Comparison of Short-Term Outcomes and Survivorship of Three Modular Dual Mobility Implants in Primary Total Hip Surgery
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Collection and Outcome Measures
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cram, P.; Lu, X.; Callaghan, J.J.; Vaughan-Sarrazin, M.S.; Cai, X.; Li, Y. Long-Term Trends in Hip Arthroplasty Use and Volume. J. Arthroplasty 2012, 27, 278–285.e2. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, I.N.; Bohensky, M.A.; de Steiger, R.; Brand, C.A.; Eskelinen, A.; Fenstad, A.M.; Furnes, O.; Graves, S.E.; Haapakoski, J.; Mäkelä, K.; et al. Lifetime Risk of Primary Total Hip Replacement Surgery for Osteoarthritis from 2003 to 2013: A Multinational Analysis Using National Registry Data. Arthritis Care Res. 2017, 69, 1659–1667. [Google Scholar] [CrossRef]
- Burn, E.; Murray, D.W.; Hawker, G.A.; Pinedo-Villanueva, R.; Prieto-Alhambra, D. Lifetime risk of knee and hip replacement following a GP diagnosis of osteoarthritis: A real-world cohort study. Osteoarthr. Cartil. 2019, 27, 1627–1635. [Google Scholar] [CrossRef]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JBJS Open Access 2023, 8, e22.00112. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Sirignano, M.N.; Nessler, J.M.; Rhea, E.B.; Ong, K.L.; Watson, H.N.; Yakkanti, M.R.; Malkani, A.L. Incidence of Instability Following Primary Total Hip Arthroplasty Continues to Decline in the Medicare Population. J. Arthroplasty 2023, 38, S89–S94.e1. [Google Scholar] [CrossRef]
- Gillinov, S.M.; Joo, P.Y.; Zhu, J.R.; Moran, J.; Rubin, L.E.; Grauer, J.N. Incidence, Timing, and Predictors of Hip Dislocation After Primary Total Hip Arthroplasty for Osteoarthritis. JAAOS J. Am. Acad. Orthop. Surg. 2022, 30, 1047–1053. [Google Scholar] [CrossRef]
- Falez, F.; Papalia, M.; Favetti, F.; Panegrossi, G.; Casella, F.; Mazzotta, G. Total hip arthroplasty instability in Italy. Int. Orthop. 2017, 41, 635–644. [Google Scholar] [CrossRef]
- Hermansen, L.L.; Viberg, B.; Hansen, L.; Overgaard, S. “True” Cumulative Incidence of and Risk Factors for Hip Dislocation Within 2 Years After Primary Total Hip Arthroplasty Due to Osteoarthritis: A Nationwide Population-Based Study from the Danish Hip Arthroplasty Register. J. Bone Jt. Surg. 2021, 103, 295–302. [Google Scholar] [CrossRef]
- van Erp, J.H.J.; Hüsken, M.F.T.; Filipe, M.D.; Snijders, T.E.; Kruyt, M.C.; de Gast, A.; Schlösser, T.P.C. Did the dislocation risk after primary total hip arthroplasty decrease over time? A meta-analysis across six decades. Arch. Orthop. Trauma Surg. 2023, 143, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Wetters, N.G.; Murray, T.G.; Moric, M.; Sporer, S.M.; Paprosky, W.G.; Della Valle, C.J. Risk Factors for Dislocation After Revision Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 2013, 471, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.B.; Hinkle, A.J.; Liu, Y.; Sambandam, S.N. Multivariate Analysis of Risk Factors for In-Hospital Dislocation Following Primary Total Hip Arthroplasty. J. Clin. Med. 2024, 13, 3456. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.H.; Ferreira, A. Dual-mobility cup: A new French revolution. Int. Orthop. (SICOT) 2017, 41, 433–437. [Google Scholar] [CrossRef]
- Guyen, O.; Pibarot, V.; Vaz, G.; Chevillotte, C.; Carret, J.-P.; Bejui-Hugues, J. Unconstrained Tripolar Implants for Primary Total Hip Arthroplasty in Patients at Risk for Dislocation. J. Arthroplasty 2007, 22, 849–858. [Google Scholar] [CrossRef]
- Boyer, B.; Philippot, R.; Geringer, J.; Farizon, F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: A 22-year follow-up of 240 hips. Int. Orthop. (SICOT) 2012, 36, 511–518. [Google Scholar] [CrossRef]
- Hoskins, W.; McDonald, L.; Claireaux, H.; Bingham, R.; Griffin, X. Dual-mobility constructs versus large femoral head bearings in primary and revision total hip arthroplasty: A systematic review and meta-analysis of comparative studies. HIP Int. 2023, 33, 685–696. [Google Scholar] [CrossRef]
- Samy, A.M.; Mahmoud, A.A.; El-Tantawy, A. Dual Mobility Cup: Does It Improve Patient’s Satisfaction After Total Hip Arthroplasty? A Prospective Comparative Randomized Study. JAAOS J. Am. Acad. Orthop. Surg. 2021, 29, e1141. [Google Scholar] [CrossRef]
- Romagnoli, M.; Grassi, A.; Costa, G.G.; Lazaro, L.E.; Lo Presti, M.; Zaffagnini, S. The efficacy of dual-mobility cup in preventing dislocation after total hip arthroplasty: A systematic review and meta-analysis of comparative studies. Int. Orthop. (SICOT) 2019, 43, 1071–1082. [Google Scholar] [CrossRef]
- Peuchot, H.; Simmons, E.H.; Fabre-Aubrespy, M.; Flecher, X.; Jacquet, C.; Argenson, J.-N. The use of both conventional and dual-mobility components in primary total hip arthroplasty is safe in a university hospital practice. Bone Jt. J. 2025, 107-B, 76–81. [Google Scholar] [CrossRef]
- Dubin, J.A.; Westrich, G.H. Anatomic dual mobility compared to modular dual mobility in primary total hip arthroplasty: A matched cohort study. Arthroplasty Today 2019, 5, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Duhil, A.; Delfosse, G.; Servien, E.; Batailler, C.; Lustig, S. Excellent survival of second-generation uncemented dual mobility cups compared with first-generation cups at a minimum of 10 years follow-up in primary total hip arthroplasty. SICOT-J. 2024, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Loloi, J.; Macaulay, W.; Hepinstall, M.S.; Schwarzkopf, R.; Aggarwal, V.K. Dual-mobility versus Fixed-bearing in Primary Total Hip Arthroplasty: Outcome Comparison. Hip Pelvis 2022, 34, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Dankert, J.F.; Lygrisse, K.; Mont, M.A.; Schwarzkopf, R. Dual Mobility Total Hip Arthroplasty in the United States: A Review of Current and Novel Designs. Surg. Technol. Int. 2020, 36, 379–387. [Google Scholar]
- Smith+Nephew USA. OR3O Dual Mobility. Available online: https://www.smith-nephew.com/en-us/health-care-professionals/products/orthopaedics/or3o?utm_source=chatgpt.com (accessed on 18 September 2025).
- Modular Dual Mobility. Available online: https://www.stryker.com/us/en/joint-replacement/products/modular-dual-mobility.html?utm_source=chatgpt.com (accessed on 18 September 2025).
- Zimmer Biomet. Vivacit-E® Vitamin E Crosslinked Polyethylene. Available online: https://www.zimmerbiomet.com/en/products-and-solutions/specialties/hip/vivacit-e-vitamin-e-highly-crosslinked-polyethylene.html?utm_source=chatgpt.com (accessed on 18 September 2025).
- Siljander, M.P.; Gausden, E.B.; Wooster, B.M.; Karczewski, D.; Sierra, R.J.; Trousdale, R.T.; Abdel, M.P. Liner malseating is rare with two modular dual-mobility designs. Bone Jt. J. 2022, 104-B, 598–603. [Google Scholar] [CrossRef]
- Minelli, M.; Kon, E.; D’Addona, A.; Rosolani, M.; Di Matteo, B.; Della Rocca, F. Minimum five years outcomes of modular dual mobility in primary total hip arthroplasty: A systematic review. Int. Orthop. (SICOT) 2025, 49, 1699–1707. [Google Scholar] [CrossRef]
- Tigani, D.; Banci, L.; Stallone, S.; Melucci, G.; Pieratelli, G.; Castiello, E. Evolution and New Generation of Dual Mobility Cups. Orthopedics 2023, 46, e273–e280. [Google Scholar] [CrossRef]
- Manson, T.T.; Adrados, M.; Gililland, J.M.; Mahmood, B.M.; Samuel, L.T.; Moskal, J.T. The Role of Dual-Mobility Components in Total Hip Arthroplasty. J. Bone Jt. Surg. 2023, 105, 250–261. [Google Scholar] [CrossRef]
- Smith, P.N.; Gill, D.R.; McAuliffe, M.J.; McDougall, C.; Stoney, J.D.; Vertullo, C.J.; Wall, C.J.; Corfield, S.; Page, R.; Cuthbert, A.R.; et al. Hip, Knee & Shoulder Arthroplasty: 2023 Annual Report; Australian Orthopaedic Association: Adelaide, Australia, 2023. [Google Scholar] [CrossRef]
- Chow, S.C.; Song, F. On Selection of Margin in Non-Inferiority Trials. J. Biom. Biostat. 2016, 7, 2. [Google Scholar] [CrossRef]
- Butler, J.T.; Stegelmann, S.D.; Butler, J.L.; Bullock, M.; Miller, R. Comparing dislocation rates by approach following elective primary dual mobility total hip arthroplasty: A systematic review. J. Orthop. Surg. Res. 2023, 18, 226. [Google Scholar] [CrossRef]
- Galvain, T.; Mantel, J.; Kakade, O.; Board, T.N. Treatment patterns and clinical and economic burden of hip dislocation following primary total hip arthroplasty in England. Bone Jt. J. 2022, 104-B, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J.; Haidukewych, G.J.; Boberg, C.J. Hospital Cost of Dislocation After Primary Total Hip Arthroplasty. J. Bone Jt. Surg. 2006, 88, 290–294. [Google Scholar] [CrossRef] [PubMed]
- de Palma, L.; Procaccini, R.; Soccetti, A.; Marinelli, M. Hospital Cost of Treating Early Dislocation following Hip Arthroplasty. HIP Int. 2012, 22, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Horberg, J.V.; Coobs, B.R.; Jiwanlal, A.K.; Betzle, C.J.; Capps, S.G.; Moskal, J.T. Dislocation rates following total hip arthroplasty via the direct anterior approach in a consecutive, non-selective cohort. Bone Jt. J. 2021, 103-B, 38–45. [Google Scholar] [CrossRef]
- Patil, N.; Deshmane, P.; Deshmukh, A.; Mow, C. Dual Mobility in Total Hip Arthroplasty: Biomechanics, Indications and Complications–Current Concepts. Indian J. Orthop. 2021, 55, 1202–1207. [Google Scholar] [CrossRef]
- Tigani, D.; Banci, L.; Valtorta, R.; Amendola, L. Hip stability parameters with dual mobility, modular dual mobility and fixed bearing in total hip arthroplasty: An analytical evaluation. BMC Musculoskelet. Disord. 2022, 23, 373. [Google Scholar] [CrossRef]
- Karidakis, G.K.; Karachalios, T. Oxidized Zirconium Head on Crosslinked Polyethylene Liner in Total Hip Arthroplasty: A 7- to 12-year In Vivo Comparative Wear Study. Clin. Orthop. Relat. Res. 2015, 473, 3836–3845. [Google Scholar] [CrossRef]
- Buckner, B.C.; Urban, N.D.; Cahoy, K.M.; Lyden, E.R.; Deans, C.F.; Garvin, K.L. Long-term polyethylene wear rates and clinical outcomes of oxidized zirconium femoral heads on highly cross-linked polyethylene in total hip arthroplasty. Bone Jt. J. 2024, 106-B, 38–43. [Google Scholar] [CrossRef]
- Civinini, R.; Cozzi Lepri, A.; Carulli, C.; Matassi, F.; Villano, M.; Innocenti, M. Patients Following Revision Total Hip Arthroplasty with Modular Dual Mobility Components and Cobalt-Chromium Inner Metal Head are at Risk of Increased Serum Metal Ion Levels. J. Arthroplast. 2020, 35, S294–S298. [Google Scholar] [CrossRef]
- Hampton, C.; Weitzler, L.; Baral, E.; Wright, T.M.; Bostrom, M.P.G. Do oxidized zirconium heads decrease tribocorrosion in total hip arthroplasty?: A study of retrieved components. Bone Jt. J. 2019, 101-B, 386–389. [Google Scholar] [CrossRef]
- Malahias, M.-A.; Atrey, A.; Gu, A.; Chytas, D.; Nikolaou, V.S.; Waddell, J.P. Is Oxidized Zirconium Femoral Head Superior to Other Bearing Types in Total Hip Arthroplasty? A Systematic Review and Meta-Analysis. J. Arthroplasty 2019, 34, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Baawa-Ameyaw, J.; Fontalis, A.; Tahmassebi, J.; Wardle, N.; Middleton, R.; Stephen, A.; Hutchinson, J.; Haddad, F.S. Oxidized zirconium versus cobalt-chrome femoral heads in total hip arthroplasty: A multicentre prospective randomized controlled trial with ten years’ follow-up. Bone Jt. J. 2022, 104-B, 833–843. [Google Scholar] [CrossRef]
- Lyman, S.; Lee, Y.-Y.; McLawhorn, A.S.; Islam, W.; MacLean, C.H. What Are the Minimal and Substantial Improvements in the HOOS and KOOS and JR Versions After Total Joint Replacement? Clin. Orthop. Relat. Res. 2018, 476, 2432–2441. [Google Scholar] [CrossRef]
- Emara, A.K.; Pasqualini, I.; Jin, Y.; Klika, A.K.; Orr, M.N.; Rullán, P.J.; Khan, S.T.; Murray, T.G.; Molloy, R.M.; Stearns, K.L.; et al. What Are the Diagnosis-Specific Thresholds of Minimal Clinically Important Difference and Patient Acceptable Symptom State in Hip Disability and Osteoarthritis Outcome Score After Primary Total Hip Arthroplasty? J. Arthroplasty 2024, 39, 1783–1788.e2. [Google Scholar] [CrossRef]
- Ruusiala, M.; Miettinen, H.; Kettunen, J.; Kröger, H.; Miettinen, S. Short-term primary and revision modular dual-mobility cup total hip arthroplasty outcomes in high-risk dislocation patients: A retrospective study. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 3981–3988. [Google Scholar] [CrossRef]
- Khatod, M.; Chan, P.H.; Prentice, H.A.; Fasig, B.H.; Paxton, E.W.; Reddy, N.C.; Kelly, M.P. Dual-Mobility Articulations in Revision Total Hip Arthroplasty: A Comparison with Metal or Ceramic on Highly Cross-Linked Polyethylene and Constrained Articulations. J. Bone Jt. Surg. 2024, 106, 2313. [Google Scholar] [CrossRef]
- Schaffler, B.C.; Raymond, H.E.; Black, C.S.; Habibi, A.A.; Ehlers, M.; Duncan, S.T.; Schwarzkopf, R. Two-Year Outcomes of Novel Dual-Mobility Implant in Primary Total Hip Arthroplasty. Adv. Orthop. 2024, 2024, 4125965. [Google Scholar] [CrossRef]

| Component | Implant A | Implant B | Implant C |
|---|---|---|---|
| Insert | Highly Cross-Linked UHMWPE (proprietary crosslinking process) | X3 Highly Cross-Linked UHMWPE (gamma irradiation + thermal treatment) | Vivacit-E Highly Cross-Linked UHMWPE (electron beam irradiation with vitamin E stabilization) |
| Modular Liner | DH Oxidized Zirconium | Cobalt–Chromium | Cobalt–Chromium |
| Acetabular Shell | Titanium alloy shell | Titanium alloy shell | Titanium alloy shell |
| Implant A (n = 142) | Implant B (n = 110) | Implant C (n = 45) | Total (n = 297) | ||
|---|---|---|---|---|---|
| Age (years), mean [range] | 62.3 [26–87] | 63.2 [22–84] | 63.1 [25–91] | 62.7 [22–91] | p = 0.809 |
| BMI (kg/m2), mean [range] | 29.9 [19.2–51.1] | 27.8 [15.4–44.6] | 29.9 [19.9–46.6] | 29.3 [15.4–51.1] | p = 0.023 |
| Sex, n (%) | p = 0.057 | ||||
| Female | 73 (51.4) | 69 (62.7) | 31 (68.9) | 173 (58.2) | |
| Male | 69 (48.6) | 41 (37.3) | 14 (31.1) | 124 (41.8) | |
| Race, n (%) | p = 0.044 | ||||
| White | 85 (59.9) | 79 (71.8) | 32 (71.1) | 196 (66.0) | |
| Black or African American | 31 (21.8) | 7 (6.4) | 5 (11.1) | 43 (14.5) | |
| Hispanic or Latino or Spanish | 5 (3.5) | 4 (3.6) | 2 (4.4) | 11 (3.7) | |
| Other | 21 (14.8) | 20 (18.2) | 6 (13.3) | 47 (15.8) | |
| Smoking Status, n (%) | p = 0.289 | ||||
| Never | 64 (45.0) | 61 (55.5) | 27 (60.0) | 151 (51.2) | |
| Former | 61 (43.0) | 41 (37.3) | 15 (33.3) | 117 (39.9) | |
| Current | 17 (12.0) | 8 (7.3) | 3 (6.7) | 28 (9.4) | |
| ASA Score, n (%) | p = 0.061 | ||||
| I | 15 (10.6) | 10 (9.1) | 5 (11.1) | 30 (10.1) | |
| II | 88 (62.0) | 70 (63.6) | 18 (40) | 176 (59.3) | |
| III | 36 (25.4) | 30 (27.3) | 20 (44.4) | 86 (29.0) | |
| IV | 3 (2.1) | 0 (0.0) | 2 (4.4) | 5 (1.7) | |
| Follow-Up (years), mean [range] | 3.1 [2.0–4.9] | 3.8 [2.0–8.2] | 3.4 [2.0–6.8] | 3.4 [2–8.2] | p < 0.001 |
| Laterality Right Left | 79 63 | 55 55 | 27 18 | 161 136 | p = 0.860 |
| Fixation Method Cemented Uncemented | 16 126 | 3 107 | 9 36 | 28 269 | p = 0.275 |
| Outcome | Implant A | Implant B | Implant C | Total |
|---|---|---|---|---|
| All-cause Acetabular Component Revision, n (%) | 7 (4.9) | 7 (6.4) | 4 (8.9) | 18 (6.1) |
| Dislocation/Instability | 3 | 0 | 0 | 3 |
| Infection | 2 | 1 | 1 | 4 |
| PPF | 1 | 2 | 1 | 4 |
| Malposition | 1 | 0 | 0 | 1 |
| Aseptic Loosening | 0 | 3 | 2 | 5 |
| Pseudotumor | 0 | 1 | 0 | 1 |
| 90-day readmissions, n (%) | 7 (4.9) | 4 (3.6) | 6 (13.3) | 17 (5.7) |
| Infection | 2 | 0 | 1 | 3 |
| PPF | 1 | 0 | 0 | 1 |
| Mechanical Loosening | 0 | 0 | 2 | 2 |
| DVT | 0 | 0 | 2 | 2 |
| Femur Fracture | 1 | 2 | 0 | 3 |
| Anesthesia of Skin | 1 | 0 | 0 | 1 |
| Primary Osteoarthritis | 0 | 2 | 0 | 2 |
| Other * | 2 | 0 | 1 | 3 |
| Implant A | Implant B | Implant C | p-Value | |
|---|---|---|---|---|
| Preoperative | 48.7 (16.1) [72] | 47.9 (14.4) [42] | 44.4 (15.0) [12] | 0.676 |
| 6 weeks | 63.4 (14.9) [73] | 64.8 (13.4) [41] | 67.0 (14.2) [13] | 0.667 |
| 3 months | 73.6 (18.5) [71] | 62.1 (31.9) [31] | 71.7 (11.4) [12] | 0.064 |
| 1 year | 79.6 (18.2) [50] | 78.8 (15.7) [29] | 69.6 (14.3) [14] | 0.147 |
| 2 years | 78.9 (21.6) [51] | 67.4 (26.2) [19] | 80.3 (16.6) [8] | 0.142 |
| Implant A | Dual Mobility Control | p-Value | |
|---|---|---|---|
| Preoperative | 48.7 (16.1) [72] | 47.1 (14.5) [54] | 0.578 |
| 6 weeks | 63.4 (14.9) [73] | 65.4 (13.5) [54] | 0.441 |
| 3 months | 73.6 (18.5) [71] | 64.8 (27.9) [43] | 0.072 |
| 1 year | 79.6 (18.2) [50] | 75.8 (15.7) [43] | 0.276 |
| 2 years | 78.9 (21.6) [51] | 71.2 (24.0) [27] | 0.167 |
| Implant Group | 3-Year KM Survivorship (%) | 95% CI for Survivorship (%) |
|---|---|---|
| Implant A | 98.26 | 96.97–99.55 |
| Implant B | 98.36 | 97.16–99.57 |
| Implant C | 96.86 | 93.91–99.57 |
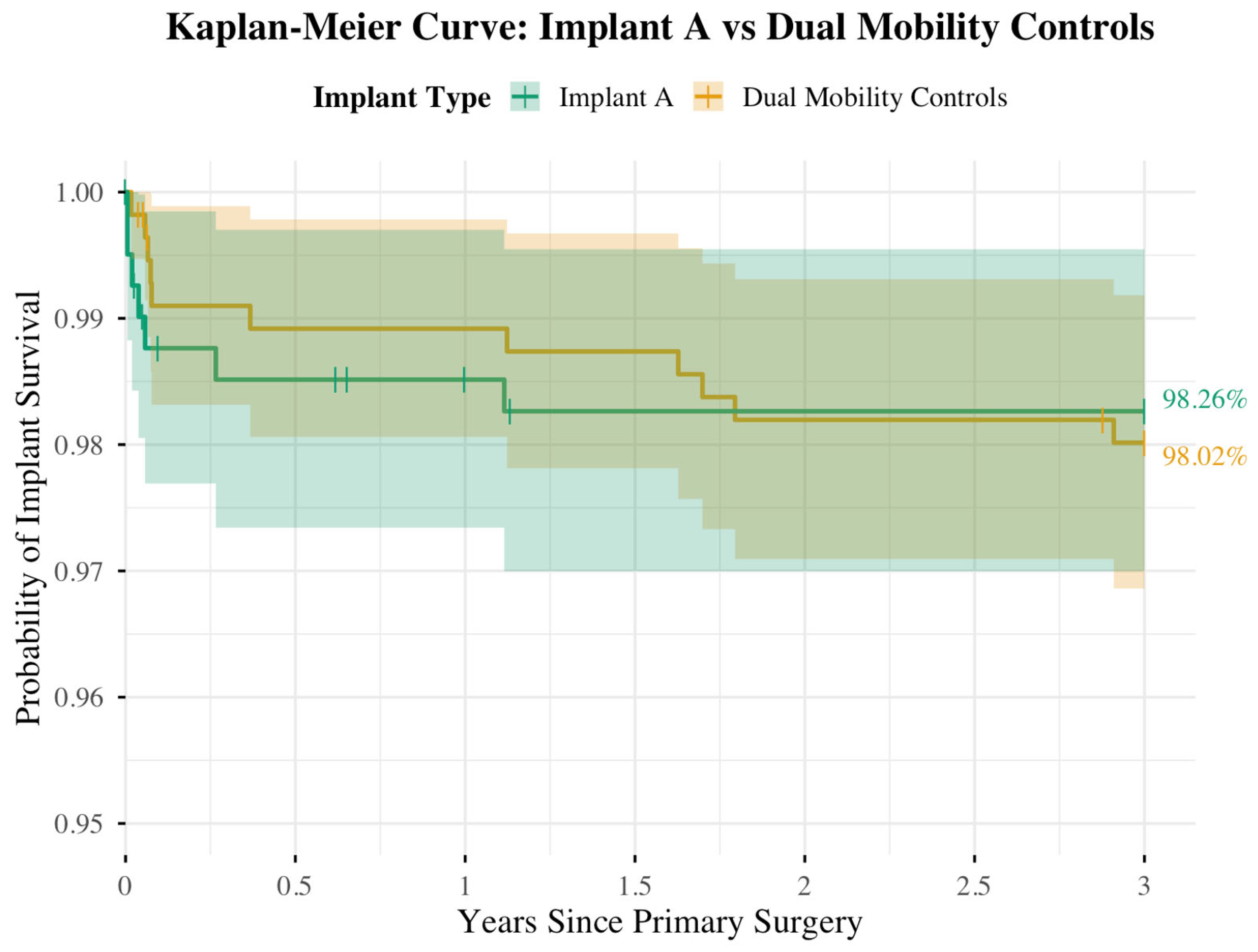
| Implant Group | 3-Year KM Survivorship (%) | 95% CI for Survivorship (%) | 95% CI for Difference (%) |
|---|---|---|---|
| Implant A | 98.26 | 96.97–99.55 | −2.19–2.69 |
| DM Controls | 98.02 | 96.86–99.18 |
| Implant Group | 0 Yrs | 0.5 Yrs | 1 Yrs | 1.5 Yrs | 2 Yrs | 2.5 Yrs | 3 Yrs |
|---|---|---|---|---|---|---|---|
| Implant A | |||||||
| n, at risk | 406 | 396 | 393 | 391 | 391 | 391 | 391 |
| n, events | 0 | 6 | 0 | 1 | 0 | 0 | 0 |
| n, censored | 0 | 4 | 3 | 1 | 0 | 0 | 0 |
| DM Controls | |||||||
| n, at risk | 557 | 548 | 548 | 547 | 544 | 544 | 542 |
| n, events | 0 | 6 | 0 | 1 | 3 | 0 | 1 |
| n, censored | 0 | 3 | 0 | 0 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, M.; Terner, B.; Gwam, C.; Schwarzkopf, R. Comparison of Short-Term Outcomes and Survivorship of Three Modular Dual Mobility Implants in Primary Total Hip Surgery. J. Clin. Med. 2025, 14, 6977. https://doi.org/10.3390/jcm14196977
Kennedy M, Terner B, Gwam C, Schwarzkopf R. Comparison of Short-Term Outcomes and Survivorship of Three Modular Dual Mobility Implants in Primary Total Hip Surgery. Journal of Clinical Medicine. 2025; 14(19):6977. https://doi.org/10.3390/jcm14196977
Chicago/Turabian StyleKennedy, Mitchell, Braden Terner, Chukwuweike Gwam, and Ran Schwarzkopf. 2025. "Comparison of Short-Term Outcomes and Survivorship of Three Modular Dual Mobility Implants in Primary Total Hip Surgery" Journal of Clinical Medicine 14, no. 19: 6977. https://doi.org/10.3390/jcm14196977
APA StyleKennedy, M., Terner, B., Gwam, C., & Schwarzkopf, R. (2025). Comparison of Short-Term Outcomes and Survivorship of Three Modular Dual Mobility Implants in Primary Total Hip Surgery. Journal of Clinical Medicine, 14(19), 6977. https://doi.org/10.3390/jcm14196977






