Endocrinology and the Lung: Exploring the Bidirectional Axis and Future Directions
Abstract
1. Introduction
2. Methods
3. Physiological Pathways of Dual Crosstalk
3.1. Hormonal Control of Lung Development
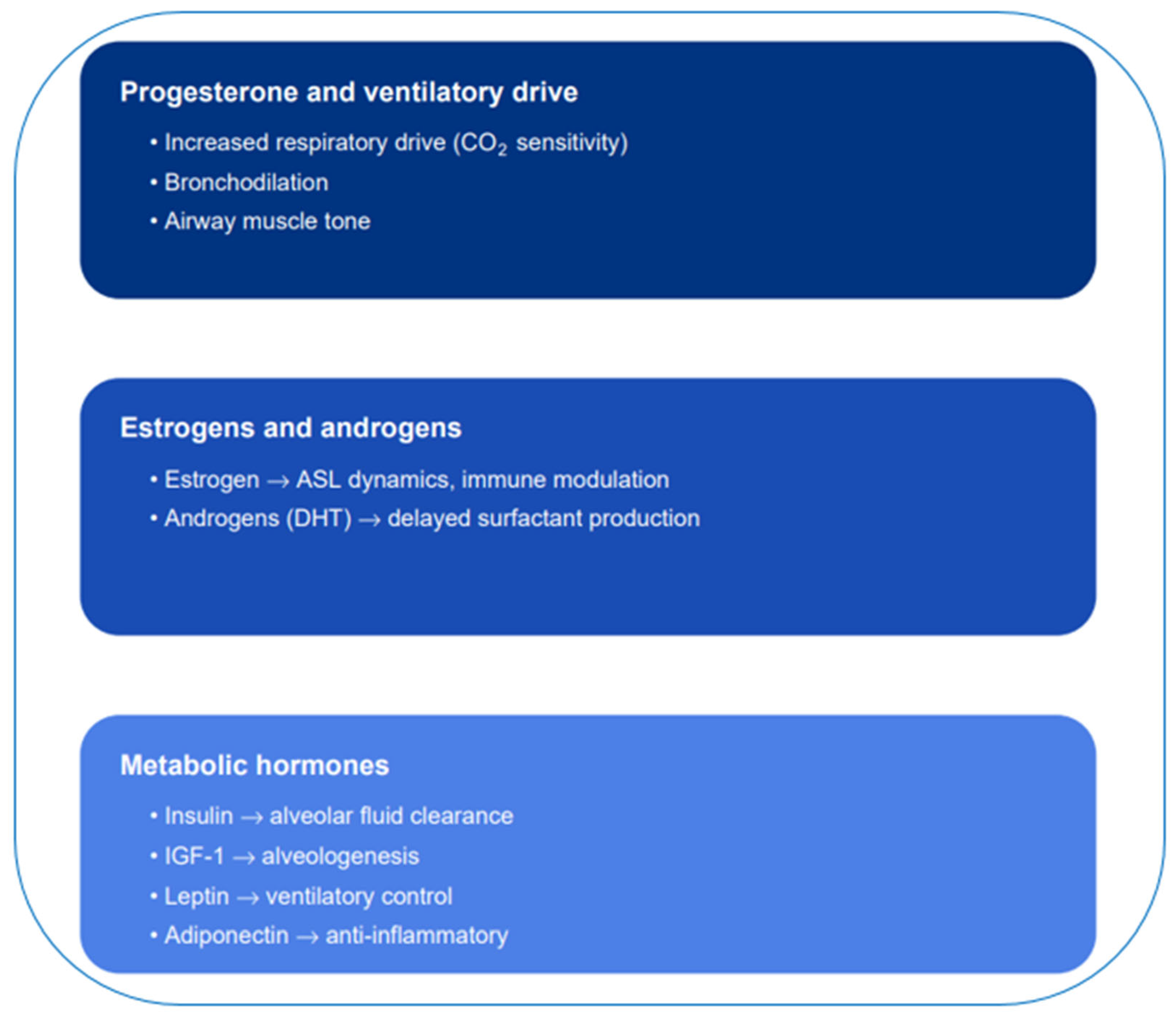
3.2. Hormonal Regulation of Respiratory Function
3.3. Hormone Target Cells and Receptors in the Lung
3.3.1. Thyroid Hormone Receptors (TRa, TRβ)
3.3.2. Glucocorticoid Receptors (GRa, GRβ)
3.3.3. Estrogen and Progesterone Receptors (ERa, ERβ, PR)
3.3.4. Insulin and IGF-1 Receptors (IR, IGF1R)
3.3.5. Endothelin-1 Receptors (ETA, ETB)
3.3.6. Leptin Receptor (Ob-Rb)
3.3.7. Parathyroid Hormone-Related Protein (PTHrP) Receptor (PTH1R)
3.3.8. Vitamin D Receptor (VDR)
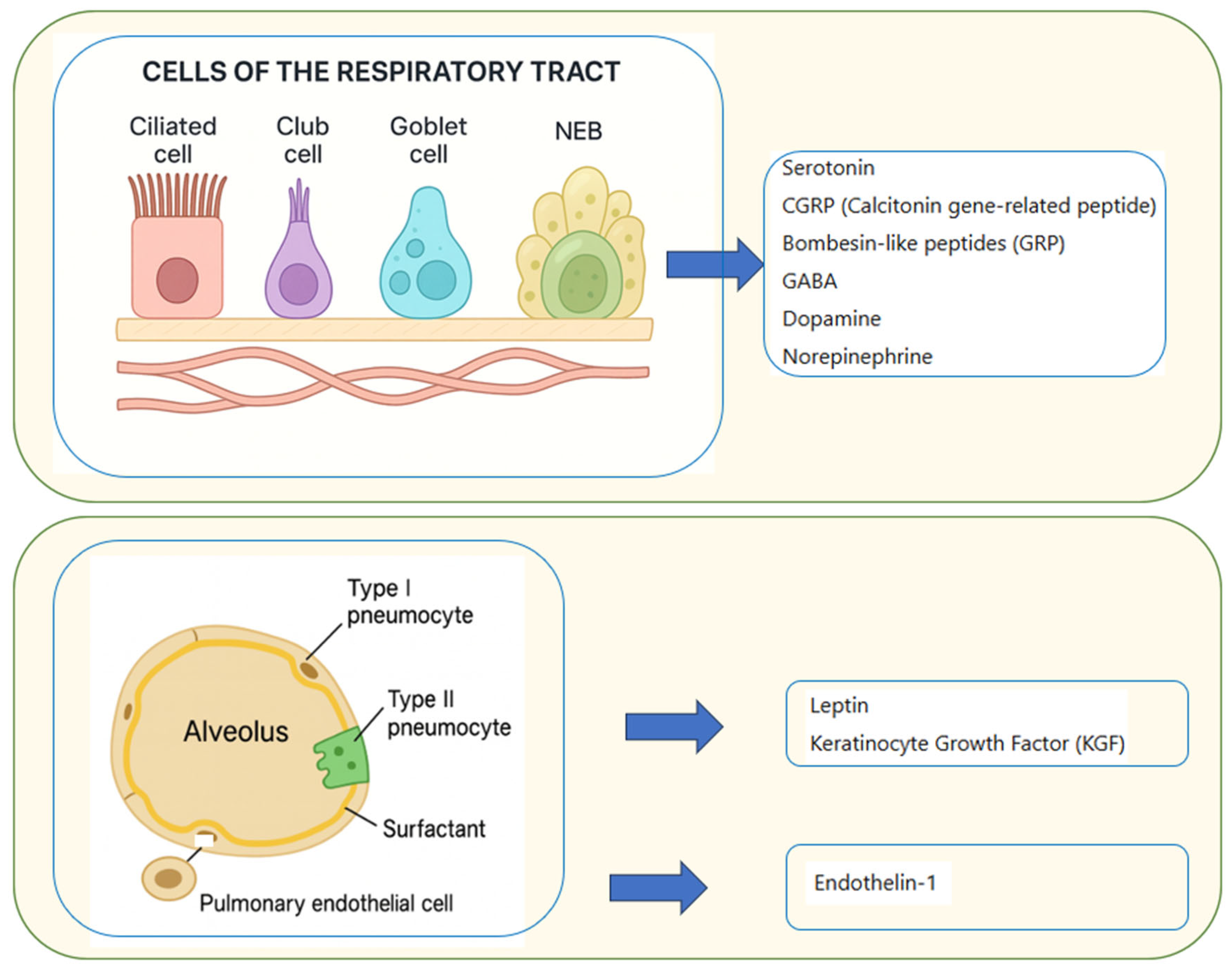
3.4. Paracrine Pulmonary Activity
3.4.1. Pulmonary Neuroendocrine Cells (PNECs)
3.4.2. Pulmonary Endothelial Cells
3.4.3. Type II Pneumocytes
4. Endocrine Ailments with Pulmonary Involvement
4.1. Acromegaly
4.2. Cushing’s Syndrome
4.3. Growth Hormone (GH) Deficiency
4.4. Thyroid Dysfunction
4.5. Diabetes Mellitus
4.6. Obesity
4.7. Hypoparathyroidism
5. Lung Conditions with Endocrine Manifestations
5.1. Non-Cancerous Lung Conditions
5.1.1. Chronic Obstructive Pulmonary Disease (COPD)
5.1.2. Obstructive Sleep Apnea (OSA)
5.1.3. Interstitial Lung Diseases and Chronic Hypoxemia States
5.2. Pulmonary Cancer/Neuroendocrine Tumors
5.2.1. Pulmonary Neuroendocrine Tumors
5.2.2. Pulmonary Endocrine Paraneoplastic Syndromes
6. Therapeutic Interferences
6.1. Inhaled Corticosteroids
6.2. Endocrinopathies Induced by Immunotherapy
6.3. Inhaled Insulin
7. Discussion and Future Directions
8. Conclusions
Funding
Conflicts of Interest
References
- Rajatapiti, P.; Kester, M.H.A.; de Krijger, R.R.; Rottier, R.; Visser, T.J.; Tibboel, D. Expression of Glucocorticoid, Retinoid, and Thyroid Hormone Receptors during Human Lung Development. J. Clin. Endocrinol. Metab. 2005, 90, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Gnanalingham, M.G.; Mostyn, A.; Gardner, D.S.; Stephenson, T.; Symonds, M.E. Developmental Regulation of the Lung in Preparation for Life after Birth: Hormonal and Nutritional Manipulation of Local Glucocorticoid Action and Uncoupling Protein-2. J. Endocrinol. 2006, 188, 375–386. [Google Scholar] [CrossRef]
- Provost, P.R.; Boucher, E.; Tremblay, Y. Glucocorticoid Metabolism in the Developing Lung: Adrenal-like Synthesis Pathway. J. Steroid Biochem. Mol. Biol. 2013, 138, 72–80. [Google Scholar] [CrossRef]
- Gerber, A.N. Glucocorticoids and the Lung. In Glucocorticoid Signaling; Springer: New York, NY, USA, 2015; Volume 872, pp. 279–298. [Google Scholar] [CrossRef]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef]
- González-Arenas, A.; Agramonte-Hevia, J. Sex Steroid Hormone Effects in Normal and Pathologic Conditions in Lung Physiology. Mini Rev. Med. Chem. 2012, 12, 1055–1062. [Google Scholar] [CrossRef]
- Sathish, V.; Martin, Y.N.; Prakash, Y.S. Sex Steroid Signaling: Implications for Lung Diseases. Pharmacol. Ther. 2015, 150, 94–108. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Endocrine Regulation of Lung Disease and Inflammation. Exp. Biol. Med. 2018, 243, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Montaño, L.M.; Carbajal-García, A.; Wang, Y.-X. Sex Hormones and Lung Inflammation. In Lung Inflammation in Health and Disease, Volume II; Springer: Cham, Switzerland, 2021; Volume 1304, pp. 259–321. [Google Scholar] [CrossRef]
- Ambhore, N.S.; Kalidhindi, R.S.R.; Sathish, V. Sex-Steroid Signaling in Lung Diseases and Inflammation. In Lung Inflammation in Health and Disease, Volume I; Springer: Cham, Switzerland, 2021; Volume 1303, pp. 243–273. [Google Scholar] [CrossRef]
- Silveyra, P.; Babayev, M.; Ekpruke, C.D. Sex, Hormones, and Lung Health. Physiol. Rev. 2025, 106, 53–86. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Forno, E.; Celedón, J.C. Sex Steroid Hormones and Asthma in a Nationwide Study of U.S. Adults. Am. J. Respir. Crit. Care Med. 2020, 201, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.D.; Oliver, B.G.G. Sexual Dimorphism in Chronic Respiratory Diseases. Cell Biosci. 2023, 13, 47. [Google Scholar] [CrossRef]
- Sorensen, J.R.; Winther, K.H.; Bonnema, S.J.; Godballe, C.; Hegedüs, L. Respiratory Manifestations of Hypothyroidism: A Systematic Review. Thyroid 2016, 26, 1519–1527. [Google Scholar] [CrossRef]
- Bottini, P.; Tantucci, C. Sleep Apnea Syndrome in Endocrine Diseases. Respiration 2003, 70, 320–327. [Google Scholar] [CrossRef]
- Attal, P.; Chanson, P. Endocrine Aspects of Obstructive Sleep Apnea. J. Clin. Endocrinol. Metab. 2010, 95, 483–495. [Google Scholar] [CrossRef]
- Ceccato, F.; Bernkopf, E.; Scaroni, C. Sleep Apnea Syndrome in Endocrine Clinics. J. Endocrinol. Investig. 2015, 38, 827–834. [Google Scholar] [CrossRef]
- Amorim, M.R.; Aung, O.; Mokhlesi, B.; Polotsky, V.Y. Leptin-Mediated Neural Targets in Obesity Hypoventilation Syndrome. Sleep 2022, 45, zsac153. [Google Scholar] [CrossRef]
- Machado, F.V.C.; Pitta, F.; Hernandes, N.A.; Bertolini, G.L. Physiopathological Relationship between Chronic Obstructive Pulmonary Disease and Insulin Resistance. Endocrine 2018, 61, 17–22. [Google Scholar] [CrossRef]
- Akset, M.; Poppe, K.G.; Kleynen, P.; Bold, I.; Bruyneel, M. Endocrine Disorders in Obstructive Sleep Apnoea Syndrome: A Bidirectional Relationship. Clin. Endocrinol. 2023, 98, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Laghi, F.; Adiguzel, N.; Tobin, M.J. Endocrinological Derangements in COPD. Eur. Respir. J. 2009, 34, 975–996. [Google Scholar] [CrossRef]
- Martins, F.O.; Conde, S.V. Gender Differences in the Context of Obstructive Sleep Apnea and Metabolic Diseases. Front. Physiol. 2021, 12, 792633. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mei, S.; Zhang, N.; Zhang, K.; Taslakjian, B.; Lian, J.; Wu, S.; Chen, B.; Solway, J.; Chen, H.J. Pulmonary Neuroendocrine Cells: Crucial Players in Respiratory Function and Airway-Nerve Communication. Front. Neurosci. 2024, 18, 1438188. [Google Scholar] [CrossRef] [PubMed]
- Soomro, Z.; Youssef, M.; Yust-Katz, S.; Jalali, A.; Patel, A.J.; Mandel, J. Paraneoplastic Syndromes in Small Cell Lung Cancer. J. Thorac. Dis. 2020, 12, 6253–6263. [Google Scholar] [CrossRef]
- Rosenow, F.; McCarthy, V.; Caruso, A.C. Sleep Apnoea in Endocrine Diseases. J. Sleep Res. 1998, 7, 3–11. [Google Scholar] [CrossRef]
- Ballard, P.L.; Hovey, M.L.; Gonzales, L.K. Thyroid Hormone Stimulation of Phosphatidylcholine Synthesis in Cultured Fetal Rabbit Lung. J. Clin. Investig. 1984, 74, 898–905. [Google Scholar] [CrossRef]
- Gonzales, L.W.; Ballard, P.L.; Ertsey, R.; Williams, M.C. Glucocorticoids and Thyroid Hormones Stimulate Biochemical and Morphological Differentiation of Human Fetal Lung in Organ Culture. J. Clin. Endocrinol. Metab. 1986, 62, 678–691. [Google Scholar] [CrossRef]
- van Tuyl, M.; Blommaart, P.E.; de Boer, P.A.J.; Wert, S.E.; Ruijter, J.M.; Islam, S.; Schnitzer, J.; Ellison, A.R.; Tibboel, D.; Moorman, A.F.M.; et al. Prenatal Exposure to Thyroid Hormone Is Necessary for Normal Postnatal Development of Murine Heart and Lungs. Dev. Biol. 2004, 272, 104–117. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Chang, M.; Lee, B. Association between Thyroid Function and Respiratory Distress Syndrome in Preterm Infants. Pediatr. Rep. 2022, 14, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bolt, R.J.; van Weissenbruch, M.M.; Lafeber, H.N.; Delemarre-van de Waal, H.A. Glucocorticoids and Lung Development in the Fetus and Preterm Infant. Pediatr. Pulmonol. 2001, 32, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Boland, R.; Joyce, B.J.; Wallace, M.J.; Stanton, H.; Fosang, A.J.; Pierce, R.A.; Harding, R.; Hooper, S.B. Cortisol Enhances Structural Maturation of the Hypoplastic Fetal Lung in Sheep. J. Physiol. 2004, 554, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Hooper, S.B.; Harding, R. Role of the Adrenal Glands in the Maturation of Lung Liquid Secretory Mechanisms in Fetal Sheep. AJP Regul. Integr. Comp. Physiol. 1996, 270, R33–R40. [Google Scholar] [CrossRef]
- Laube, M.; Thome, U.H. Y It Matters-Sex Differences in Fetal Lung Development. Biomolecules 2022, 12, 437. [Google Scholar] [CrossRef]
- Jensen, D.; Wolfe, L.A.; Slatkovska, L.; Webb, K.A.; Davies, G.A.L.; O’Donnell, D.E. Effects of Human Pregnancy on the Ventilatory Chemoreflex Response to Carbon Dioxide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1369–R1375. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, D.A.; Millhorn, D.E.; Gallman, E.A.; Cidlowski, J.A. Progesterone Stimulates Respiration through a Central Nervous System Steroid Receptor-Mediated Mechanism in Cat. Proc. Natl. Acad. Sci. USA 1987, 84, 7788–7792. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Redline, S.; Young, T.; Boland, L.L.; Baldwin, C.M.; Nieto, F.J.; O’Connor, G.T.; Rapoport, D.M.; Robbins, J.A. Hormone Replacement Therapy and Sleep-Disordered Breathing. Am. J. Respir. Crit. Care Med. 2003, 167, 1186–1192. [Google Scholar] [CrossRef]
- Lee, J.; Eklund, E.E.; Lambert-Messerlian, G.; Palomaki, G.E.; Butterfield, K.; Curran, P.; Bourjeily, G. Serum Progesterone Levels in Pregnant Women with Obstructive Sleep Apnea: A Case Control Study. J. Womens Health 2017, 26, 259–265. [Google Scholar] [CrossRef]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Austin, D.; Peterson, A. Menopausal Status and Sleep-Disordered Breathing in the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2003, 167, 1181–1185. [Google Scholar] [CrossRef]
- Sigurðardóttir, E.S.; Gislason, T.; Benediktsdottir, B.; Hustad, S.; Dadvand, P.; Demoly, P.; Franklin, K.A.; Heinrich, J.; Holm, M.; van der Plaat, D.A.; et al. Female Sex Hormones and Symptoms of Obstructive Sleep Apnea in European Women of a Population-Based Cohort. PLoS ONE 2022, 17, e0269569. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.J.; McElvaney, N.G. Sex Differences in Airway Disease: Estrogen and Airway Surface Liquid Dynamics. Biol. Sex. Differ. 2024, 15, 56. [Google Scholar] [CrossRef]
- Torday, J.S. Androgens Delay Human Fetal Lung Maturation in Vitro. Endocrinology 1990, 126, 3240–3244. [Google Scholar] [CrossRef]
- Deng, W.; Li, C.-Y.; Tong, J.; Zhang, W.; Wang, D.-X. Regulation of ENaC-Mediated Alveolar Fluid Clearance by Insulin via PI3K/Akt Pathway in LPS-Induced Acute Lung Injury. Respir. Res. 2012, 13, 29. [Google Scholar] [CrossRef]
- Li, J.; Masood, A.; Yi, M.; Lau, M.; Belcastro, R.; Ivanovska, J.; Jankov, R.P.; Tanswell, A.K. The IGF-I/IGF-R1 Pathway Regulates Postnatal Lung Growth and Is a Nonspecific Regulator of Alveologenesis in the Neonatal Rat. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L626–L637. [Google Scholar] [CrossRef]
- Bassi, M.; Giusti, H.; Leite, C.M.; Anselmo-Franci, J.A.; do Carmo, J.M.; da Silva, A.A.; Hall, J.E.; Colombari, E.; Glass, M.L. Central Leptin Replacement Enhances Chemorespiratory Responses in Leptin-Deficient Mice Independent of Changes in Body Weight. Pflug. Arch. 2012, 464, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Furuya, W.I.; Menani, J.V.; Colombari, D.S.A.; do Carmo, J.M.; da Silva, A.A.; Hall, J.E.; Moreira, T.S.; Wenker, I.C.; Mulkey, D.K.; et al. Leptin into the Ventrolateral Medulla Facilitates Chemorespiratory Response in Leptin-Deficient (Ob/Ob) Mice. Acta Physiol. 2014, 211, 240–248. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Templeton, S.P. Regulation of Lung Inflammation by Adiponectin. Front. Immunol. 2023, 14, 1244586. [Google Scholar] [CrossRef]
- Yu, G.; Tzouvelekis, A.; Wang, R.; Herazo-Maya, J.D.; Ibarra, G.H.; Srivastava, A.; de Castro, J.P.W.; DeIuliis, G.; Ahangari, F.; Woolard, T.; et al. Thyroid Hormone Inhibits Lung Fibrosis in Mice by Improving Epithelial Mitochondrial Function. Nat. Med. 2018, 24, 39–49. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Wan, R.; Pan, X.; Li, B.; Zhao, H.; Yang, J.; Zhao, W.; Wang, S.; Wang, Q.; et al. Single-Cell RNA Sequencing Provides New Insights into Therapeutic Roles of Thyroid Hormone in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 69, 456–469. [Google Scholar] [CrossRef]
- Pan, X.; Wang, L.; Yang, J.; Li, Y.; Xu, M.; Liang, C.; Liu, L.; Li, Z.; Xia, C.; Pang, J.; et al. TRβ Activation Confers AT2-to-AT1 Cell Differentiation and Anti-Fibrosis during Lung Repair via KLF2 and CEBPA. Nat. Commun. 2024, 15, 8672. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Tuffin, L.J.; Cidlowski, J.A. The Physiology of Human Glucocorticoid Receptor β (hGRβ) and Glucocorticoid Resistance. Ann. N. Y. Acad. Sci. 2006, 1069, 1–9. [Google Scholar] [CrossRef]
- Bhallamudi, S.; Connell, J.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Estrogen Receptors Differentially Regulate Intracellular Calcium Handling in Human Nonasthmatic and Asthmatic Airway Smooth Muscle Cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L112–L124. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, B.S.; Sommer, B.; Solís-Chagoyán, H.; Calixto, E.; Aquino-Gálvez, A.; Jaimez, R.; Gomez-Verjan, J.C.; González-Avila, G.; Flores-Soto, E.; Montaño, L.M. Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 7879. [Google Scholar] [CrossRef]
- Townsend, E.A.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Rapid Effects of Estrogen on Intracellular Ca2+ Regulation in Human Airway Smooth Muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L521–L530. [Google Scholar] [CrossRef]
- Townsend, E.A.; Sathish, V.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Estrogen Effects on Human Airway Smooth Muscle Involve cAMP and Protein Kinase A. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L923–L928. [Google Scholar] [CrossRef]
- Jain, R.; Ray, J.M.; Pan, J.; Brody, S.L. Sex Hormone-Dependent Regulation of Cilia Beat Frequency in Airway Epithelium. Am. J. Respir. Cell Mol. Biol. 2012, 46, 446–453. [Google Scholar] [CrossRef]
- Choi, E.; Duan, C.; Bai, X.-C. Regulation and Function of Insulin and Insulin-like Growth Factor Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2025, 26, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Krein, P.M.; Winston, B.W. Roles for Insulin-like Growth Factor I and Transforming Growth Factor-Beta in Fibrotic Lung Disease. Chest 2002, 122, 289S–293S. [Google Scholar] [CrossRef]
- Davie, N.; Haleen, S.J.; Upton, P.D.; Polak, J.M.; Yacoub, M.H.; Morrell, N.W.; Wharton, J. ETA and ETB Receptors Modulate the Proliferation of Human Pulmonary Artery Smooth Muscle Cells. Am. J. Respir. Crit. Care Med. 2002, 165, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Bergen, H.T.; Cherlet, T.C.; Manuel, P.; Scott, J.E. Identification of Leptin Receptors in Lung and Isolated Fetal Type II Cells. Am. J. Respir. Cell Mol. Biol. 2002, 27, 71–77. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, H.; Zhang, J.-R.; Zeng, W.; Hu, X. Leptin Receptor Signaling Sustains Metabolic Fitness of Alveolar Macrophages to Attenuate Pulmonary Inflammation. Sci. Adv. 2022, 8, eabo3064. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Sanchez-Esteban, J.; Rubin, L.P. Paracrine Mediators of Mechanotransduction in Lung Development. Am. J. Med. Sci. 1998, 316, 205–208. [Google Scholar] [CrossRef]
- Torday, J.S.; Rehan, V.K. Stretch-Stimulated Surfactant Synthesis Is Coordinated by the Paracrine Actions of PTHrP and Leptin. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L130–L135. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M. Vitamin D Effects on Lung Immunity and Respiratory Diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [CrossRef]
- Hamza, F.N.; Daher, S.; Fakhoury, H.M.A.; Grant, W.B.; Kvietys, P.R.; Al-Kattan, K. Immunomodulatory Properties of Vitamin D in the Intestinal and Respiratory Systems. Nutrients 2023, 15, 1696. [Google Scholar] [CrossRef]
- Pujols, L.; Mullol, J.; Torrego, A.; Picado, C. Glucocorticoid Receptors in Human Airways. Allergy 2004, 59, 1042–1052. [Google Scholar] [CrossRef]
- Aravamudan, B.; Goorhouse, K.J.; Unnikrishnan, G.; Thompson, M.A.; Pabelick, C.M.; Hawse, J.R.; Prakash, Y.S.; Sathish, V. Differential Expression of Estrogen Receptor Variants in Response to Inflammation Signals in Human Airway Smooth Muscle. J. Cell Physiol. 2017, 232, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Ambhore, N.S.; Kalidhindi, R.S.R.; Loganathan, J.; Sathish, V. Role of Differential Estrogen Receptor Activation in Airway Hyperreactivity and Remodeling in a Murine Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2019, 61, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Pujols, L.; Mullol, J.; Picado, C. Alpha and Beta Glucocorticoid Receptors: Relevance in Airway Diseases. Curr. Allergy Asthma Rep. 2007, 7, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pujols, L.; Mullol, J.; Picado, C. Importance of Glucocorticoid Receptors in Upper and Lower Airways. Front. Biosci. Landmark Ed. 2010, 15, 789–800. [Google Scholar] [CrossRef]
- Lockett, J.; Inder, W.J.; Clifton, V.L. The Glucocorticoid Receptor: Isoforms, Functions, and Contribution to Glucocorticoid Sensitivity. Endocr. Rev. 2024, 45, 593–624. [Google Scholar] [CrossRef]
- Pujolsa, L.; Mullol, J.; Picado, C. Glucocorticoid Receptor in Human Respiratory Epithelial Cells. Neuroimmunomodulation 2009, 16, 290–299. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Libert, C. Comprehensive Overview of the Structure and Regulation of the Glucocorticoid Receptor. Endocr. Rev. 2014, 35, 671–693. [Google Scholar] [CrossRef]
- Chen, X.; He, H. Expression Profiles of Glucocorticoid Receptor α- and β-Isoforms in Diverse Physiological and Pathological Conditions. Horm. Metab. Res. 2025, 57, 359–365. [Google Scholar] [CrossRef]
- Nicolaides, N.C. The Human Glucocorticoid Receptor Beta: From Molecular Mechanisms to Clinical Implications. Endocrinology 2022, 163, bqac150. [Google Scholar] [CrossRef] [PubMed]
- Kalidhindi, R.S.R.; Ambhore, N.S.; Bhallamudi, S.; Loganathan, J.; Sathish, V. Role of Estrogen Receptors α and β in a Murine Model of Asthma: Exacerbated Airway Hyperresponsiveness and Remodeling in ERβ Knockout Mice. Front. Pharmacol. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, B.S.; Flores-Soto, E.; Sommer, B.; Reyes-García, J.; Arredondo-Zamarripa, D.; Solís-Chagoyán, H.; Lemini, C.; Rivero-Segura, N.A.; Santiago-de-la-Cruz, J.A.; Pérez-Plascencia, C.; et al. 17β-Estradiol Induces Hyperresponsiveness in Guinea Pig Airway Smooth Muscle by Inhibiting the Plasma Membrane Ca2+-ATPase. Mol. Cell. Endocrinol. 2024, 590, 112273. [Google Scholar] [CrossRef]
- Noguchi, M.; Furukawa, K.T.; Morimoto, M. Pulmonary Neuroendocrine Cells: Physiology, Tissue Homeostasis and Disease. Dis. Model. Mech. 2020, 13, dmm046920. [Google Scholar] [CrossRef]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of Endothelin-1 in the Lungs of Patients with Pulmonary Hypertension. N. Engl. J. Med. 1993, 328, 1732–1739. [Google Scholar] [CrossRef]
- Dhaun, N.; Webb, D.J. Endothelins in Cardiovascular Biology and Therapeutics. Nat. Rev. Cardiol. 2019, 16, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Van Lommel, A. Pulmonary Neuroendocrine Cells (PNEC) and Neuroepithelial Bodies (NEB): Chemoreceptors and Regulators of Lung Development. Paediatr. Respir. Rev. 2001, 2, 171–176. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Sui, P.; Chen, J.; Moya, E.A.; Hume, P.; Janssen, W.J.; Duran, J.M.; Thistlethwaite, P.; Carlin, A.; et al. Excess Neuropeptides in Lung Signal through Endothelial Cells to Impair Gas Exchange. Dev. Cell 2022, 57, 839–853.e6. [Google Scholar] [CrossRef]
- Gaur, P.; Saini, S.; Vats, P.; Kumar, B. Regulation, Signalling and Functions of Hormonal Peptides in Pulmonary Vascular Remodelling during Hypoxia. Endocrine 2018, 59, 466–480. [Google Scholar] [CrossRef]
- Kostyunina, D.S.; Rowan, S.C.; Pakhomov, N.V.; Dillon, E.; Rochfort, K.D.; Cummins, P.M.; O’Rourke, M.J.; McLoughlin, P. Shear Stress Markedly Alters the Proteomic Response to Hypoxia in Human Pulmonary Endothelial Cells. Am. J. Respir. Cell Mol. Biol. 2023, 68, 551–565. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; Roerink, S.H.P.P.; Drenthen, L.C.A.; van Haren-Willems, J.H.G.M.; Wagenmakers, M.A.E.M.; Smit, J.W.A.; Hermus, A.R.M.M.; Netea-Maier, R.T. The Course of Obstructive Sleep Apnea Syndrome in Patients With Acromegaly During Treatment. J. Clin. Endocrinol. Metab. 2020, 105, 290–304. [Google Scholar] [CrossRef]
- Pilarska, M.; Dżaman, K.; Szczepański, D.; Węgrzecki, T. Craniofacial Parameters and Obstructive Sleep Apnea in Newly Diagnosed Acromegaly. Front. Endocrinol. 2025, 16, 1632944. [Google Scholar] [CrossRef]
- Störmann, S.; Gutt, B.; Roemmler-Zehrer, J.; Bidlingmaier, M.; Huber, R.M.; Schopohl, J.; Angstwurm, M.W. Assessment of Lung Function in a Large Cohort of Patients with Acromegaly. Eur. J. Endocrinol. 2017, 177, 15–23. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Gao, L.; Wang, Z.; Feng, C.; Jia, M.; Xing, B. Lung Function and Blood Gas Abnormalities in Patients with Acromegaly. J. Clin. Neurosci. 2020, 73, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Monzani, F.; Caraccio, N.; Siciliano, G.; Manca, L.; Murri, L.; Ferrannini, E. Clinical and Biochemical Features of Muscle Dysfunction in Subclinical Hypothyroidism. J. Clin. Endocrinol. Metab. 1997, 82, 3315–3318. [Google Scholar] [CrossRef] [PubMed]
- Akkoyunlu, M.E.; Ilhan, M.M.; Bayram, M.; Taşan, E.; Yakar, F.; Ozçelik, H.K.; Karakose, F.; Kart, L. Does Hormonal Control Obviate Positive Airway Pressure Therapy in Acromegaly with Sleep-Disordered Breathing? Respir. Med. 2013, 107, 1803–1809. [Google Scholar] [CrossRef]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s Syndrome: State of the Art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Hasenmajer, V.; Sbardella, E.; Sciarra, F.; Minnetti, M.; Isidori, A.M.; Venneri, M.A. The Immune System in Cushing’s Syndrome. Trends Endocrinol. Metab. 2020, 31, 655–669. [Google Scholar] [CrossRef]
- Attia, A.; Bertherat, J. Cushing’s Syndrome and COVID-19. Pituitary 2024, 27, 945–954. [Google Scholar] [CrossRef]
- Braun, L.T.; Vogel, F.; Reincke, M. Long-Term Morbidity and Mortality in Patients with Cushing’s Syndrome. J. Neuroendocrinol. 2022, 34, e13113. [Google Scholar] [CrossRef]
- Reincke, M.; Fleseriu, M. Cushing Syndrome: A Review. JAMA 2023, 330, 170–181. [Google Scholar] [CrossRef]
- Merola, B.; Sofia, M.; Longobardi, S.; Fazio, S.; Micco, A.; Esposito, V.; Colao, A.; Biondi, B.; Lombardi, G. Impairment of Lung Volumes and Respiratory Muscle Strength in Adult Patients with Growth Hormone Deficiency. Eur. J. Endocrinol. 1995, 133, 680–685. [Google Scholar] [CrossRef]
- Merola, B.; Longobardi, S.; Sofia, M.; Pivonello, R.; Micco, A.; Di Rella, F.; Esposito, V.; Colao, A.; Lombardi, G. Lung Volumes and Respiratory Muscle Strength in Adult Patients with Childhood- or Adult-Onset Growth Hormone Deficiency: Effect of 12 Months’ Growth Hormone Replacement Therapy. Eur. J. Endocrinol. 1996, 135, 553–558. [Google Scholar] [CrossRef]
- Cilluffo, G.; Ferrante, G.; Fasola, S.; Ciresi, A.; Cardillo, I.; Tancredi, G.; Viegi, G.; Giordano, C.; Scichilone, N.; La Grutta, S. Direct and Indirect Effects of Growth Hormone Deficiency (GHD) on Lung Function in Children: A Mediation Analysis. Respir. Med. 2018, 137, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, J.; Huang, R.; Xiao, Y. Nonlinear Association of TSH with Pulmonary Ventilation: Insights from Bidirectional Mendelian Randomization and Cross-Sectional Study. BMC Pulm. Med. 2025, 25, 126. [Google Scholar] [CrossRef]
- Hanke, L.; Poeten, P.; Spanke, L.; Britz, S.; Diel, P. The Influence of Levothyroxine on Body Composition and Physical Performance in Subclinical Hypothyroidism. Horm. Metab. Res. 2023, 55, 51–58. [Google Scholar] [CrossRef]
- Sachdev, Y.; Hall, R. Effusions into Body Cavities in Hypothyroidism. Lancet 1975, 305, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Zwillich, C.W.; Pierson, D.J.; Hofeldt, F.D.; Lufkin, E.G.; Weil, J.V. Ventilatory Control in Myxedema and Hypothyroidism. N. Engl. J. Med. 1975, 292, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Siafakas, N.M.; Salesiotou, V.; Filaditaki, V.; Tzanakis, N.; Thalassinos, N.; Bouros, D. Respiratory Muscle Strength in Hypothyroidism. Chest 1992, 102, 189–194. [Google Scholar] [CrossRef]
- Kahaly, G.; Olshausen, K.V.; Mohr-Kahaly, S.; Erbel, R.; Boor, S.; Beyer, J.; Meyer, J. Arrhythmia Profile in Acromegaly. Eur. Heart J. 1992, 13, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Small, D.; Gibbons, W.; Levy, R.D.; de Lucas, P.; Gregory, W.; Cosio, M.G. Exertional Dyspnea and Ventilation in Hyperthyroidism. Chest 1992, 101, 1268–1273. [Google Scholar] [CrossRef]
- Pino-García, J.M.; García-Río, F.; Díez, J.J.; Gómez-Mendieta, M.A.; Racionero, M.A.; Díaz-Lobato, S.; Villamor, J. Regulation of Breathing in Hyperthyroidism: Relationship to Hormonal and Metabolic Changes. Eur. Respir. J. 1998, 12, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Nieswandt, J.; Wagner, S.; Schlegel, J.; Mohr-Kahaly, S.; Hommel, G. Ineffective Cardiorespiratory Function in Hyperthyroidism. J. Clin. Endocrinol. Metab. 1998, 83, 4075–4078. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Kampmann, C.; Mohr-Kahaly, S. Cardiovascular Hemodynamics and Exercise Tolerance in Thyroid Disease. Thyroid 2002, 12, 473–481. [Google Scholar] [CrossRef]
- Goswami, R.; Guleria, R.; Gupta, A.K.; Gupta, N.; Marwaha, R.K.; Pande, J.N.; Kochupillai, N. Prevalence of Diaphragmatic Muscle Weakness and Dyspnoea in Graves’ Disease and Their Reversibility with Carbimazole Therapy. Eur. J. Endocrinol. 2002, 147, 299–303. [Google Scholar] [CrossRef]
- van den Borst, B.; Gosker, H.R.; Zeegers, M.P.; Schols, A.M.W.J. Pulmonary Function in Diabetes: A Metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.L.; Krishnan, J.A.; Glick, S.; Smith, L.J. Systematic Review of the Association between Lung Function and Type 2 Diabetes Mellitus. Diabet. Med. 2010, 27, 977–987. [Google Scholar] [CrossRef]
- Sonoda, N.; Morimoto, A.; Tatsumi, Y.; Asayama, K.; Ohkubo, T.; Izawa, S.; Ohno, Y. A Prospective Study of the Impact of Diabetes Mellitus on Restrictive and Obstructive Lung Function Impairment: The Saku Study. Metabolism 2018, 82, 58–64. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Cai, Y.-H.; Shu, L.-P.; Yang, J.; Qi, L.; Han, M.; Zhou, J.; Simó, R.; Lecube, A. Bidirectional Relationship between Diabetes and Pulmonary Function: A Systematic Review and Meta-Analysis. Diabetes Metab. 2021, 47, 101186. [Google Scholar] [CrossRef]
- Lecube, A.; Simó, R.; Pallayova, M.; Punjabi, N.M.; López-Cano, C.; Turino, C.; Hernández, C.; Barbé, F. Pulmonary Function and Sleep Breathing: Two New Targets for Type 2 Diabetes Care. Endocr. Rev. 2017, 38, 550–573. [Google Scholar] [CrossRef] [PubMed]
- Fuso, L.; Pitocco, D.; Antonelli-Incalzi, R. Diabetic Lung, an Underrated Complication from Restrictive Functional Pattern to Pulmonary Hypertension. Diabetes Metab. Res. Rev. 2019, 35, e3159. [Google Scholar] [CrossRef] [PubMed]
- Maan, H.B.; Meo, S.A.; Al Rouq, F.; Meo, I.M.U.; Gacuan, M.E.; Alkhalifah, J.M. Effect of Glycated Hemoglobin (HbA1c) and Duration of Disease on Lung Functions in Type 2 Diabetic Patients. Int. J. Environ. Res. Public Health 2021, 18, 6970. [Google Scholar] [CrossRef] [PubMed]
- Abbas, U.; Shah, S.A.; Babar, N.; Agha, P.; Khowaja, M.A.; Nasrumminallah, M.; Arif, H.E.; Hussain, N.; Hasan, S.M.; Baloch, I.A. Cardiorespiratory Dynamics of Type 2 Diabetes Mellitus: An Extensive View of Breathing and Fitness Challenges in a Diabetes Prevalent Population. PLoS ONE 2024, 19, e0303564. [Google Scholar] [CrossRef]
- Al-Sayyar, A.; Hulme, K.D.; Thibaut, R.; Bayry, J.; Sheedy, F.J.; Short, K.R.; Alzaid, F. Respiratory Tract Infections in Diabetes—Lessons From Tuberculosis and Influenza to Guide Understanding of COVID-19 Severity. Front. Endocrinol. 2022, 13, 919223. [Google Scholar] [CrossRef]
- Salome, C.M.; King, G.G.; Berend, N. Physiology of Obesity and Effects on Lung Function. J. Appl. Physiol. 2010, 108, 206–211. [Google Scholar] [CrossRef]
- Hegewald, M.J. Impact of Obesity on Pulmonary Function: Current Understanding and Knowledge Gaps. Curr. Opin. Pulm. Med. 2021, 27, 132–140. [Google Scholar] [CrossRef] [PubMed]
- McNeill, J.N.; Lau, E.S.; Zern, E.K.; Nayor, M.; Malhotra, R.; Liu, E.E.; Bhat, R.R.; Brooks, L.C.; Farrell, R.; Sbarbaro, J.A.; et al. Association of Obesity-Related Inflammatory Pathways with Lung Function and Exercise Capacity. Respir. Med. 2021, 183, 106434. [Google Scholar] [CrossRef]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef]
- McClean, K.M.; Kee, F.; Young, I.S.; Elborn, J.S. Obesity and the Lung: 1. Epidemiology. Thorax 2008, 63, 649–654. [Google Scholar] [CrossRef]
- Buras, E.D.; Converso-Baran, K.; Davis, C.S.; Akama, T.; Hikage, F.; Michele, D.E.; Brooks, S.V.; Chun, T.-H. Fibro-Adipogenic Remodeling of the Diaphragm in Obesity-Associated Respiratory Dysfunction. Diabetes 2019, 68, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Pugliese, G.; Laudisio, D.; Castellucci, B.; Barrea, L.; Savastano, S.; Colao, A. The Impact of Obesity on Immune Response to Infection: Plausible Mechanisms and Outcomes. Obes. Rev. 2021, 22, e13216. [Google Scholar] [CrossRef] [PubMed]
- Hornung, F.; Rogal, J.; Loskill, P.; Löffler, B.; Deinhardt-Emmer, S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int. J. Mol. Sci. 2021, 22, 3456. [Google Scholar] [CrossRef]
- Peters, U.; Suratt, B.T.; Bates, J.H.T.; Dixon, A.E. Beyond BMI: Obesity and Lung Disease. Chest 2018, 153, 702–709. [Google Scholar] [CrossRef]
- Lo Mauro, A.; Tringali, G.; Codecasa, F.; Abbruzzese, L.; Sartorio, A.; Aliverti, A. Pulmonary and Chest Wall Function in Obese Adults. Sci. Rep. 2023, 13, 17753. [Google Scholar] [CrossRef]
- Joosen, D.A.W.A.; van de Laar, R.J.J.M.; Koopmans, R.P.; Stassen, P.M. Acute Dyspnea Caused by Hypocalcemia-Related Laryngospasm. J. Emerg. Med. 2015, 48, 29–30. [Google Scholar] [CrossRef]
- Gafni, R.I.; Collins, M.T. Hypoparathyroidism. N. Engl. J. Med. 2019, 380, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khan, A.A. Hypoparathyroidism: Diagnosis, Management and Emerging Therapies. Nat. Rev. Endocrinol. 2025, 21, 360–374. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Patraș, D.; Ciubară, A.B. Chronic Obstructive Pulmonary Disease and Type 2 Diabetes Mellitus: Complex Interactions and Clinical Implications. J. Clin. Med. 2025, 14, 1809. [Google Scholar] [CrossRef]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar] [CrossRef]
- Iglesias, P. Muscle in Endocrinology: From Skeletal Muscle Hormone Regulation to Myokine Secretion and Its Implications in Endocrine–Metabolic Diseases. J. Clin. Med. 2025, 14, 4490. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, S.; Zhou, R. Osteoporosis in Patients With Respiratory Diseases. Front. Physiol. 2022, 13, 939253. [Google Scholar] [CrossRef]
- Lippi, L.; Folli, A.; Curci, C.; D’Abrosca, F.; Moalli, S.; Mezian, K.; de Sire, A.; Invernizzi, M. Osteosarcopenia in Patients with Chronic Obstructive Pulmonary Diseases: Which Pathophysiologic Implications for Rehabilitation? Int. J. Environ. Res. Public Health 2022, 19, 14314. [Google Scholar] [CrossRef]
- Chopra, S.; Rathore, A.; Younas, H.; Pham, L.V.; Gu, C.; Beselman, A.; Kim, I.-Y.; Wolfe, R.R.; Perin, J.; Polotsky, V.Y.; et al. Obstructive Sleep Apnea Dynamically Increases Nocturnal Plasma Free Fatty Acids, Glucose, and Cortisol During Sleep. J. Clin. Endocrinol. Metab. 2017, 102, 3172–3181. [Google Scholar] [CrossRef]
- Ken-Dror, G.; Fry, C.H.; Murray, P.; Fluck, D.; Han, T.S. Changes in Cortisol Levels by Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea: Meta-Analysis of 637 Individuals. Clin. Endocrinol. 2021, 95, 909–917. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.; Xu, L.; Yang, Y.; Meng, Y.; Li, Y.; Liu, B. Obstructive Sleep Apnea and Serum Total Testosterone: A System Review and Meta-Analysis. Sleep Breath. 2023, 27, 789–797. [Google Scholar] [CrossRef]
- Alvarenga, T.A.; Fernandes, G.L.; Bittencourt, L.R.; Tufik, S.; Andersen, M.L. The Effects of Sleep Deprivation and Obstructive Sleep Apnea Syndrome on Male Reproductive Function: A Multi-Arm Randomised Trial. J. Sleep Res. 2023, 32, e13664. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Usategui-Martín, R.; Rigual, R.; Ruiz-Mambrilla, M.; Fernández-Gómez, J.-M.; Dueñas, A.; Pérez-Castrillón, J.L. Molecular Mechanisms Involved in Hypoxia-Induced Alterations in Bone Remodeling. Int. J. Mol. Sci. 2022, 23, 3233. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Farwell, A.P. Euthyroid Sick Syndrome. Compr. Physiol. 2016, 6, 1071–1080. [Google Scholar] [CrossRef]
- Fisseler-Eckhoff, A.; Demes, M. Neuroendocrine Tumors of the Lung. Cancers 2012, 4, 777–798. [Google Scholar] [CrossRef]
- Metovic, J.; Bianchi, F.; Rossi, G.; Barella, M.; Sonzogni, A.; Harari, S.; Papotti, M.; Pelosi, G. Recent Advances and Current Controversies in Lung Neuroendocrine Neoplasms. Semin. Diagn. Pathol. 2021, 38, 90–97. [Google Scholar] [CrossRef]
- Sung, S.; Heymann, J.J.; Politis, M.G.; Baine, M.K.; Rekhtman, N.; Saqi, A. Small Biopsy and Cytology of Pulmonary Neuroendocrine Neoplasms: Brief Overview of Classification, Immunohistochemistry, Molecular Profiles, and World Health Organization Updates. Adv. Anat. Pathol. 2022, 29, 329–336. [Google Scholar] [CrossRef]
- Crinò, L. ES 09.05 Management of Paraneoplastic Syndromes in SCLC. J. Thorac. Oncol. 2017, 12, S1630. [Google Scholar] [CrossRef]
- Anwar, A.; Jafri, F.; Ashraf, S.; Jafri, M.A.S.; Fanucchi, M. Paraneoplastic Syndromes in Lung Cancer and Their Management. Ann. Transl. Med. 2019, 7, 359. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Laskey, J.; Evans, W.K.; Goss, P.E.; Johansen, E.; Khamsi, F. Cushing’s Syndrome Associated with Ectopic Corticotropin Production and Small-Cell Lung Cancer. J. Clin. Oncol. 1992, 10, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Donovan, P.J.; Achong, N.; Griffin, K.; Galligan, J.; Pretorius, C.J.; McLeod, D.S.A. PTHrP-Mediated Hypercalcemia: Causes and Survival in 138 Patients. J. Clin. Endocrinol. Metab. 2015, 100, 2024–2029. [Google Scholar] [CrossRef]
- Ost, D.E.; Jim Yeung, S.-C.; Tanoue, L.T.; Gould, M.K. Clinical and Organizational Factors in the Initial Evaluation of Patients with Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e121S–e141S. [Google Scholar] [CrossRef]
- Beuschlein, F.; Else, T.; Bancos, I.; Hahner, S.; Hamidi, O.; van Hulsteijn, L.; Husebye, E.S.; Karavitaki, N.; Prete, A.; Vaidya, A.; et al. European Society of Endocrinology and Endocrine Society Joint Clinical Guideline: Diagnosis and Therapy of Glucocorticoid-Induced Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 2024, 109, 1657–1683. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Findling, J.; Bancos, I. Adrenal Insufficiency in Adults: A Review. JAMA 2025, 334, 714. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine Toxicities of Immune Checkpoint Inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Sánchez, J.C.; Díez, J.J. Isolated ACTH Deficiency Induced by Cancer Immunotherapy: A Systematic Review. Pituitary 2021, 24, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Johnson, D.B. Approach to the Patient With Immune Checkpoint Inhibitor-Associated Endocrine Dysfunction. J. Clin. Endocrinol. Metab. 2023, 108, 1514–1525. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, T.; Yu, L.; Ma, W.; Liu, W.; Zhang, C. The Risk of Endocrine Immune-Related Adverse Events Induced by PD-1 Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2024, 14, 1381250. [Google Scholar] [CrossRef]
- DailyMed—AFREZZA–Insulin Human Powder, Metered AFREZZA–Insulin Human Kit. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29f4637b-e204-425b-b89c-7238008d8c10 (accessed on 22 August 2025).
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S181–S206. [Google Scholar] [CrossRef]
| Hormone/Mediator | Receptor | Pulmonary Localization | Main Functions | References |
|---|---|---|---|---|
| Thyroid hormones (T3/T4) | TRa, TRβ | Alveolar type II (AT2), regenerating epithelium | Alveologenesis, AT2→AT1 differentiation, antifibrotic | [48,49,50] |
| Glucocorticoids | GRa, GRβ | Epithelium, endothelium, airway smooth muscle | Anti-inflammatory, transcriptomic reprogramming, resistance (GRβ) | [51,52,53,54,55,56,57] |
| Estrogens | ERa, ERβ | Airway smooth muscle, bronchial epithelium | Bronchodilation, Ca2+ regulation | [58,59,60,61,62,63,64,65,66] |
| Progesterone | PR | Ciliated epithelium | Decreases ciliary beat (transcriptionally mediated) | [56] |
| Insulin | IR | AT2, airway epithelium | Alveolar fluid clearance, metabolic regulation | [57] |
| IGF-1 | IGF1R | Epithelium, fibroblasts, endothelium | Cell proliferation, repair, fibrosis | [57,58] |
| Endothelin-1 | ETA, ETB | Vascular smooth muscle (ETA), endothelium (ETB) | Vasoconstriction, vascular tone, remodeling | [59] |
| Leptin | Ob-Rb | AT2, bronchial epithelium, alveolar macrophages | Immune modulation, alveolar homeostasis | [60,61] |
| PTHrP | PTH1R | Lipofibroblasts (via AT2 secretion) | Surfactant synthesis, alveolar stability | [62,63] |
| Vitamin D (1,25(OH)2D3) | VDR | Epithelium, alveolar macrophages | Antimicrobial peptide induction, immune modulation | [67,68] |
| Hormone/Mediator | Cell of Origin | Stimuli | Main Functions | Ref. |
|---|---|---|---|---|
| Serotonin | Pulmonary neuroendocrine cells (PNECs) | Hypoxia, neural stimulation | Pulmonary vasoconstriction, ventilation regulation, immune modulation | [23,79] |
| CGRP (Calcitonin gene-related peptide) | PNECs | Hypoxia, epithelial injury | Vasodilation, activation of ILC2, goblet cell differentiation, airway inflammation | [23,79] |
| Bombesin-like peptides (GRP) | PNECs | Hypoxia, neural inputs | Epithelial repair, immune modulation | [23,79] |
| Endothelin-1 | Pulmonary endothelial cells | Shear stress, inflammation | Vasoconstriction, vascular tone regulation, pulmonary hypertension | [80,81] |
| Leptin | Type II pneumocytes | Inflammation, mechanical stress | Immune modulation, surfactant regulation, alveolar homeostasis | [73,74] |
| Keratinocyte growth factor (KGF) | Type II pneumocytes | Epithelial injury | Epithelial proliferation, tissue repair, surfactant regulation | [77,78] |
| GABA, dopamine, norepinephrine | PNECs | Hypoxia | Neurotransmission, ventilation regulation, CNS-lung communication | [23,79] |
| Syndrome | Associated Tumor | Prevalence | Clinical Features | Diagnostic Clues | Treatment | References |
|---|---|---|---|---|---|---|
| SIADH (Syndrome of Inappropriate Antidiuretic Hormone Secretion) | Small Cell Lung Carcinoma (SCLC) | ~10–15% | Hyponatraemia, low serum osmolarity, high urine osmolarity, no edema | Unexplained hyponatraemia in smokers | Fluid restriction, hypertonic saline (if severe), vasopressin receptor antagonists (e.g., tolvaptan) | [148,149] |
| Ectopic Cushing’s Syndrome (ECS) | Mainly SCLC; also bronchial carcinoids | ~1–5% | Rapid muscle weakness, hyperglycaemia, hypertension, hypokalaemia, metabolic alkalosis | High cortisol and ACTH; no suppression on high-dose dexamethasone | Ketoconazole, metyrapone, etomidate; oncologic therapy | [150] |
| Humoral Hypercalcemia of Malignancy (HHM) | Squamous cell carcinoma (NSCLC) | Less common than SIADH/ECS | Nausea, constipation, polyuria, confusion, coma (in severe cases) | Hypercalcaemia, low PTH, high PTHrP | IV hydration, bisphosphonates (e.g., zoledronic acid), denosumab (if refractory) | [151] |
| Carcinoid Syndrome | Bronchial carcinoid tumors (with liver metastases) | Rare (<1%) | Flushing, watery diarrhea, bronchospasm | Elevated 5-HIAA (urine), high chromogranin A | Somatostatin analogues (e.g., octreotide) | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, P. Endocrinology and the Lung: Exploring the Bidirectional Axis and Future Directions. J. Clin. Med. 2025, 14, 6985. https://doi.org/10.3390/jcm14196985
Iglesias P. Endocrinology and the Lung: Exploring the Bidirectional Axis and Future Directions. Journal of Clinical Medicine. 2025; 14(19):6985. https://doi.org/10.3390/jcm14196985
Chicago/Turabian StyleIglesias, Pedro. 2025. "Endocrinology and the Lung: Exploring the Bidirectional Axis and Future Directions" Journal of Clinical Medicine 14, no. 19: 6985. https://doi.org/10.3390/jcm14196985
APA StyleIglesias, P. (2025). Endocrinology and the Lung: Exploring the Bidirectional Axis and Future Directions. Journal of Clinical Medicine, 14(19), 6985. https://doi.org/10.3390/jcm14196985







