A Structured Low-Intensity Home-Based Walking Program to Improve Physical and Mental Functioning After Hospitalization for Severe COVID-19: A Pragmatic Nonrandomized Controlled Trial
Abstract
1. Introduction
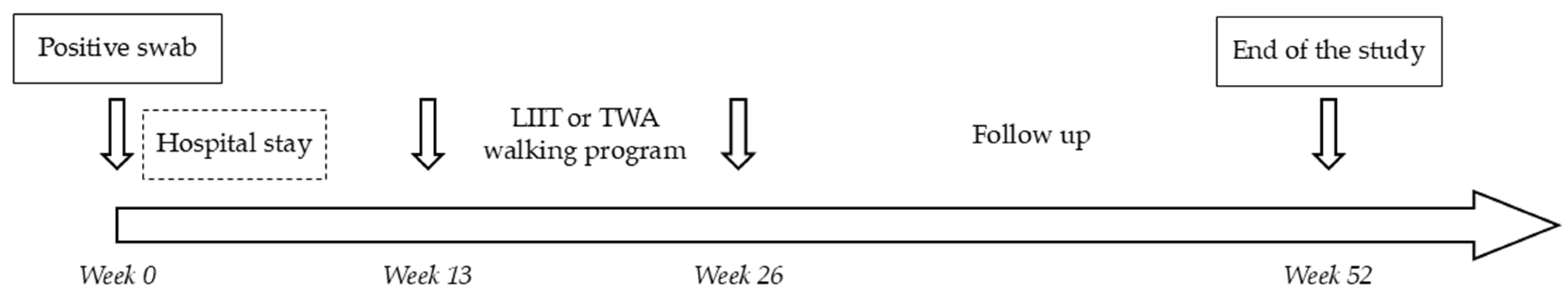
2. Materials and Methods
2.1. Subjects
2.2. Interventions
2.3. Structured Low-Intensity Interval Training
2.4. Traditional Walking Advice
2.5. Outcome Measures
2.6. Statistical Analysis
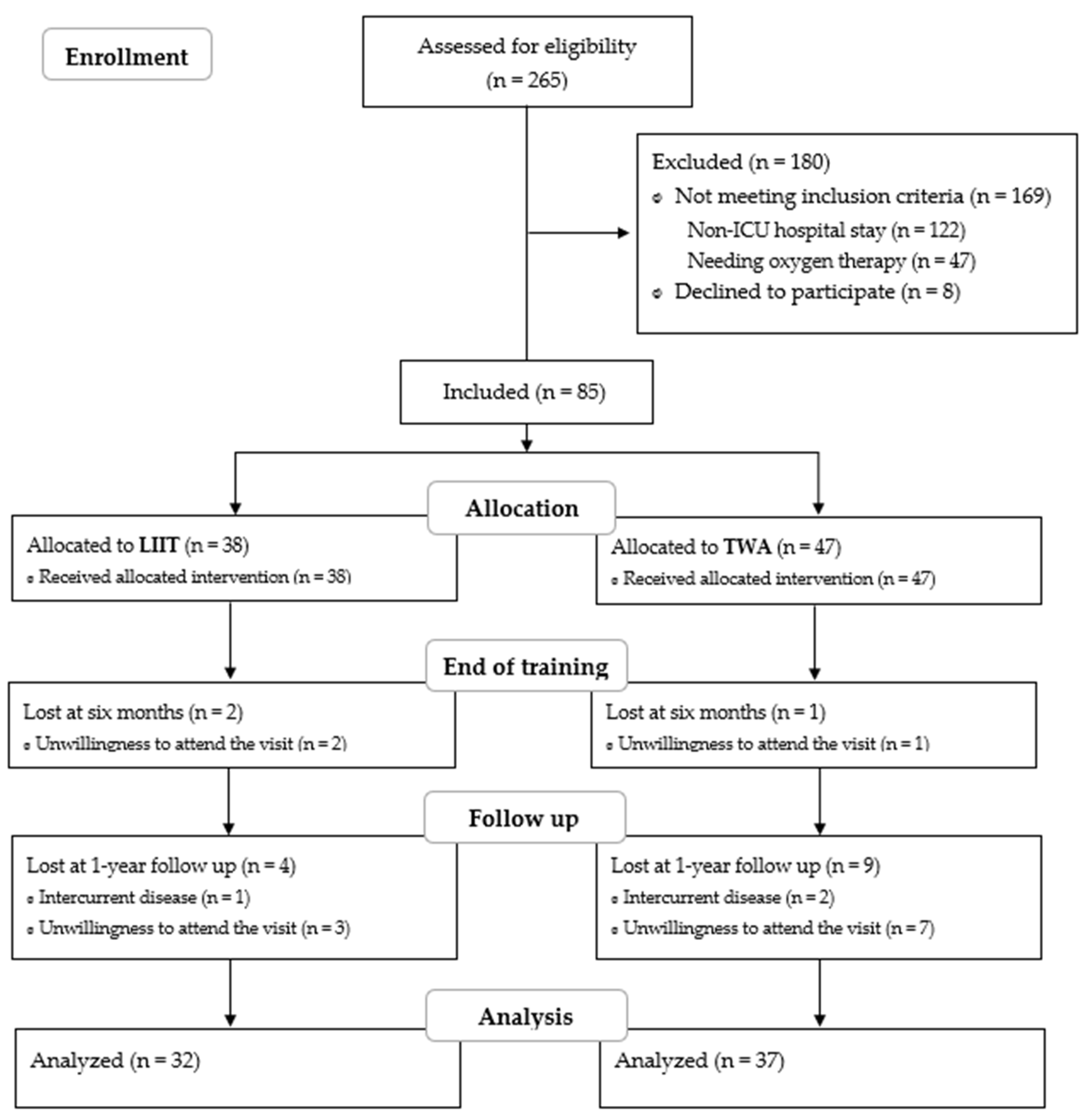
3. Results
3.1. Training Features
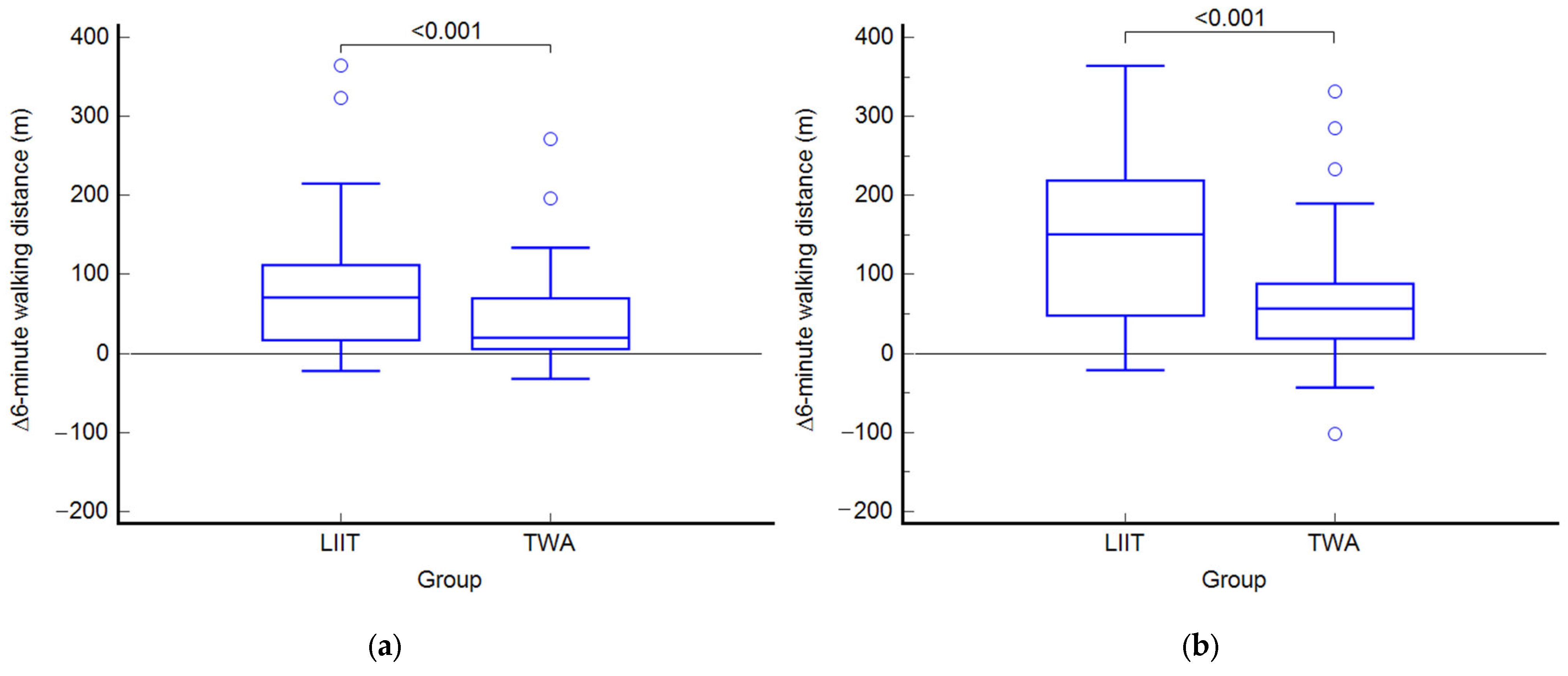
3.2. Outcomes at the End of Treatment (T1)
3.3. Within-Group Variations at the End of Treatment (T1)
3.4. Outcomes at Follow-Up (T2)
3.5. Within-Group Variations at Follow-Up (T2)
3.6. Power Calculation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QoL | Quality of life |
| LIIT | Low-intensity interval training |
| TWA | Traditional walking advice |
| ACSM | American College of Sports Medicine |
| 6MWD | 6 min walking distance |
| HR | Heart rate |
| O2Sat | Oxygen saturation |
| 30STS | 30-s sit-to-stand test |
| SF-12 | Medical Outcomes Study 12-Item Short-Form Health Survey |
| PCS | Physical component summary |
| MCS | Mental component summary |
| BAI | Beck Anxiety Inventory |
| PHQ-9 | Patient Health Questionnaire-9 |
| MoCA | Montreal Cognitive Assessment |
| PSQI | Pittsburgh Sleep Quality Index |
| PAD | Peripheral artery disease |
| MS | Multiple sclerosis |
References
- Halle, M.; Bloch, W.; Niess, A.M.; Predel, H.G.; Reinsberger, C.; Scharhag, J.; Steinacker, J.; Wolfarth, B.; Scherr, J.; Niebauer, J. Exercise and sports after COVID-19-Guidance from a clinical perspective. Transl. Sport Med. 2021, 4, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, V.A.; Allan, L.; Bethel, A.; Cowley, A.; Cross, J.L.; Day, J.; Drummond, A.; Hall, A.J.; Howard, M.; Morley, N.; et al. Rehabilitation to enable recovery from COVID-19: A rapid systematic review. Physiotherapy 2021, 111, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, 5427. [Google Scholar] [CrossRef] [PubMed]
- Salman, D.; Vishnubala, D.; Le Feuvre, P.; Beaney, T.; Korgaonkar, J.; Majeed, A.; McGregor, A.H. Returning to physical activity after COVID-19. BMJ 2021, 372, m4721. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Levine, B.D.; Baptiste, D.; Bhave, N.; Desai, S.; Dineen, E.; Durstenfeld, M.; Edward, J.; Huang, M.; Jacobsen, R.; et al. Exercise Intolerance and Response to Training in Patients With Postacute Sequelae of SARS-CoV2 (Long COVID): A Scientific Statement From the American Heart Association. Circulation 2025, 152, e50–e62. [Google Scholar] [CrossRef]
- Tryfonos, A.; Pourhamidi, K.; Jörnåker, G.; Engvall, M.; Eriksson, L.; Elhallos, S.; Asplund, N.; Mandic, M.; Sundblad, P.; Sepic, A.; et al. Functional Limitations and Exercise Intolerance in Patients With Post-COVID Condition: A Randomized Crossover Clinical Trial. JAMA Netw. Open 2024, 7, e244386. [Google Scholar] [CrossRef]
- Ceravolo, M.G.; Arienti, C.; De Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.G.; Patrini, M.; Negrini, S. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef]
- Baker, T.L.; Greiner, J.V. Guidelines: Discharge Instructions for COVID-19 Patients. J. Prim. Care Community Health 2021, 12, 21501327211024400. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Fairbank, R. Long COVID exercise trials proposed by NIH raise alarm. Nature 2023, 616, 228–229. [Google Scholar] [CrossRef]
- Jahn, K.; Sava, M.; Sommer, G.; Schumann, D.M.; Bassetti, S.; Siegemund, M.; Battegay, M.; Stolz, D.; Tamm, M.; Khanna, N.; et al. Exercise capacity impairment after COVID-19 pneumonia is mainly caused by deconditioning. Eur. Respir. J. 2022, 59, 2101136. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- White, P.D.; Goldsmith, K.; Johnson, A.L.; Potts, L.; Walwyn, R.; DeCesare, J.C.; Baber, H.L.; Burgess, M.; Clark, L.V.; Cox, D.L.; et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, Z.; Li, Z.; Huang, L.; Liang, P.; Liu, L.; Li, D. Meta-analysis of the impact of physical activity on the recovery of physical function in COVID-19 patients. Heliyon 2023, 9, e19339. [Google Scholar] [CrossRef] [PubMed]
- Nantakool, S.; Sa-Nguanmoo, P.; Konghakote, S.; Chuatrakoon, B. Effects of Exercise Rehabilitation on Cardiorespiratory Fitness in Long-COVID-19 Survivors: A Meta-Analysis. J. Clin. Med. 2024, 13, 3621. [Google Scholar] [CrossRef]
- McDowell, C.P.; Tyner, B.; Shrestha, S.; McManus, L.; Comaskey, F.; Harrington, P.; Walsh, K.A.; O’Neill, M.; Ryan, M. Effectiveness and tolerance of exercise interventions for long COVID: A systematic review of randomised controlled trials. BMJ Open 2025, 15, e082441. [Google Scholar] [CrossRef]
- Deng, J.; Qin, C.; Lee, M.; Lee, Y.; You, M.; Liu, J. Effects of rehabilitation interventions for old adults with long COVID: A systematic review and meta-analysis of randomised controlled trials. J. Glob. Health 2024, 14, 05025. [Google Scholar] [CrossRef]
- Pouliopoulou, D.V.; Macdermid, J.C.; Saunders, E.; Peters, S.; Brunton, L.; Miller, E.; Quinn, K.L.; Pereira, T.V.; Bobos, P. Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2333838. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, X.K.; Sit, C.H.; Liang, X.; Li, M.H.; Ma, A.C.; Wong, S.H. Effect of Physical Exercise-Based Rehabilitation on Long COVID: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2024, 56, 143–154. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical Activity, Exercise, and Chronic Diseases: A Brief Review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in patients on dialysis: A multicenter, randomized clinical trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Malagoni, A.M.; Argirò, M.; Felisatti, M.; Nardini, E.; Zambon, C.; Basaglia, N.; Manfredini, F. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: A pilot randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 228–239. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Manfredini, R.; De Giorgi, A.; Gasbarro, V.; Zamboni, P.; Manfredini, F. Don’t stop walking: The in-home rehabilitation program for peripheral artery disease patients during the COVID-19 pandemic. Intern. Emerg. Med. 2021, 16, 1307–1315. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Vagnoni, E.; Felisatti, M.; Mandini, S.; Heidari, M.; Mascoli, F.; Basaglia, N.; Manfredini, R.; Zamboni, P.; Manfredini, F. Evaluation of patient compliance, quality of life impact and cost-effectiveness of a “test in-train out” exercise-based rehabilitation program for patients with intermittent claudication. Circ. J. 2011, 75, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Paneroni, M.; Scalvini, S.; Perger, E.; Zampogna, E.; Govetto, S.; Oliva, F.M.; Matrone, A.; Bernocchi, P.; Rosa, D.; Vitacca, M. Home-based exercise program for people with residual disability following hospitalization for COVID-19: Randomized control trial. Ann. Phys. Rehabil. Med. 2024, 67, 101815. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Malagoni, A.M.; Mascoli, F.; Mandini, S.; Taddia, M.C.; Basaglia, N.; Manfredini, R.; Conconi, F.; Zamboni, P. Training rather than walking—The test in-train out program for home-based rehabilitation in peripheral arteriopathy. Circ. J. 2008, 72, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Traina, L.; Ficarra, V.; Gandolfi, G.; Argentoni, A.; Straudi, S.; Gasbarro, V.; Lamberti, N. A “test in-train out” program versus a “go home and walk” intervention for home-based exercise therapy in patients with peripheral artery disease: A randomized controlled trial. Scand. J. Med. Sci. Sports 2024, 34, e14584. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott, Williams & Wilkin: Philadelphia, PA, USA, 2013. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P.; Quattrociocchi, L.; Gianicolo, E.; Groth, N.; Ware, J.E. Questionario Sullo Stato di Salute SF-12. Versione Italiana; Guerini e Associati Editore: Milan, Italy, 2001. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Zieliński, G. Effect Size Guidelines for Individual and Group Differences in Physiotherapy. Arch. Phys. Med. Rehabil. 2025; in press. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Yi, Q.L. How Do I Interpret a Confidence Interval? Transfusion 2016, 56, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, A.; Paneroni, M.; Carotenuto, M.; Bertella, E.; Cirio, S.; Gandolfo, A.; Simonelli, C.; Vigna, M.; Lastoria, C.; Malovini, A.; et al. Prevalence of exercise-induced oxygen desaturation after recovery from SARS-CoV-2 pneumonia and use of lung ultrasound to predict need for pulmonary rehabilitation. Pulmonology 2023, 29, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Cortés, R.; Rivera-Lillo, G.; Arias-Campoverde, M.; Soto-García, D.; García-Palomera, R.; Torres-Castro, R. Use of sit-to-stand test to assess the physical capacity and exertional desaturation in patients post COVID-19. Chron. Respir. Dis. 2021, 18, 1479973121999205. [Google Scholar] [CrossRef] [PubMed]
- Fuglebjerg, N.J.U.; Jensen, T.O.; Hoyer, N.; Ryrsø, C.K.; Lindegaard, B.; Harboe, Z.B. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int. J. Infect. Dis. 2020, 99, 100–101. [Google Scholar] [CrossRef]
- Chetta, A.; Zanini, A.; Pisi, G.; Aiello, M.; Tzani, P.; Neri, M.; Olivieri, D. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir. Med. 2006, 100, 1573–1578. [Google Scholar] [CrossRef]
- Baratto, C.; Caravita, S.; Faini, A.; Perego, G.B.; Senni, M.; Badano, L.P.; Parati, G. Impact of COVID-19 on exercise pathophysiology: A combined cardiopulmonary and echocardiographic exercise study. J. Appl. Physiol. 2021, 130, 1470–1478. [Google Scholar] [CrossRef]
- McGregor, G.; Sandhu, H.; Bruce, J.; Sheehan, B.; McWilliams, D.; Yeung, J.; Jones, C.; Lara, B.; Alleyne, S.; Smith, J.; et al. Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-COVID-19 condition (REGAIN study): Multicentre randomised controlled trial. BMJ 2024, 384, e076506. [Google Scholar] [CrossRef] [PubMed]
- Daynes, E.; Evans, R.A.; Greening, N.J.; Bishop, N.C.; Yates, T.; Lozano-Rojas, D.; Ntotsis, K.; Richardson, M.; Baldwin, M.M.; Hamrouni, M.; et al. Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): A randomised controlled trial of exercise-based rehabilitation. Eur. Respir. J. 2025, 65, 2402152. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Wade, D.T. Updated NICE guidance on chronic fatigue syndrome. BMJ 2020, 371, m4774. [Google Scholar] [CrossRef] [PubMed]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2017, 4, CD003200. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Lamberti, N.; Malagoni, A.M.; Felisatti, M.; Zuccalà, A.; Torino, C.; Tripepi, G.; Catizone, L.; Mallamaci, F.; Zoccali, C. The role of deconditioning in the end-stage renal disease myopathy: Physical exercise improves altered resting muscle oxygen consumption. Am. J. Nephrol. 2015, 41, 329–336. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Ficarra, V.; Tsolaki, E.; Straudi, S.; Zamboni, P.; Basaglia, N.; Gasbarro, V. Biomarkers of Muscle Metabolism in Peripheral Artery Disease: A Dynamic NIRS-Assisted Study to Detect Adaptations Following Revascularization and Exercise Training. Diagnostics 2020, 10, 312. [Google Scholar] [CrossRef]
- Murrow, J.R.; Brizendine, J.T.; Djire, B.; Young, H.J.; Rathbun, S.; Nilsson, K.R., Jr.; McCully, K.K. Near infrared spectroscopy-guided exercise training for claudication in peripheral arterial disease. Eur. J. Prev. Cardiol. 2019, 26, 471–480. [Google Scholar] [CrossRef]
- Baggetta, R.; D’Arrigo, G.; Torino, C.; ElHafeez, S.A.; Manfredini, F.; Mallamaci, F.; Zoccali, C.; Tripepi, G. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: A secondary analysis of the EXCITE trial. BMC Geriatr. 2018, 18, 248. [Google Scholar] [CrossRef]
- Pomidori, L.; Lamberti, N.; Malagoni, A.M.; Manfredini, F.; Pozzato, E.; Felisatti, M.; Catizone, L.; Barillà, A.; Zuccalà, A.; Tripepi, G.; et al. Respiratory muscle impairment in dialysis patients: Can minimal dose of exercise limit the damage? A Preliminary study in a sample of patients enrolled in the EXCITE trial. J. Nephrol. 2016, 29, 863–869. [Google Scholar] [CrossRef]
- Devasahayam, A.J.; Downer, M.B.; Ploughman, M. The Effects of Aerobic Exercise on the Recovery of Walking Ability and Neuroplasticity in People with Multiple Sclerosis: A Systematic Review of Animal and Clinical Studies. Mult. Scler. Int. 2017, 2017, 4815958. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Donadi, M.; Tanaka, H.; Basaglia, N.; Manfredini, F. Effectiveness of blood flow-restricted slow walking on mobility in severe multiple sclerosis: A pilot randomized trial. Scand. J. Med. Sci. Sports 2020, 30, 1999–2009. [Google Scholar] [CrossRef]
- Baroni, A.; Fregna, G.; Lamberti, N.; Manfredini, F.; Straudi, S. Fatigue can influence the development of late-onset pain in post-COVID-19 syndrome: An observational study. Eur. J. Pain 2024, 28, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Coswig, V.S.; Gentil, P.; Naves, J.P.; Viana, R.B.; Bartel, C.; Del Vecchio, F.B. Commentary: The Effects of High Intensity Interval Training vs Steady State Training on Aerobic and Anaerobic Capacity. Front. Physiol. 2016, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Kasper, K. Sports Training Principles. Curr. Sports Med. Rep. 2019, 18, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, B.J.; Sartini, C.; Lee, I.M.; Choi, M.; Amuzu, A.; Gutierrez, C.; Casas, J.P.; Ash, S.; Lennnon, L.T.; Wannamethee, S.G.; et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health 2014, 14, 382. [Google Scholar] [CrossRef]
- Booth, M.L.; Bauman, A.; Owen, N. Perceived Barriers to Physical Activity among Older Australians. J. Aging Phys. Act. 2002, 10, 271–280. [Google Scholar] [CrossRef]
- Brandon, C.A.; Gill, D.P.; Speechley, M.; Gilliland, J.; Jones, G.R. Physical activity levels of older community-dwelling adults are influenced by summer weather variables. Appl. Physiol. Nutr. Metab. 2009, 34, 182–190. [Google Scholar] [CrossRef]
- Cheng, X.; Cao, M.; Yeung, W.F.; Cheung, D.S.T. The effectiveness of exercise in alleviating long COVID symptoms: A systematic review and meta-analysis. Worldviews Evid Based Nurs. 2024, 21, 561–574. [Google Scholar] [CrossRef]
- Kogel, A.; Machatschek, M.; Scharschmidt, R.; Wollny, C.; Lordick, F.; Ghanem, M.; Laufs, U.; Fikenzer, S. Physical exercise as a treatment for persisting symptoms post-COVID infection: Review of ongoing studies and prospective randomized controlled training study. Clin. Res. Cardiol. 2023, 112, 1699–1709. [Google Scholar] [CrossRef]
- Manfredini, F.; D’arrigo, G.; Lamberti, N.; Torino, C.; Tripepi, G.; Mallamaci, F.; Zoccali, C. The legacy effect of a home walking exercise programme in kidney failure patients on dialysis. Nephrol. Dial. Transplant. 2022, 37, 1974–1981. [Google Scholar] [CrossRef]
| LIIT (n = 32) | TWA (n = 37) | p | |
|---|---|---|---|
| Age, years | 63 ± 11 | 65 ± 10 | 0.29 |
| Males, n (%) | 23 (72) | 23 (62) | 0.40 |
| Obesity, n (%) | 7 (22) | 8 (22) | 0.98 |
| Hypertension, n (%) | 15 (47) | 24 (65) | 0.14 |
| Diabetes, n (%) | 5 (16) | 7 (19) | 0.72 |
| Total hospital stay, days | 55 ± 35 | 52 ± 46 | 0.76 |
| ICU stay, days | 20 ± 17 | 19 ± 25 | 0.98 |
| Mechanical invasive ventilation, n (%) | 11 (34) | 10 (27) | 0.76 |
| Noninvasive ventilation, n (%) | 21 (66) | 27 (73) | 0.47 |
| 6MWD (m) | 296 ± 101 | 340 ± 116 | 0.10 |
| ΔHR (bpm) | 11 ± 12 | 9 ± 11 | 0.19 |
| ΔO2Sat (%) | −2 ± 3 | −2 ± 2 | 0.66 |
| 30STS (reps) | 8.2 ± 4.2 | 9.2 ± 4.2 | 0.34 |
| PCS-12 | 39.6 ± 8.9 | 42.5 ± 8.6 | 0.18 |
| MCS-12 | 49.5 ± 9.0 | 49.2 ± 8.8 | 0.90 |
| BAI | 7.5 ± 4.6 | 9.4 ± 7.8 | 0.23 |
| PHQ-9 | 5.3 ± 2.9 | 6.7 ± 5.2 | 0.17 |
| PSQI | 11.3 ± 6.1 | 12.4 ± 5.4 | 0.42 |
| MoCA | 22.4 ± 3.7 | 24.3 ± 2.5 | 0.014 |
| LIIT (n = 32) | TWA (n = 37) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End | Follow up | Baseline | End | Follow up | Between-group ΔT1-T0 Cohen’s d η2 | Between-group ΔT2-T0 Cohen’s d η2 | Two-way ANOVA p value | |
| 6MWT (m) | 296 (260 to 333) | 383 * (346 to 420) | 434 *† (394 to 473) | 340 (302 to 379) | 382 * (349 to 415) | 409 *† (366 to 453) | 45 ‡ (8 to 82) d = 0.60 η2 = 0.083 | 68 ‡ (21 to 115) d = 0.71 η2 = 0.112 | 0.001 |
| ΔHR (bpm) | 11 (7 to 15) | 9 (6 to 12) | 8 (6 to 11) | 9 (5 to 14) | 10 (8 to 13) | 9 (6 to 12) | −3 (−8 to 2) d = 0.45 η2 = 0.048 | −3 (−9 to 4) d = 0.48 η2 = 0.055 | 0.24 |
| ΔO2Sat (%) | −2.0 (−3.0 to −1.0) | −1.0 * (−1.6 to −0.5) | 0.4 * (−1.1 to 2.1) | −1.6 (−2.3 to −0.9) | −1.1 (−1.6 to −0.5) | −0.6 (−1.2 to 0.0) | 0.5 (−2.0 to 1.0) d = 0.28 η2 = 0.019 | 1.0 (−0.2 to 2.2) d = 0.75 η2 = 0.123 | 0.14 |
| 30STS reps | 8.2 (6.7 to 9.7) | 11.2 * (9.7 to 12.6) | 13.1 *† (11.2 to 15.0) | 9.2 (7.8 to 10.5) | 10.9 * (9.7 to 12.2) | 12.3 *† (10.7 to 13.9) | 1.2 (−0.3 to 2.7) d = 0.39 η2 = 0.037 | 1.8 (−0.3 to 3.9) d = 0.41 η2 = 0.040 | 0.087 |
| PCS-12 | 39.6 (36.4 to 42.9) | 47.2 * (44.2 to 50.2) | 44.9 * (41.6 to 48.2) | 42.5 (39.6 to 45.3) | 46.9 * (44.3 to 49.5) | 46.3 * (43.1 to 49.5) | 3.1 (−0.8 to 7.0) d = 0.40 η2 = 0.039 | 1.4 (−3.3 to 6.2) d = 0.14 η2 = 0.005 | 0.32 |
| MCS-12 | 49.5 (46.3 to 52.7) | 49.4 (46.6 to 52.2) | 51.5 (48.7 to 54.4) | 49.2 (46.3 to 52.2) | 48.9 (45.8 to 52.1) | 50.7 (47.3 to 54.0) | 0.2 (−4.1 to 4.5) d = 0.02 η2 = 0.000 | 0.6 (−4.9 to 6.2) d = 0.06 η2 = 0.001 | 0.97 |
| BAI | 7.5 (5.8 to 9.2) | 7.8 (5.1 to 10.6) | 6.1 (4.0 to 8.1) | 9.4 (6.8 to 12.0) | 6.9 (3.5 to 10.4) | 6.8 (4.6 to 8.9) | −2.8 (−1.7 to 7.4) d = −0.29 η2 = 0.021 | −1.2 (−2.2 to 4.6) d = −0.17 η2 = 0.007 | 0.36 |
| PHQ-9 | 5.3 (4.2 to 6.3) | 4.7 (3.4 to 6.0) | 4.4 (3.2 to 5.7) | 6.7 (5.0 to 8.4) | 5.2 (3.4 to 7.0) | 4.3 * (3.2 to 5.3) | −0.9 (−1.6 to 3.4) d = −0.17 η2 = 0.007 | −1.6 (−0.5 to 3.7) d = −0.36 η2 = 0.031 | 0.38 |
| PSQI | 11.3 (9.1 to 13.4) | 9.7 (7.6 to 11.8) | 8.3 * (6.6 to 10.0) | 12.4 (10.6 to 14.2) | 9.9 (7.6 to 12.2) | 10.5 (8.3 to 12.7) | −1.0 (−2.2 to 4.1) d = −0.13 η2 = 0.004 | 1.1 (−4.0 to 1.8) d = 0.18 η2 = 0.008 | 0.37 |
| MoCA | 22.4 (21.1 to 23.8) | 23.6 * (22.5 to 24.7) | 23.8 * (22.5 to 25.1) | 24.3 (23.5 to 25.2) | 24.0 (23.1 to 24.9) | 24.7 (23.7 to 25.7) | 1.5 ‡ (0.0 to 2.9) d = 0.53 η2 = 0.066 | 1.1 (−0.7 to 2.9) d = 0.29 η2 = 0.021 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, N.; Baroni, A.; Piva, G.; Fregna, G.; Schincaglia, N.; Crepaldi, A.; Gamberini, L.; Occhi, A.; Straudi, S.; Manfredini, F. A Structured Low-Intensity Home-Based Walking Program to Improve Physical and Mental Functioning After Hospitalization for Severe COVID-19: A Pragmatic Nonrandomized Controlled Trial. J. Clin. Med. 2025, 14, 6938. https://doi.org/10.3390/jcm14196938
Lamberti N, Baroni A, Piva G, Fregna G, Schincaglia N, Crepaldi A, Gamberini L, Occhi A, Straudi S, Manfredini F. A Structured Low-Intensity Home-Based Walking Program to Improve Physical and Mental Functioning After Hospitalization for Severe COVID-19: A Pragmatic Nonrandomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(19):6938. https://doi.org/10.3390/jcm14196938
Chicago/Turabian StyleLamberti, Nicola, Andrea Baroni, Giovanni Piva, Giulia Fregna, Nicola Schincaglia, Anna Crepaldi, Lorenzo Gamberini, Antonella Occhi, Sofia Straudi, and Fabio Manfredini. 2025. "A Structured Low-Intensity Home-Based Walking Program to Improve Physical and Mental Functioning After Hospitalization for Severe COVID-19: A Pragmatic Nonrandomized Controlled Trial" Journal of Clinical Medicine 14, no. 19: 6938. https://doi.org/10.3390/jcm14196938
APA StyleLamberti, N., Baroni, A., Piva, G., Fregna, G., Schincaglia, N., Crepaldi, A., Gamberini, L., Occhi, A., Straudi, S., & Manfredini, F. (2025). A Structured Low-Intensity Home-Based Walking Program to Improve Physical and Mental Functioning After Hospitalization for Severe COVID-19: A Pragmatic Nonrandomized Controlled Trial. Journal of Clinical Medicine, 14(19), 6938. https://doi.org/10.3390/jcm14196938









