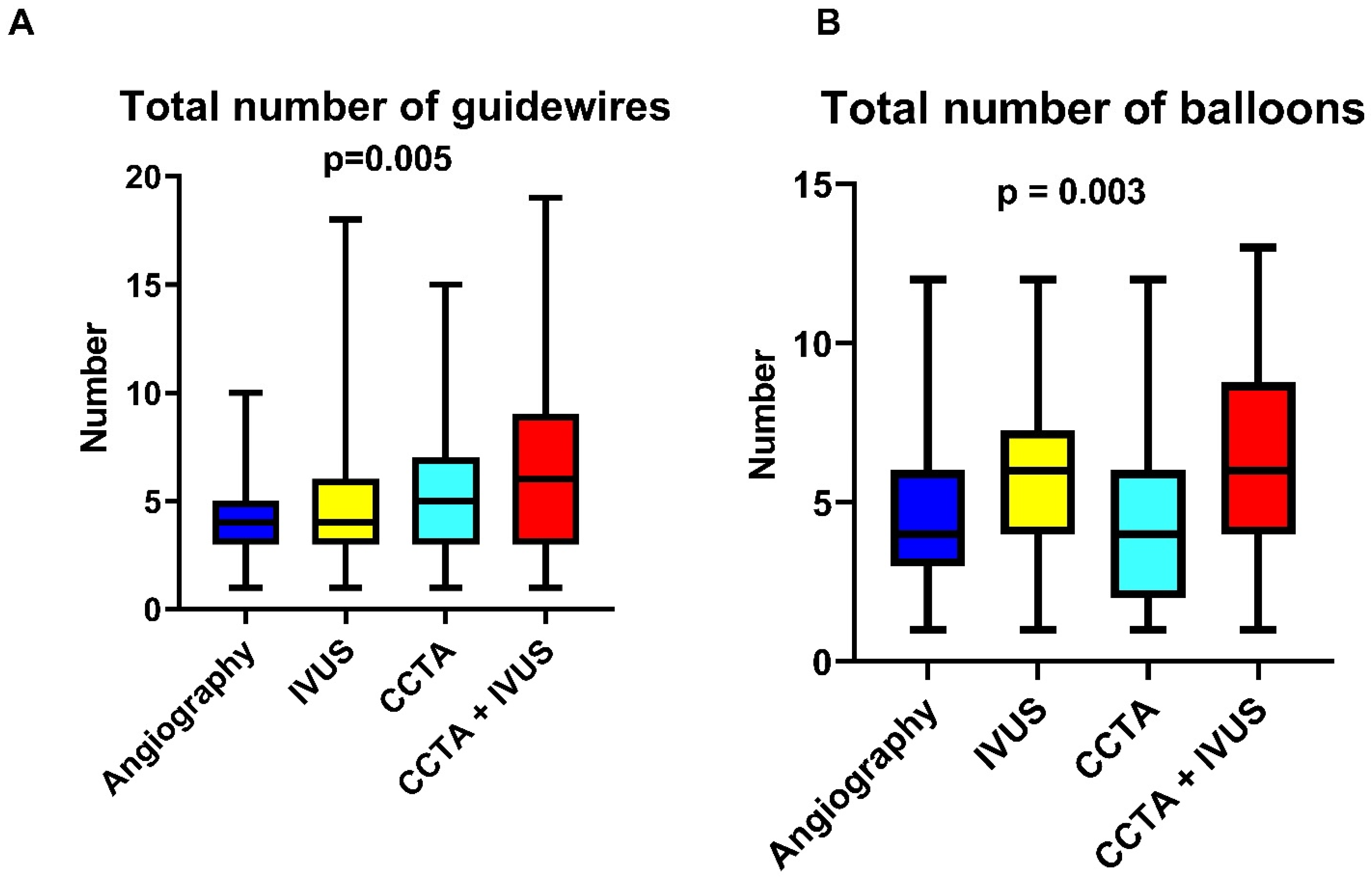
| Total number of guidewires | 5 [3–7] | 4 [3–5] | 4 [3–6] | 5 [3–7] | 6 [3–9] | 0.005 |
| Total number of balloons | 5 [3–7] | 4 [3–6] | 6 [4–7.25] | 4 [2–6] | 6 [4–8.75] | 0.003 |
| Outcome | All N = 171 | Angiography-Guided N = 48 | IVUS-Guided N = 42 | CCTA-Guided N = 40 | CT + IVUS-Guided N = 41 | p Value |
|---|---|---|---|---|---|---|
| Procedural success | 154 (90.0) | 45 (93.8) | 42 (100) | 28 (70.0) | 39 (95.1) | 0.97 |
| Coronary perforations | 10 (5.8) | 1 (2.0) | 2 (4.7) | 5 (12.5) | 2 (4.8) | 0.42 |
| Intense debulking (rotational atherectomy/coronary lithotripsy) | 24 (14.0) | 2 (4.1) | 6 (14.2) | 5 (12.5) | 11 (26.8) | 0.005 |
| Intravascular lithotripsy | 16 (9.3) | 1 (2.0) | 5 (11.9) | 3 (7.5) | 7 (17.0) | 0.03 |
| Rotational atherectomy | 8 (4.6) | 1 (2.0) | 2 (4.7) | 2 (5.0) | 3 (7.3) | 0.26 |
| MACE | 2 (1.5) | 1 (2.0) | 0 | 0 (0) | 1 (2.4) | 0.91 |
| Creatinine levels post CTO-PCI | 1.1 (0.93–1.37) | 1.1 (0.97–1.64) | 1.16 (0.91–1.36) | 1.3 [1.1–1.6] | 1.06 (0.89–1.5) | 0.77 |
| Contrast-induced nephropathy | 1 (0.5) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Variable | Univariable Analysis | p Value | Multivariable Analysis | p Value |
|---|---|---|---|---|
| Coronary imaging | 1.7 (1.2–2.7) | 0.006 | 1.6 (1.02–2.4) | 0.04 |
| J-CTO score | 2.2(1.4–3.3) | <0.001 | 2.0 (1.3–3.0) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panuccio, G.; Mashayekhi, K.; Werner, G.S.; Ichibori, Y.; Carabetta, N.; Skurk, C.; Göktekin, Ö.; Siegrist, P.T.; Leistner, D.M.; De Rosa, S.; et al. Comparative Impact of Coronary Imaging Strategies in CTO-PCI: A Retrospective Single-Center Analysis. J. Clin. Med. 2025, 14, 6976. https://doi.org/10.3390/jcm14196976
Panuccio G, Mashayekhi K, Werner GS, Ichibori Y, Carabetta N, Skurk C, Göktekin Ö, Siegrist PT, Leistner DM, De Rosa S, et al. Comparative Impact of Coronary Imaging Strategies in CTO-PCI: A Retrospective Single-Center Analysis. Journal of Clinical Medicine. 2025; 14(19):6976. https://doi.org/10.3390/jcm14196976
Chicago/Turabian StylePanuccio, Giuseppe, Kambis Mashayekhi, Gerald S. Werner, Yasuhiro Ichibori, Nicole Carabetta, Carsten Skurk, Ömer Göktekin, Patrick T. Siegrist, David M. Leistner, Salvatore De Rosa, and et al. 2025. "Comparative Impact of Coronary Imaging Strategies in CTO-PCI: A Retrospective Single-Center Analysis" Journal of Clinical Medicine 14, no. 19: 6976. https://doi.org/10.3390/jcm14196976
APA StylePanuccio, G., Mashayekhi, K., Werner, G. S., Ichibori, Y., Carabetta, N., Skurk, C., Göktekin, Ö., Siegrist, P. T., Leistner, D. M., De Rosa, S., Torella, D., Landmesser, U., & Abdelwahed, Y. S. (2025). Comparative Impact of Coronary Imaging Strategies in CTO-PCI: A Retrospective Single-Center Analysis. Journal of Clinical Medicine, 14(19), 6976. https://doi.org/10.3390/jcm14196976







