Trends in Etiology and Mortality in Severe Polytrauma Patients with Traumatic Brain Injury: A 25-Year Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
- Adult patients (≥18 years);
- Polytrauma, defined as ISS > 15 or NISS > 15;
- Presence of TBI confirmed by clinical or radiological findings;
- Admission to our center within the study period (1993–2018);
- Received prehospital or in-hospital acute management;
- Complete medical records with accessible data.
2.3. Exclusion Criteria
- Patients under 18 years;
- Low-energy trauma or non-traumatic brain injuries (e.g., stroke, hypoxic encephalopathy);
- Isolated TBI without systemic polytrauma (ISS ≤ 15);
- Patients declared dead at the scene;
- Missing key variables or incomplete prehospital or in-hospital records;
- Other specific exclusions included patients with burns, hanging or drowning due to different pathophysiology and outcomes.
2.4. Variables Analyzed
- Epidemiological factors: age, sex, date of injury;
- Medical history and trauma characteristics: mechanism (blunt/penetrating), intent (assault, self-inflicted, or accidental);
- Trauma cause: traffic accidents (car, motorcycle, pedestrian), falls, assaults, others;
- Severity indicators and protective factors: seatbelt or helmet use, fall height, prehospital vital signs, emergency medical team care (SAMUR-061), initial shock, cardiopulmonary resuscitation (CPR), intubation, lactate, fluid resuscitation;
- In-hospital parameters: vital signs, emergency surgery, transfusions, injury location, Abbreviated Injury Scale (AIS), ISS, and NISS;
- Outcomes: ICU admission, complications, case fatality rate (CFR) (day 1, 30-day, overall), including preventable deaths.
2.5. Statistical Analysis
3. Results
3.1. General Analysis
3.2. Initial Care
3.3. Period-Based Analysis
3.4. Case Fatality Rates
4. Discussion
- The incidence of PTBI progressively declined, largely reflecting advances in road safety legislation and preventive strategies.
- Patient demographics shifted toward an older and more comorbid population, a trend that may counterbalance therapeutic progress and complicate outcomes.
- Overall case fatality decreased, mainly due to improvements in prehospital management, hemorrhage control, and systemic trauma care; however, changes in case-mix and hospital-level practices may influence these trends.
- TBI-related fatality persisted at 28%, with this apparent stability likely reflecting an older, more comorbid population, a higher burden of severe cases, and the inherent limitations of crude mortality rates, reinforcing the need to emphasize functional outcomes and to develop targeted neuroprotective strategies.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Abbreviated Injury Scale |
| CFR | Case Fatality Rates |
| CNS | Central nervous system |
| CRASH | Corticosteroid Randomization After Significant Head Injury |
| GCS | Glasgow Coma Scale |
| IMPACT | International Mission for Prognosis and Analysis of Clinical Trials in TBI |
| ICU | Intensive Care Unit |
| ISS | Injury Severity Score |
| NISS | New Injury Severity Score |
| PTBI | Polytrauma with Traumatic Brain Injury |
| RTA | Road Traffic Accidents |
| SAMUR-061 | Madrid Emergency Medical Service |
| TBI | Traumatic Brain Injury |
| TRISS | Trauma and Injury Severity Score |
| WHO | World Health Organization |
References
- Schubert, F.D.; Gabbe, L.J.; Bjurlin, M.A.; Renson, A. Differences in trauma mortality between ACS-verified and state-designated trauma centers in the US. Injury 2019, 50, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- World Health Organization. The Global Burden of Disease: 2004 Update 2004. Available online: https://www.who.int/publications/i/item/9789241563710 (accessed on 11 September 2025).
- Chico-Fernández, M.; Llompart-Pou, J.; Guerrero-López, F.; Sánchez-Casado, M.; García-Sáez, I.; García, M.; Egea-Guerrero, J.; Fernández-Ortega, J.; Bueno-González, A.; González-Robledo, J.; et al. Epidemiología del trauma grave en España. REgistro de TRAuma en UCI (RETRAUCI). Fase piloto. Med. Intensiv. 2016, 40, 327–347. [Google Scholar] [CrossRef]
- Clark, E.C.; Neumann, S.; Hopkins, S.; Kostopoulos, A.; Hagerman, L.; Dobbins, M. Changes to Public Health Surveillance Methods Due to the COVID-19 Pandemic: Scoping Review. JMIR Public Heal. Surveill. 2024, 10, e49185. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Stelfox, H.T.; Evans, D.; Hameed, S.M.; Yanchar, N.L.; Simons, R.; Kortbeek, J.; Bourgeois, G.; Clément, J.; Turgeon, A.F.; et al. Trends in Injury Outcomes Across Canadian Trauma Systems. JAMA Surg. 2017, 152, 168–174. [Google Scholar] [CrossRef]
- Scarborough, K.; Slone, D.S.; Uribe, P.; Craun, M.; Bar-Or, R.; Bar-Or, D. Reduced Mortality at a Community Hospital Trauma Center. Arch. Surg. 2008, 143, 22–27. [Google Scholar] [CrossRef]
- Ministerio de Sanidad-Asuntos Sociales-Igualdad. Lesiones en España Análisis de la Legislación Sobre Prevención de Lesiones no Intencionales. 2012. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/lesiones/legislacion/docs/LESIONES_Espana.pdf (accessed on 1 August 2025).
- Pineda-Jaramillo, J.; Barrera-Jiménez, H.; Mesa-Arango, R. Unveiling the relevance of traffic enforcement cameras on the severity of vehicle–pedestrian collisions in an urban environment with machine learning models. J. Saf. Res. 2022, 81, 225–238. [Google Scholar] [CrossRef]
- Border, J.R.; LaDuca, J.; Seibel, R. Priorities in the management of the patient with polytrauma. Prog. Surg. 1975, 14, 84–120. [Google Scholar] [CrossRef]
- Pape, H.; Leenen, L. Polytrauma management - What is new and what is true in 2020? J. Clin. Orthop. Trauma 2021, 12, 88–95. [Google Scholar] [CrossRef]
- Pape, H.-C.; Lefering, R.; Butcher, N.; Peitzman, A.; Leenen, L.; Marzi, I.; Lichte, P.; Josten, C.; Bouillon, B.; Schmucker, U.; et al. The definition of polytrauma revisited. J. Trauma Acute Care Surg. 2014, 77, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B.M.; Enninghorst, N.; King, K.L.; Balogh, Z.J. The most critically injured polytrauma patient mortality: Should it be a measurement of trauma system performance? Eur. J. Trauma Emerg. Surg. 2022, 50, 115–119. [Google Scholar] [CrossRef]
- Hamill, V.; Barry, S.J.; McConnachie, A.; McMillan, T.M.; Teasdale, G.M. Mortality from Head Injury over Four Decades in Scotland. J. Neurotrauma 2015, 32, 689–703. [Google Scholar] [CrossRef]
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Zahed, C. Prediction of outcome in severe traumatic brain injury. Curr. Opin. Crit. Care 2009, 15, 437–441. [Google Scholar] [CrossRef]
- Picetti, E.; Rossi, S.; Abu-Zidan, F.M.; Ansaloni, L.; Armonda, R.; Baiocchi, G.L.; Bala, M.; Balogh, Z.J.; Berardino, M.; Biffl, W.L.; et al. WSES consensus conference guidelines: Monitoring and management of severe adult traumatic brain injury patients with polytrauma in the first 24 hours. World J. Emerg. Surg. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Ahmed, N.; Kuo, Y.-H. Prediction of Trauma Mortality Incorporating Pre-injury Comorbidities Into Existing Mortality Scoring Indices. Am. Surg. 2022, 88, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, E.; Naumann, R.B.; Deveaux, G.; Wang, L.; Stringfellow, E.J.; Lich, K.H.; Jalali, M.S. Impacts of alcohol and opioid polysubstance use on road safety: Systematic review. Accid. Anal. Prev. 2022, 173, 106713. [Google Scholar] [CrossRef]
- Brinck, T.; Heinänen, M.; Söderlund, T.; Lefering, R.; Handolin, L. Does arrival time affect outcomes among severely injured blunt trauma patients at a tertiary trauma centre? Injury 2019, 50, 1929–1933. [Google Scholar] [CrossRef]
- Hildebrand, F.; Giannoudis, P.V.; van Griensven, M.; Zelle, B.; Ulmer, B.; Krettek, C.; Bellamy, M.C.; Pape, H.-C. Management of polytraumatized patients with associated blunt chest trauma: A comparison of two European countries. Injury 2005, 36, 293–302. [Google Scholar] [CrossRef]
- MacKenzie, E.J.; Rivara, F.P.; Jurkovich, G.J.; Nathens, A.B.; Frey, K.P.; Egleston, B.L.; Salkever, D.S.; Scharfstein, D.O. A National Evaluation of the Effect of Trauma-Center Care on Mortality. New Engl. J. Med. 2006, 354, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Aharonson-Daniel, L.; Giveon, A.; Stein, M.; Peleg, K. Different AIS Triplets: Different Mortality Predictions in Identical ISS and NISS. J. Trauma Acute Care Surg. 2006, 61, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Tang, B.; Xue, C.; Liu, Y.; Liu, X.; Lv, Y.; Zhang, L. Comparison of the Ability to Predict Mortality between the Injury Severity Score and the New Injury Severity Score: A Meta-Analysis. Int. J. Environ. Res. Public Heal. 2016, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Lossius, H.M.; Rehn, M.; E Tjosevik, K.; Eken, T. Calculating trauma triage precision: Effects of different definitions of major trauma. J. Trauma Manag. Outcomes 2012, 6, 9. [Google Scholar] [CrossRef]
- Serviá, L.; Montserrat, N.; Badia, M.; Llompart-Pou, J.A.; Barea-Mendoza, J.A.; Chico-Fernández, M.; Sánchez-Casado, M.; Jiménez, J.M.; Mayor, D.M.; Trujillano, J. Machine learning techniques for mortality prediction in critical traumatic patients: Anatomic and physiologic variables from the RETRAUCI study. BMC Med Res. Methodol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Larkin, E.J.; Jones, M.K.; Young, S.D.; Young, J.S. Interest of the MGAP score on in-hospital trauma patients: Comparison with TRISS, ISS and NISS scores. Injury 2022, 53, 3059–3064. [Google Scholar] [CrossRef]
- Salehi, O.; Dezfuli, S.A.T.; Namazi, S.S.; Khalili, M.D.; Saeedi, M. A New Injury Severity Score for Predicting the Length of Hospital Stay in Multiple Trauma Patients. Trauma Mon. 2016, 21, e20349. [Google Scholar] [CrossRef]
- Carver, D.; Kirkpatrick, A.W.; D’aMours, S.; Hameed, S.M.; Beveridge, J.; Ball, C.G. A Prospective Evaluation of the Utility of a Hybrid Operating Suite for Severely Injured Patients. Ann. Surg. 2020, 271, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Hynes, A.M.; Geng, Z.; Schmulevich, D.; Fox, E.E.; Meador, C.L.; Scantling, D.R.; Holena, D.N.; Abella, B.S.; Young, A.J.; Holland, S.; et al. Staying on target: Maintaining a balanced resuscitation during damage-control resuscitation improves survival. J. Trauma Acute Care Surg. 2021, 91, 841–848. [Google Scholar] [CrossRef]
- Ball, C.G.; Kirkpatrick, A.W.; D’Amours, S.K. The RAPTOR: Resuscitation with angiography, percutaneous techniques and oper-ative repair. Transforming the discipline of trauma surgery. Can. J. Surg. 2011, 54, E3–E4. [Google Scholar]
- Holcomb, J.B.; Jenkins, D.; Rhee, P.; Johannigman, J.; Mahoney, P.; Mehta, S.; Cox, E.D.; Gehrke, M.J.; Beilman, G.J.; Schreiber, M.; et al. Damage Control Resuscitation: Directly Addressing the Early Coagulopathy of Trauma. J. Trauma: Inj. Infect. Crit. Care 2007, 62, 307–310. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Santiago, L.A.; Oh, B.C.; Dash, P.K.; Holcomb, J.B.; Wade, C.E. A clinical comparison of penetrating and blunt traumatic brain injuries. Brain Inj. 2012, 26, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Sun, Y.-R.; Wu, X.; Yu, J.; Li, Z.-Q.; Du, Z.-Y.; Wu, X.-H.; Zhou, L.-F.; Hu, J. Coagulopathy in Traumatic Brain Injury and Its Correlation with Progressive Hemorrhagic Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2016, 33, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.H.; Joseph, D.; Castater, C.; A Kuhls, D.; Kirkendoll, S.; Albini, P.; Duncan, T.K. Growing injury threats to longevity in the older population: American Association for the Surgery of Trauma Prevention Committee topical update. Trauma Surg. Acute Care Open 2025, 10, e001526. [Google Scholar] [CrossRef]
- Glynn, R.; Edwards, F.; Wullschleger, M.; Gardiner, B.; Laupland, K.B. Major trauma and comorbidity: A scoping review. Eur. J. Trauma Emerg. Surg. 2025, 51, 1–16. [Google Scholar] [CrossRef]
- Veerapaneni, D.; Sakthiyendran, N.B.A.; Du, Y.; Mallinger, L.A.B.; Reinert, A.B.; Kim, S.Y.; Nguyen, C.B.; Daneshmand, A.; Abdalkader, M.; Mohammed, S.; et al. Early Pupil Abnormality Frequency Predicts Poor Outcomes and Enhances International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Model Prognostication in Traumatic Brain Injury. Crit. Care Explor. 2025, 7, e1257. [Google Scholar] [CrossRef]
- Piñeiro, P.; Calvo, A.; Pérez-Díaz, M.D.; Ramos, S.; García-Ramos, S.; Power, M.; Solchaga, I.; Rey, C.; Hortal, J.; Turégano, F.; et al. Early Thrombocytopenia at Hospital Admission Predicts Mortality in Patients with Non-Isolated Severe Traumatic Brain Injury. Biomedicines 2024, 12, 2702. [Google Scholar] [CrossRef]
- Mekkodathil, A.; El-Menyar, A.; Hakim, S.; Al Jogol, H.; Parchani, A.; Peralta, R.; Rizoli, S.; Al-Thani, H. Initial Serum Levels of Magnesium and Calcium as Predictors of Mortality in Traumatic Brain Injury Patients: A Retrospective Study. Diagnostics 2023, 13, 1172. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Rau, C.-S.; Huang, C.-Y.; Su, W.-T.; Hsu, S.-Y.; Hsieh, C.-H. The Stress Index as a Predictor of Mortality in Patients with Isolated Moderate to Severe Traumatic Brain Injury. Diagnostics 2024, 14, 1244. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Yen, Y.-H.; Tsai, C.-H.; Hsu, S.-Y.; Tsai, P.-L.; Hsieh, C.-H. Geriatric Trauma Outcome Score as a Mortality Predictor in Isolated Moderate to Severe Traumatic Brain Injury: A Single-Center Retrospective Study. Healthcare 2024, 12, 1680. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, O.H. Recent Advances in Prehospital and In-Hospital Management of Patients with Severe Trauma. J. Clin. Med. 2025, 14, 2208. [Google Scholar] [CrossRef] [PubMed]
- Isgrò, S.; Giani, M.; Antolini, L.; Giudici, R.; Valsecchi, M.G.; Bellani, G.; Chiara, O.; Bassi, G.; Latronico, N.; Cabrini, L.; et al. Identifying Trauma Patients in Need for Emergency Surgery in the Prehospital Setting: The Prehospital Prediction of In-Hospital Emergency Treatment (PROPHET) Study. J. Clin. Med. 2023, 12, 6660. [Google Scholar] [CrossRef] [PubMed]
- Kapapa, T.; Petkov, M.; Pala, A.; Woischneck, D.; Schiller, F.; Jesuthasan, S.; Schiller, F.; Bracht, H.; Mayer, B.; Oehmichen, M. Mortality During In-Hospital Stay and the First 24 h After Decompressive Craniectomy in Severe Traumatic Brain Injury: A Multi-Center, Retrospective Propensity Score-Matched Study. J. Clin. Med. 2025, 14, 5540. [Google Scholar] [CrossRef]
- Ministerio de Presidencia. RD 1428/2003 Para Aprobación de la ley Sobre Tráfico, Circulación de Vehículos a Motor y Seguridad Vial. 2003. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2003-23514 (accessed on 1 August 2025).
- Ministerio del Interior. Real Decreto Legislativo 6/2015, de 30 de Octubre, por el que se Aprueba el Texto Refundido de la Ley Sobre Tráfico, Circulación de Vehículos a Motor y Seguridad Vial. BOE 2015. Ars Iuris Salmant. 2016, 4, 232–233. [Google Scholar]
- European Road Safety Observatory. European Road Safety Observatory. European Road Safety Observatory Facts and Figures – Pedestrians - 2021. Facts and Figures, European Comission. 2021. Available online: https://road-safety.transport.ec.europa.eu/system/files/2022-03/FF_pedestrians_20220209.pdf (accessed on 13 September 2025).
- Governors Highway Safety Association. Governors Highway Safety Association. Pedestrian Traffic Fatalities by State: 2022 Preliminary Data (January-December). GHSA’s Annual Spotlight Report. 2023. Available online: https://www.ghsa.org/resource-hub/pedestrian-traffic-fatalities-state-2022-preliminary-data-january-december (accessed on 1 August 2025).
- Naumann, R.B.; West, B.A.; Barry, V.; Matthews, S.; Lee, R. Pedestrian and Overall Road Traffic Crash Deaths — United States and 27 Other High-Income Countries, 2013–2022. Mmwr-Morbidity Mortal. Wkly. Rep. 2025, 74, 134–139. [Google Scholar] [CrossRef]
- Blanco, C. El suicidio en España. Respuesta institucional y social. Rev. Cienc. Soc. 2020, 33, 79–106. [Google Scholar]
- World Health Organization. Preventing suicide: A Global Imperative. World Health Organization Mental Health, Brain Health and Substance Use. 2014. Available online: https://www.who.int/publications/i/item/9789241564779 (accessed on 11 September 2025).
- Martínez-Herrera, E.; Galindo-Oseguera, E.; Castillo-Cruz, J.; Fuentes-Venado, C.E.; Gasca-López, G.A.; Calzada-Mendoza, C.C.; Ocharan-Hernández, E.; Zúñiga-Cruz, C.A.; Farfán-García, E.D.; Arellano-Ramírez, A.; et al. Mortality-Associated Factors in a Traumatic Brain Injury Population in Mexico. Biomedicines 2024, 12, 2037. [Google Scholar] [CrossRef]
- Benz, D.; Balogh, Z.J. Damage control surgery: Current state and future directions. Curr. Opin. Crit. Care 2017, 23, 491–497. [Google Scholar] [CrossRef]

| Patients | Neurological Condition | ||||
| Age in years (mean, SD) | 43 | 20 | Initial assessment (n/%) | ||
| Sex (n/%) | Pupillary abnormality | 129 | 17 | ||
| Female | 220 | 29 | Deficit | 52 | 7 |
| Male | 548 | 71 | INJURIES BY REGION/SEVERITY (median, IQR) | ||
| Comorbidities (n/%) | Head AIS | 4 | 3–5 | ||
| 0 | 409 | 53 | Face AIS | 0 | 0 |
| 1 | 145 | 19 | Thorax AIS | 2 | 0–3 |
| ≥2 | 158 | 28 | Abdomen AIS | 0 | 0–2 |
| Type of comorbidities (n/%) | Extremities AIS | 2 | 0–3 | ||
| Hypertension | 65 | 9 | Skin AIS | 0 | 0–1 |
| Cardiopathy | 23 | 3 | ISS | 27 | 19–38 |
| Ischemic heart disease | 23 | 3 | NISS | 34 | 24–41 |
| Diabetes mellitus | 32 | 4 | INITIAL SURGERIES (n/%) | ||
| Anticoagulation | 33 | 4 | Chest tube insertion | 169 | 22 |
| Substance abuse | 38 | 5 | Emergent surgery | 388 | 51 |
| Alcoholism | 32 | 4 | Neurosurgery | 193 | 25 |
| Psychiatric disorder | 61 | 8 | CASE FATALITY RATES (n/%) | ||
| INITIAL MANAGEMENT (n/%) | Total (30 days) | 262 | 34 | ||
| 061-SAMUR team | 722 | 94 | Death upon arrival | 64 | 8 |
| Prehospital status | Death on first day | 137 | 19 | ||
| Intubation | 488 | 64 | CAUSE OF DEATH (n/%) | ||
| CPR | 36 | 5 | CNS injury | 212 | 28 |
| Apnea | 115 | 15 | Exsanguination | 40 | 5 |
| Shock | 136 | 9 | Sepsis | 13 | 2 |
| CAUSES OF SHOCK AT ADMISSION (n/%) | Multiorgan failure | 13 | 2 | ||
| CNS | 36 | 26 | Cardiac/Lung injury | 14 | 2 |
| Multiple | 26 | 19 | Distributive shock | 5 | 0.7 |
| Hemoperitoneum | 20 | 15 | COMPLICATIONS (n/%) | ||
| Fractures | 12 | 9 | 0 | 380 | 50 |
| Other | 42 | 31 | 1 | 252 | 33 |
| No shock | 632 | 82 | >1 | 14 | 2 |
| Studyperiods | 1 | 2 | 3 | 4 |
| n | 184 | 206 | 191 | 187 |
| Age in years, mean (+/−SD) | 38 (+/−17) | 38 (+/−17) | 42 (+/−21) | 54 (+/−22) |
| Sex, n (%) | ||||
| Female | 47 (25%) | 55 (27%) | 48 (25%) | 70 (37%) |
| Male | 137 (75%) | 151 (73%) | 143 (75%) | 117 (63%) |
| Comorbidity, n (%) | ||||
| No history | 145 (79%) | 101 (49%) | 91 (48%) | 72 (39%) |
| Hypertension | 0 | 6 (3%) | 20 (10%) | 39 (21%) |
| Cardiopathy | 0 | 3 (1%) | 6 (3%) | 14 (7%) |
| Ischemic heart disease | 0 | 7 (3%) | 6 (3%) | 10 (5%) |
| Diabetes mellitus | 0 | 6 (3%) | 6 (3%) | 20 (11%) |
| Anticoagulation | 0 | 6 (3%) | 5 (3%) | 22 (12%) |
| Substance abuse | 6 (3%) | 16 (8%) | 8 (4%) | 8 (4%) |
| Alcoholism | 5 (3%) | 14 (7%) | 8 (4%) | 5 (3%) |
| Psychiatric disorder | 5 (3%) | 16 (8%) | 17 (9%) | 24 (13%) |
| Mechanism of trauma, n (%) | ||||
| Car | 52 (28%) | 59 (29%) | 24 (13%) | 12 (6%) |
| Motorcycle | 24 (13%) | 31 (15%) | 37 (19%) | 18 (10%) |
| Bicycle | 2 (1%) | 2 (1%) | 2 (1%) | 5 (3%) |
| Pedestrian | 59 (32%) | 32 (16%) | 49 (26%) | 59 (32%) |
| Fall | 40 (22%) | 36 (17%) | 53 (28%) | 68 (36%) |
| Suicide attempt | 0 | 6 (3%) | 10 (5%) | 25 (13%) |
| Firearm | 4 (2%) | 7 (3%) | 5 (3%) | 3 (2%) |
| Sharp weapon | 1 (0.5%) | 2 (1%) | 4 (2%) | 1 (0.5%) |
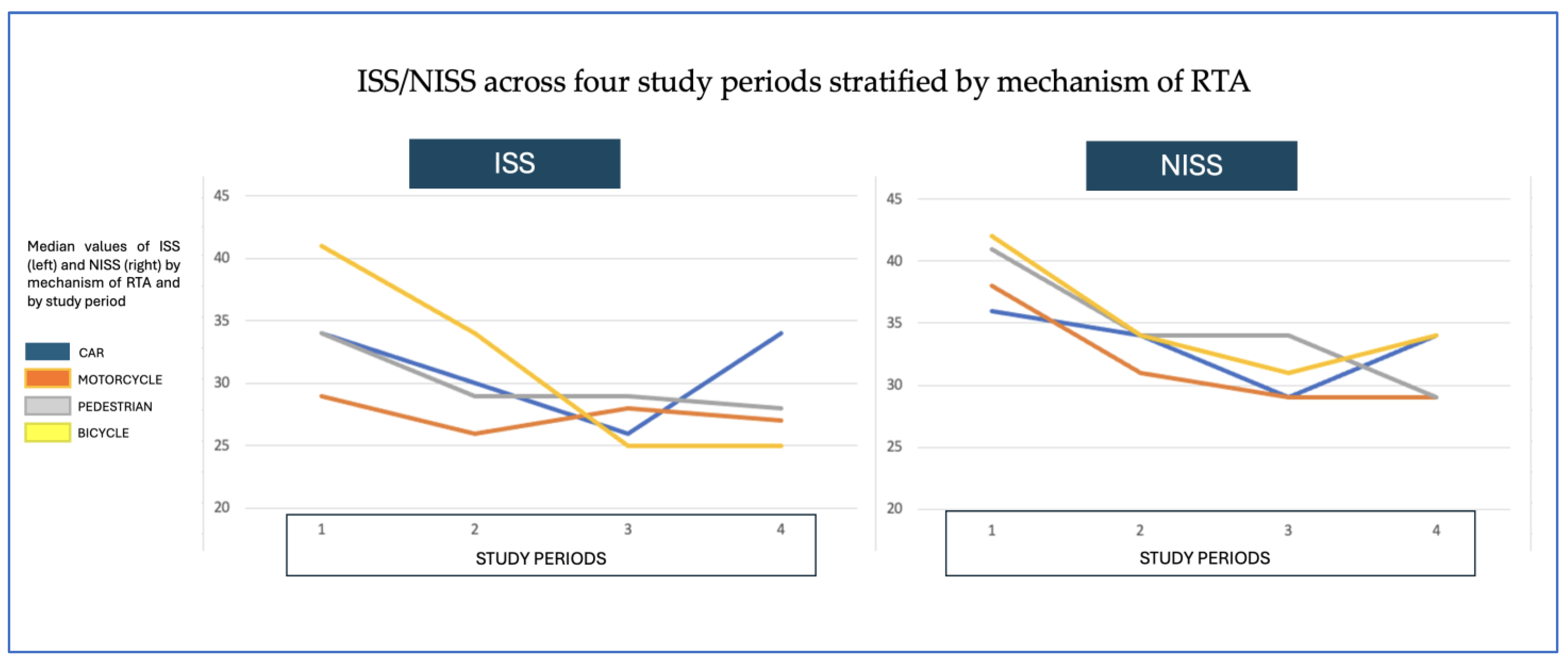
| Car-Related PTBI | Motorcycle-Related PTBI | |||||||
| Period | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| n | 52 | 59 | 24 | 12 | 24 | 31 | 37 | 18 |
| Age in years, mean (+/−SD) | 34 (+/−14) | 34 (+/−14) | 26 (+/−8) | 41 (+/−21) | 24 (+/−6) | 29 (+/−12) | 33 (+/−13) | 37 (+/−14) |
| Seatbelt/Helmet, n (%) | 7 (13%) | 18 (31%) | 12 (50%) | 9 (75%) | 8 (33%) | 11 (35%) | 21 (57%) | 16 (89%) |
| Trauma scores | ||||||||
| GCS, median (IQR) | 5 (3–9) | 8 (5–12) | 7 (3–14) | 6 (4–15) | 4 (3–10) | 6 (3–11) | 7 (3–13) | 11 (6–15) |
| IIS, median (IQR) | 34 (24–50) | 30 (19–38) | 26 (20–34) | 34 (26–36) | 29 (22–42) | 26 (20–36) | 28 (22–24) | 27 (18–34) |
| NISS, median (IQR) | 36 (25–50) | 34 (25–43) | 29 (26–41) | 34 (29–41) | 38 (25–47) | 31 (25–41) | 29 (22–37) | 29 (22–34) |
| Case fatality | ||||||||
| Total, n (%) | 17 (32%) | 18 (33%) | 4 (15%) | 4 (33%) | 5 (20%) | 9 (30%) | 4 (11%) | 2 (11%) |
| On arrival, n (%) | 3 (6%) | 1 (2%) | 1 (4%) | 0 | 0 | 0 | 1 (3%) | 0 |
| Due to CNS injury, n (%) | 13 (24%) | 13 (24%) | 4 (16%) | 4 (33%) | 3 (12%) | 9 (30%) | 4 (11%) | 2 (11%) |
| Periods | 1 | 2 | 3 | 4 |
| n | 184 | 206 | 191 | 187 |
| Trauma scores, median (IQR) | ||||
| GCS | 3 (3–9) | 8 (3–12) | 7 (3–13) | 11 (6–15) |
| ISS | 34 (25–50) | 27 (17–36) | 25 (19–34) | 25 (16–34) |
| NISS | 41 (29–50) | 32 (22–41) | 29 (24–38) | 29 (22–38) |
| Injury distribution per region (AIS), median (IQR) | ||||
| Head | 5 (4–5) | 4 (3–4) | 4 (3–4) | 4 (3–5) |
| Face | 0 | 0 (0–1) | 0 (0–2) | 0 (0–2) |
| Thorax | 3 (0–4) | 3 (0–4) | 1 (0–3) | 0 (0–3) |
| Abdomen | 0 (0–2) | 0 | 0 (0–2) | 0 (0–2) |
| Extremities | 2 (0–3) | 2 (0–3) | 0 (0–3) | 0 (0–3) |
| Skin | 0 | 0 (0–1) | 0 (0–1) | 0 |
| Initial surgeries, n (%) | ||||
| Chest tube insertion | 43 (23%) | 44 (21%) | 39 (20%) | 43 (23%) |
| Emergent surgery | 95 (52%) | 114 (55%) | 99 (52%) | 80 (43%) |
| Neurosurgery | 27 (15%) | 58 (28) | 57 (30%) | 34 (18%) |
| ICU admittance, n (%) | ||||
| ICU | 146 (79%) | 181 (88%) | 167 (87%) | 147 (79%) |
| Case Fatality Rates, n (%) | ||||
| Total | 75 (41%) | 73 (35%) | 58 (30%) | 57 (30%) |
| On arrival | 29 (16%) | 10 (5%) | 15 (8%) | 10 (5%) |
| First day | 61 (33%) | 53 (26%) | 26 (14%) | 27 (14%) |
| Cause of death, n (%) | ||||
| CNS injury | 52 (28%) | 61 (30%) | 47 (25%) | 52 (28%) |
| Exsanguination | 9 (5%) | 14 (7%) | 14 (7%) | 3 (2%) |
| Sepsis | 2 (1%) | 6 (3%) | 4 (2%) | 1 (0.5%) |
| Multiorgan failure | 3 (2%) | 8 (4%) | 1 (0.5%) | 1 (0.5%) |
| Cardiac/lung injury | 2 (1%) | 8 (4%) | 3 (1%) | 3 (2%) |
| Distributive shock | 1 (0.5%) | 2 (1%) | 2 (1%) | 0 |
| Variable | p | HR | CI 95% |
| Patients | |||
| Advanced age | <0001 | 1.01 | 1.004–1.016 |
| Period 4 | <0.001 | 0.806 | 0.717–0.906 |
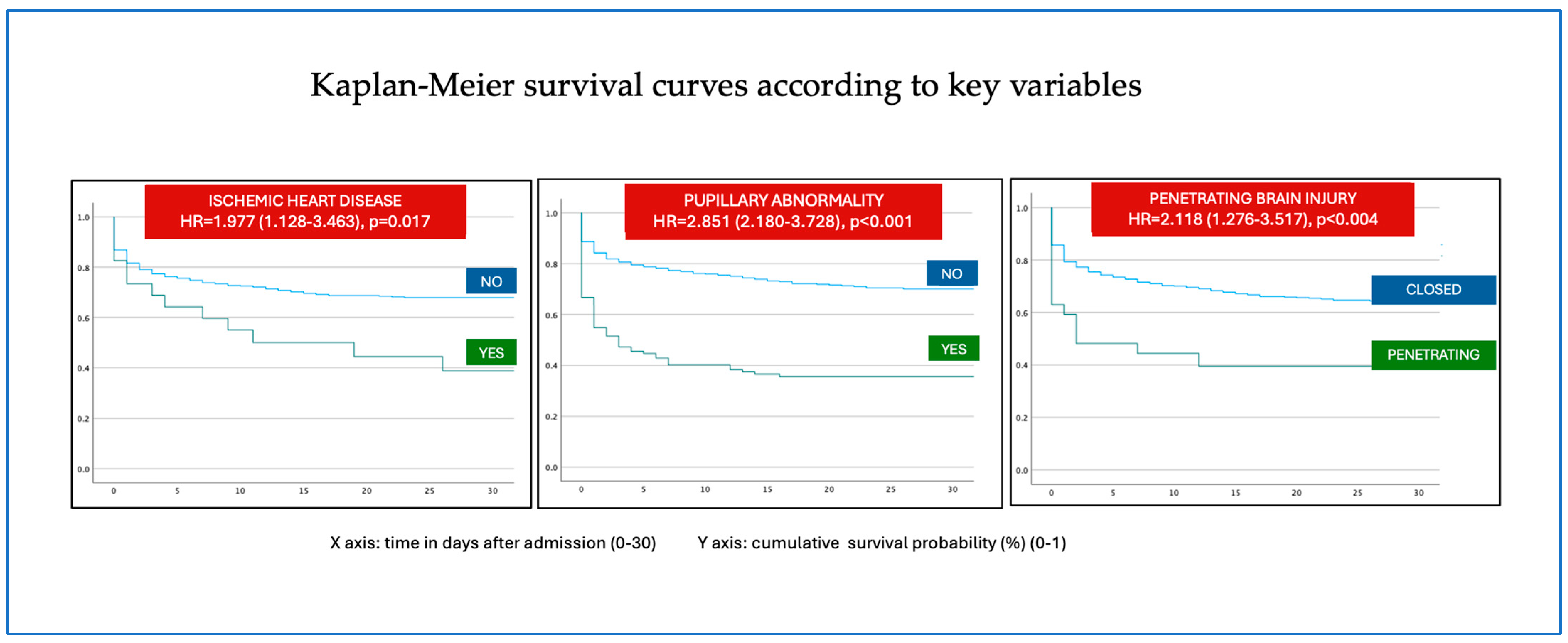
| Ischemic heart disease | 0.017 | 1.977 | 1.128–3.463 |
| Type of trauma | |||
| Penetrating trauma | <0.004004 | 2.118 | 1.276–3.517 |
| Motorcycle RTA | <0001 | 0.457 | 0.289–0.721 |
| Fall from height | 0.005 | 1.458 | 1.124–1.892 |
| Fire weapon | <0.001 | 2.549 | 1.457–4.457 |
| Initial assistance | |||
| Intubation | <0.001 | 2.677 | 1.950–3.674 |
| CPR | <0.001 | 6.323 | 4.344–9.202 |
| Initial Shock | <0.001 | 2.808 | 2.161–3.648 |
| Fixed pupil | <0.001 | 2.851 | 2.180–3.728 |
| GCS | <0.001 | 0.82 | 0.790–0.851 |
| Normal systolic pressure | <0.0101 | 0.988 | 0.984–0.991 |
| ISS | <0.001 | 1.055 | 1.045–1.064 |
| NISS | <0.001 | 1.058 | 1.049–1.067 |
| Initial surgery | |||
| Chest tube insertion | <0.001 | 1.544 | 1.185–2.011 |
| Emergent surgery | <0.001 | 0.394 | 0.303–0.511 |
| Limb surgery | <0.001 | 0.133 | 0.071–0.252 |
| Abdominal surgery | 0.002 | 1.785 | 1.231–2.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Sierra, O.; Boto, R.; Torre, A.d.l.; Montalvo, A.; Pérez-Díaz, D.; Rey, C. Trends in Etiology and Mortality in Severe Polytrauma Patients with Traumatic Brain Injury: A 25-Year Retrospective Analysis. J. Clin. Med. 2025, 14, 6986. https://doi.org/10.3390/jcm14196986
Mateo-Sierra O, Boto R, Torre Adl, Montalvo A, Pérez-Díaz D, Rey C. Trends in Etiology and Mortality in Severe Polytrauma Patients with Traumatic Brain Injury: A 25-Year Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(19):6986. https://doi.org/10.3390/jcm14196986
Chicago/Turabian StyleMateo-Sierra, Olga, Rebeca Boto, Ana de la Torre, Antonio Montalvo, Dolores Pérez-Díaz, and Cristina Rey. 2025. "Trends in Etiology and Mortality in Severe Polytrauma Patients with Traumatic Brain Injury: A 25-Year Retrospective Analysis" Journal of Clinical Medicine 14, no. 19: 6986. https://doi.org/10.3390/jcm14196986
APA StyleMateo-Sierra, O., Boto, R., Torre, A. d. l., Montalvo, A., Pérez-Díaz, D., & Rey, C. (2025). Trends in Etiology and Mortality in Severe Polytrauma Patients with Traumatic Brain Injury: A 25-Year Retrospective Analysis. Journal of Clinical Medicine, 14(19), 6986. https://doi.org/10.3390/jcm14196986





