Parathyromatosis: The Pathogenic Background (Post-Parathyroidectomy Seeding or Exceptional Embryologic Remnant) and the Importance of a Fine Clinical Index for Recurrent Primary Hyperparathyroidism (a Narrative Review)
Abstract
1. Introduction
Objective
2. Methods
3. Sample-Focused Analysis of Parathyromatosis
3.1. Parathyromatosis in Prior Surgery Candidates
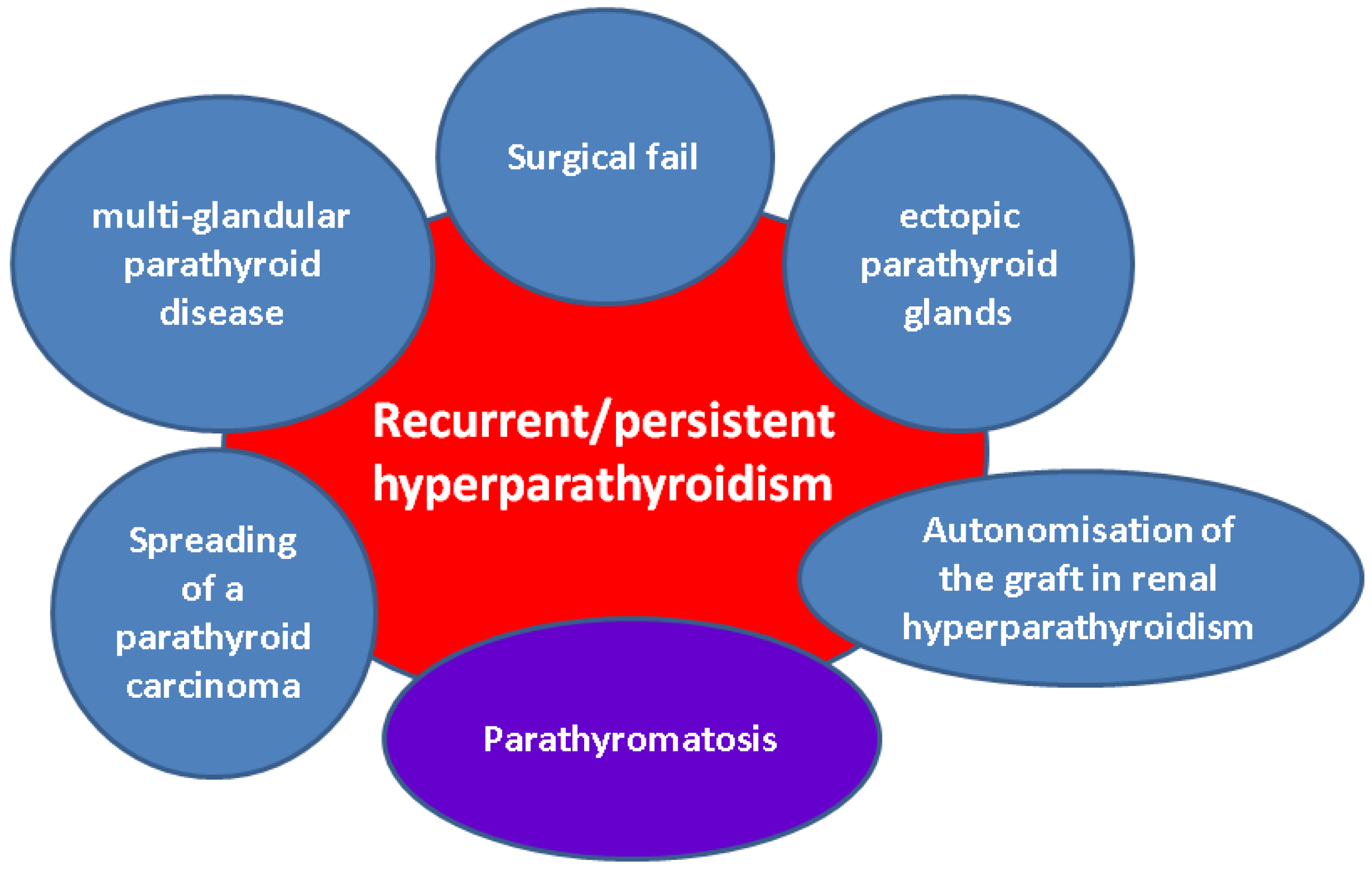
3.1.1. Recurrent Hyperparathyroidism Following Parathyroid Surgery for Primary Hyperparathyroidism
3.1.2. Secondary Hyperparathyroidism
3.2. Management Challenges Amid the Diagnosis of Parathyromatosis
3.2.1. Detection and Confirmation of Post-Parathyroidectomy Parathyromatosis
3.2.2. Parathyroidectomy and Medical Therapy for Parathyromatosis
3.3. Parathyromatosis in Patients Without a Surgical History
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | auto-transplantation |
| ACTH | Adrenocorticotropic Hormone |
| BMD | bone mineral density |
| CaSR | calcium-sensing receptor |
| CKD | chronic kidney disease |
| CT | computed tomography |
| 4D CT | four-dimensional computed tomography |
| DXA | Dual-Energy X-Ray Absorptiometry |
| F | female |
| FNAC | fine-needle aspiration cytology |
| HPTH | hyperparathyroidism |
| MEN | multiple endocrine neoplasia |
| M | male |
| mo | month |
| MRI | magnetic resonance imaging |
| NA | not available |
| PTH | parathyroid hormone |
| PHPT | primary hyperparathyroidism |
| PTx | parathyroidectomy |
| PC | parathyroid carcinoma |
| PET-CT | positron emission tomography–computed tomography |
| PEIT | percutaneous ethanol injection therapy |
| P1NP | procollagen type 1 N-terminal propeptide |
| SHPT | secondary hyperparathyroidism |
| SPECT | single-photon-emission computed tomography |
| Tc | Technetium |
| TSH | Thyroid Stimulating Hormone |
| VATS | video-assisted thoracoscopy |
| y | years |
Appendix A
| Age (Years) | 53 | 54 | 56 | 57 | 58 | 59 | |
|---|---|---|---|---|---|---|---|
| Lumbar | BMD | 0.718 | 0.771 | 0.829 | 0.797 | 0.826 | 0.841 |
| T-score | −3.0 | −2.3 | −2.0 | −2.3 | −2.0 | −1.9 | |
| Z-score | −2.1 | −1.3 | −0.8 | −1.0 | −0.7 | −0.5 | |
| Femoral neck | BMD | 0.599 | 0.650 | 0.622 | 0.613 | 0.628 | 0.603 |
| T-score | −2.2 | −1.7 | −2.0 | −2.1 | −2.0 | −2.2 | |
| Z-score | −1.2 | −0.7 | −0.9 | −0.9 | −0.8 | −1.0 | |
| Total hip | BMD | 0.680 | 0.732 | 0.744 | 0.714 | 0.729 | 0.712 |
| T-score | −2.0 | −1.6 | −1.6 | −1.9 | −1.7 | −1.9 | |
| Z-score | −1.4 | −1.0 | −0.9 | −1.0 | −0.9 | −1.0 | |
| 1/3 distal radius | BMD | NA | 0.560 | 0.548 | 0.559 | 0.544 | 0.549 |
| T-score | NA | −2.2 | −2.4 | −2.2 | −2.5 | −2.4 | |
| Z-score | NA | −1.3 | −1.4 | −1.1 | −1.3 | −1.2 | |
| Parameter (Unit) | Pre-Admission | On Admission | Normal Range |
|---|---|---|---|
| Serum total calcium (mg/dL) | 11.16 | 10.6 | 8.4–10.2 |
| Serum ionized calcium (mg/dL) ** | 4.56 | 4.32 | 3.9–4.9 |
| Phosphorus (mg/dL) | NA | 2.8 | 2.3–4.7 |
| Total proteins (g/dL) ** | 8.1 | 8.1 | 6.4–8.6 |
| PTH (pg/mL) * | 109 | 64.62 | 17.3–74.1 |
| 25-hydroxyvitamin D (ng/mL) | 31.4 (>30) | 31.8 | 20–100 |
| 24-h urinary calcium (mg/24 h) | 0.42 | 0.36 | 0.07–0.3 |
| Alkaline phosphatase (U/L) | NA | 74 | 38 –105 |
| Osteocalcin (ng/mL) | NA | 17.92 | 15–46 |
| CrossLaps (ng/mL) | NA | 0.49 | 0.33–0.782 |
| P1NP (ng/mL) | NA | 116.5 | 20.25–76.31 |
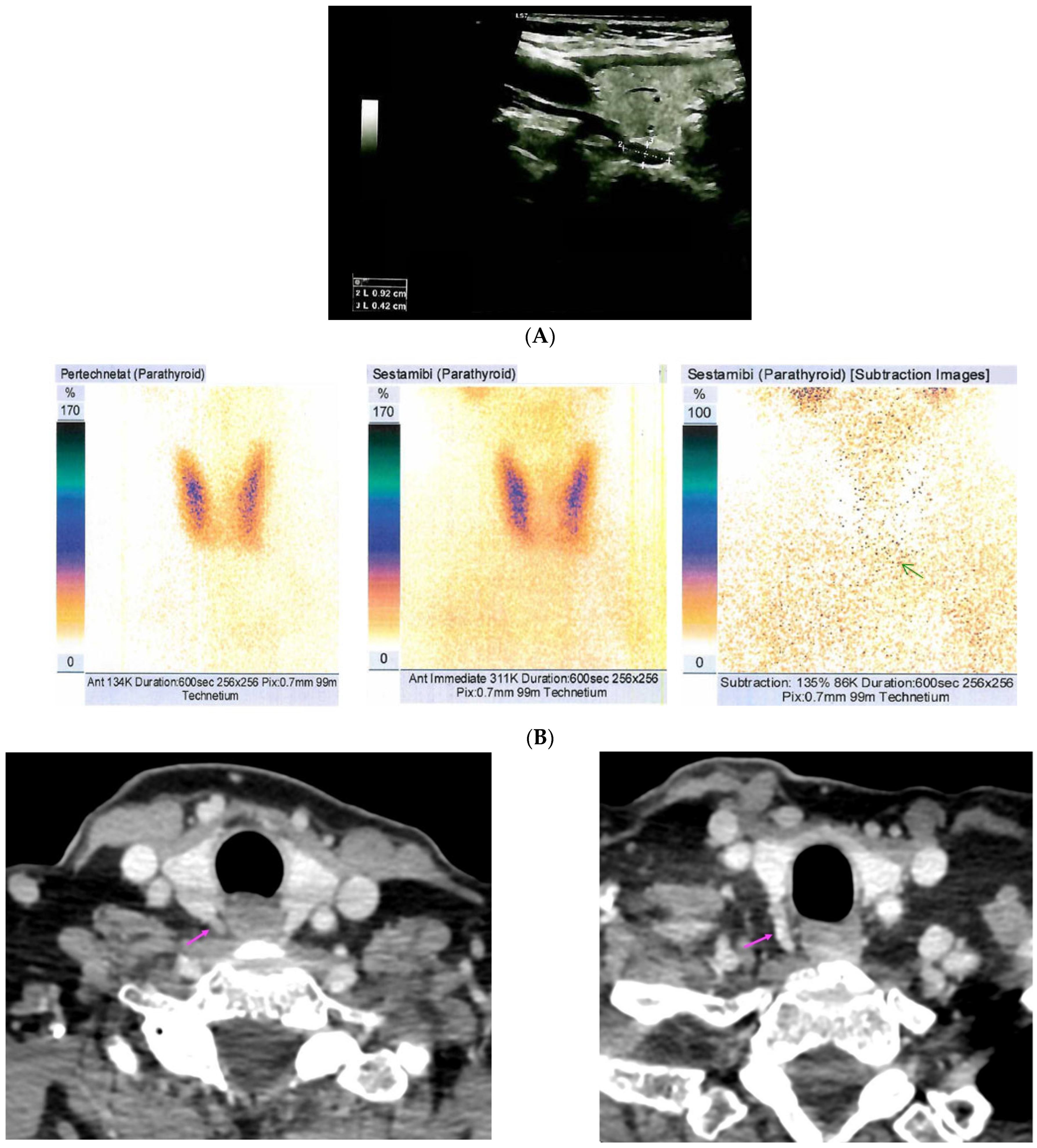
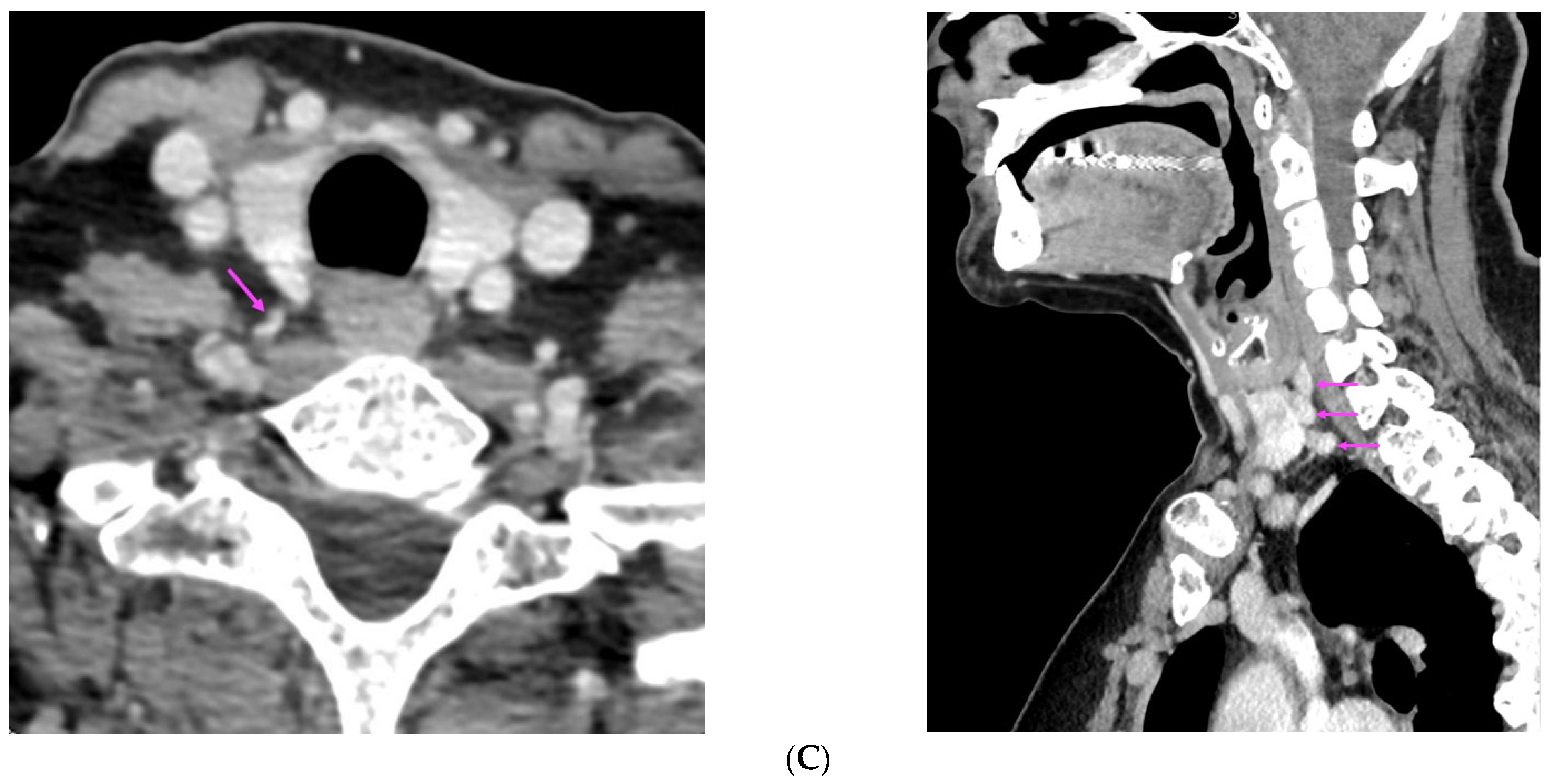
References
- Baloch, Z.W.; LiVolsi, V.A. Pathology of the parathyroid glands in hyperparathyroidism. Semin. Diagn. Pathol. 2013, 30, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Twig, B.A.; van Dalen, T.; Vroonhoven, T.J.M.V.V.; Consten, E.C.J. Recurrent hyperparathyroidism caused by benign neoplastic seeding: Two cases of parathyromatosis and a review of the literature. Acta Chir. Belg. 2013, 113, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Paduraru, D.N.; Nica, A.E.; Valea, A. Parathyroidectomy: Is vitamin D a player for a good outcome? J. Med. Life 2016, 9, 348–352. [Google Scholar] [PubMed]
- Palmer, J.A.; Brown, W.A.; Kerr, W.H.; Rosen, I.B.; Watters, N.A. The surgical aspects of hyperparathyroidism. Arch. Surg. 1975, 110, 1004–1007. [Google Scholar] [CrossRef]
- Reddick, R.; Costa, J.; Marx, S. Parathyroid hyperplasia and parathyromatosis. Lancet 1977, 1, 549. [Google Scholar] [CrossRef]
- Pipernea, R.; Popa, F.-L.; Ciortea, V.-M.; Irsay, L.; Ungur, R.A.; Pintea, A.L.; Iliescu, M.-G.; Cipăian, R.-C.; Stanciu, M. The role of rehabilitation and anabolic treatment in severe osteoporosis associated with significant vitamin D deficiency-case report. Balneo PRM Res. J. 2023, 14, 539. [Google Scholar] [CrossRef]
- Nica, S.; Sionel, R.; Măciucă, R.; Csutak, O.; Cimponeriu, D.; Ciobica, M.L.; Nica, M.I.; Chelu, I.; Radu, I.; Toma, M. Gender-Dependent Associations Between Digit Ratio and Genetic Polymorphisms, BMI, and Reproductive Factors. Rom. J. Mil. Med. 2025, 128, 78–86. [Google Scholar] [CrossRef]
- Hage, M.P.; Salti, I.; Fuleihan, G.E.-H. Parathyromatosis: A rare yet problematic etiology of recurrent and persistent hyperparathyroidism. Metabolism 2012, 61, 762–775. [Google Scholar] [CrossRef]
- Alnajmi, R.A.; Ali, D.S.; Khan, A.A. Persistence and Recurrence of Primary Hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 39, 101986. [Google Scholar] [CrossRef]
- Guerin, C.; Paladino, N.C.; Lowery, A.; Castinetti, F.; Taieb, D.; Sebag, F. Persistent and recurrent hyperparathyroidism. Updates Surg. 2017, 69, 161–169. [Google Scholar] [CrossRef]
- Popa, F.L.; Boicean, L.C.; Iliescu, M.G.; Stanciu, M. The importance of association between sex steroids deficiency, reduction of bone mineral density and falling risk in men with implications in medical rehabilitation. Balneo PRM Res. J. 2021, 12, 318–322. [Google Scholar] [CrossRef]
- Matsuoka, S.; Tominaga, Y.; Sato, T.; Uno, N.; Goto, N.; Katayama, A.; Uchida, K.; Tsuzuki, T. Recurrent renal hyperparathyroidism caused by parathyromatosis. World J. Surg. 2007, 31, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Haciyanli, S.G.; Acar, N.; Gür, E.; Çelik, S.; Karaıslı, S.; Dilek, O.; Haciyanli, M. Severe hypercalcaemia of primary hyperparathyroidism: Could giant adenoma be the real culprit rather than carcinoma? Ann. R. Coll. Surg. Engl. 2020, 102, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, N.; Carsote, M.; Cocolos, A.; Petrova, E.; Olaru, M.; Dumitrache, C.; Ghemigian, A. The Link Between Bone Osteocalcin and Energy Metabolism in a Group of Postmenopausal Women. Curr. Health Sci. J. 2019, 45, 47–51. [Google Scholar] [CrossRef]
- Ladsous, M.; Deguelte, S.; Hindié, E.; Caiazzo, R.; Delemer, B. Chapter 15: Recurrent or persistent primary hyperparathyroidism, parathyromatosis. Ann. Endocrinol. 2025, 86, 101704. [Google Scholar] [CrossRef]
- Aksoy-Altinboga, A.; Akder Sari, A.; Rezanko, T.; Haciyanli, M.; Orgen Calli, A. Parathyromatosis: Critical diagnosis regarding surgery and pathologic evaluation. Korean J. Pathol. 2012, 46, 197–200. [Google Scholar] [CrossRef]
- Mitrica, M.; Vasiliu, O.; Plesa, A.; Sirbu, O.M. Multinodular and vacuolating neuronal tumor. Rom. J. Mil. Med. 2025, 128, 10–16. [Google Scholar] [CrossRef]
- Fernandez-Ranvier, G.G.; Khanafshar, E.; Jensen, K.; Zarnegar, R.; Lee, J.; Kebebew, E.; Duh, Q.; Clark, O.H. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer 2007, 110, 255–264. [Google Scholar] [CrossRef]
- Valea, A.; Carsote, M.; Moldovan, C.; Georgescu, C. Chronic autoimmune thyroiditis and obesity. Arch. Balk. Med. Union 2018, 53, 64–69. [Google Scholar]
- Tublin, M.E.; Yim, J.H.; Carty, S.E. Recurrent hyperparathyroidism secondary to parathyromatosis: Clinical and imaging findings. J. Ultrasound Med. 2007, 26, 847–851. [Google Scholar] [CrossRef]
- Pinnamaneni, N.; Shankar, P.R.; Muthukrishnan, A. (99m)Tc MIBI SPECT findings in parathyromatosis—A rare entity causing recurrent hyperparathyroidism. Clin. Nucl. Med. 2013, 38, e443–e445. [Google Scholar] [CrossRef]
- Vellanki, P.; Lange, K.; Elaraj, D.; Kopp, P.A.; El Muayed, M. Denosumab for management of parathyroid carcinoma-mediated hypercalcemia. J. Clin. Endocrinol. Metab. 2014, 99, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Daphnis, E.; Stylianou, K.; Katsipi, I.; Stratigis, S.; Karamitopoulou, E.; Karkavitsas, N.; Kyriazis, J. Parathyromatosis and the challenge of treatment. Am. J. Kidney Dis. 2006, 48, 502–505. [Google Scholar] [CrossRef]
- Bashantoof, S.K.; Alramadhan, M.A.; Alawadh, M.H.; Samaih, N.A.B.; Alshammari, R.A.; Aodah, A. Intractable Parathyromatosis despite extensive surgical interventions: A case report with literature review. Int. J. Surg. Case Rep. 2024, 114, 109172. [Google Scholar] [CrossRef]
- Garg, Y.; Vaishnav, M.S.; Garg, N.; Muniraj, K.; Srikanta, S. Parathyroid Carcinoma Complicated by Parathyromatosis and Refractory Hypercalcemia. Cureus 2024, 16, e72584. [Google Scholar] [CrossRef]
- Spillane, C.; Calpin, G.; Singh, S.; O’Reilly, K.; Hehir, C.; Hill, A.; Magee, C.; Barrett, H. A case of mediastinal hyperparathyromatosis. J. Surg. Case Rep. 2024, 2024, rjad735. [Google Scholar] [CrossRef]
- Sapuppo, G.; Giusti, M.A.; Aricò, D.; Masucci, R.; Tavarelli, M.; Russo, M.; Pellegriti, G. Recurrent parathyromatosis in a patient with concomitant MEN1 and CASR gene alterations: Clinical management of a case report and literature review. Front. Endocrinol. 2023, 14, 1108278. [Google Scholar] [CrossRef]
- Saleh, T.; Alaswad, M.; Otry, A.; Saleh, W. Retrosternal parathyromatosis in a patient with prior total parathyroidectomy. J. Surg. Case Rep. 2023, 2023, rjad256. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Zhou, P.; Liu, H.; Wang, J.; Su, X. Recurrence hyperparathyroidism caused by synchronous parathyroid carcinoma and parathyromatosis in a patient with long-term hemodialysis. BMC Nephrol. 2023, 24, 293. [Google Scholar] [CrossRef]
- Li, L.; He, C.; Cheng, G.; Cao, J.; Wang, C.; Tang, Y.; Zhang, W. Recurrent renal secondary hyperparathyroidism caused by supernumerary mediastinal parathyroid gland and parathyromatosis: A case report. Front. Surg. 2023, 10, 1135596. [Google Scholar] [CrossRef]
- Tzotzas, T.; Goropoulos, A.; Karras, S.; Terzaki, A.; Siolos, A.; Doumas, A.; Zaramboukas, T.; Tigas, S. Effective long-term management of parathyromatosis-related refractory hypercalcemia with a combination of denosumab and cinacalcet treatment. Hormones 2022, 21, 171–176. [Google Scholar] [CrossRef]
- Kaur, J.; Drake, T. Ectopic Pleural Parathyroid Adenoma Causing Recurrent Primary Hyperparathyroidism. Cureus 2022, 14, e25101. [Google Scholar] [CrossRef] [PubMed]
- Latgé, A.; Averous, G.; Helali, M.; Bachellier, P.; Imperiale, A. Parathyromatosis: A challenging cause of recurrent primary hyperparathyroidism. QJM 2022, 115, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Ilyicheva, E.A.; Bersenev, G.A. Parathyromatosis as a cause of recurrence primary hyperparathyroidism: A case report. Int. J. Surg. Case Rep. 2021, 80, 105689. [Google Scholar] [CrossRef] [PubMed]
- Altin, O.; Sari, R. Pericardial Type 1 Parathyromatosis: A very rare cause of primary hyperparathyroidism. Acta Endocrinol. 2020, 16, 505–507. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, N.-H.; Liu, H.; Dong, M.J. Persistent secondary hyperparathyroidism caused by parathyromatosis and supernumerary parathyroid glands in a patient on haemodialysis. BMC Nephrol. 2020, 21, 257. [Google Scholar] [CrossRef]
- Haciyanli, M.; Karaisli, S.; Gucek Haciyanli, S.; Atasever, A.; Arikan Etit, D.; Gur, E.O.; Acar, T. Parathyromatosis: A very rare cause of recurrent primary hyperparathyroidism-case report and review of the literature. Ann. R. Coll. Surg. Engl. 2019, 101, e178–e183. [Google Scholar] [CrossRef]
- Cao, H.; Zeng, M.; Fang, H.; Tang, L.; Liu, W. Parathyromatosis type 2 detected by 99mTc-MIBI SPECT/CT. Gland. Surg. 2019, 8, 806–809. [Google Scholar] [CrossRef]
- Miller, M.J.; Agrawal, N.; Katz, G.; Ogilvie, J.; Melamed, J. Parathyromatosis with a papillary architecture. Histopathology 2019, 75, 598–602. [Google Scholar] [CrossRef]
- Wei, A.E.; Garrett, M.R.; Gupta, A. Parathyromatois: A rare case of recurrent hyperparathyroidism localized by four-dimensional computed tomography. AACE Clin. Case Rep. 2019, 5, e384–e387. [Google Scholar] [CrossRef]
- Aggarwal, A.; Wadhwa, R.; Aggarwal, V. Parathyromatosis Following Endoscopic Parathyroid Surgery: A Rare Occurrence. Indian J. Endocrinol. Metab. 2017, 21, 641–642. [Google Scholar] [CrossRef]
- Jain, M.; Krasne, D.L.; Singer, F.R.; Giuliano, A.E. Recurrent primary hyperparathyroidism due to Type 1 parathyromatosis. Endocrine 2017, 55, 643–650. [Google Scholar] [CrossRef]
- Nakamura, M.; Tanaka, K.; Fujii, T. Hyperparathyroidism caused by distant pulmonary lesions and parathyromatosis after ethanol injection/parathyroidectomy for secondary hyperparathyroidism. Hemodial. Int. 2017, 21, E45–E49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dey, P.; Gude, G.; Saikia, U.N. Parathyromatosis-A rare occurrence along the endoscopic tract detected on fine needle aspiration cytology. Diagn. Cytopathol. 2016, 44, 1125–1127. [Google Scholar] [CrossRef]
- Scorza, A.B.; Moore, A.G.; Terry, M.; Bricker, L.A. Secondary parathyromatosis in a patient with normal kidney function: Review of diagnostic modalities and approaches to management. Endocr. Pract. 2014, 20, e4–e7. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.-H.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Miner. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.-Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef]
- Frey, S.; Mosbah, H.; Donatini, G.; Brunaud, L.; Chabre, O.; Vezzosi, D. Chapter 9: Indications for the treatment of primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101698. [Google Scholar] [CrossRef]
- Manea, M.M.; Dragos, D.; Ghenu, M.I.; Enache, I.I.; Stoican, I.C.; Ciulavu, C.; Vasiliu, O.; Sirbu, C.A.; Tuta, S. The Neurocardiogenic Impact of Ischemic Stroke: Intricacies of Cardiac Enzymes and the Vegetative System. Rom. J. Mil. Med. 2025, 128, 36–42. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Lahart, I.M.; Viswanath, A.K.; Borumandi, F.; Sodi, R.; Metzendorf, M.-I.; Bongaerts, B. Parathyroidectomy for adults with primary hyperparathyroidism. Cochrane Database Syst. Rev. 2023, 3, CD013035. [Google Scholar] [CrossRef]
- Ünlü, M.T.; Aygun, N.; Kostek, M.; Caliskan, O.; Uludag, M. The relationship between postoperative parathormone suppression and surgical cure in primary hyperparathyroidism. Front. Endocrinol. 2025, 16, 1629719. [Google Scholar] [CrossRef]
- Uludag, M.; Unlu, M.T.; Kostek, M.; Caliskan, O.; Aygun, N.; Isgor, A. Persistent and Recurrent Primary Hyperparathyroidism: Etiological Factors and Pre-Operative Evaluation. Sisli Etfal Hastan Tip Bul. 2023, 57, 1–17. [Google Scholar] [CrossRef]
- Shires, C.B.; Parsons, A.; Dewan, K.; Sebelik, M. Controversies in thyroid and parathyroid surgery. Am. J. Otolaryngol. 2025, 46, 104703. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, R.; Lin, Z.; Donovan, P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann. Surg. 2011, 253, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, T.S.; Yen, T.W.F.; Doffek, K.; Krzywda, E.; Schaefer, S.; Sippel, R.S.; Wilson, S.D. Operative failures after parathyroidectomy for hyperparathyroidism: The influence of surgical volume. Ann. Surg. 2010, 252, 691–695. [Google Scholar] [CrossRef]
- Lenschow, C.; Pistorius, R.; Meir, M.; Schlegel, N. Parathyroid glands and their anatomical positional variations: Preoperative diagnostics and surgical planning. Chirurgie 2025. [Google Scholar] [CrossRef]
- Anghel, D.; Ciobica, L.M.; Negru, M.M.; Jurcut, C.; Otlocan, L.; Coca, A. Bone mineral density and vitamin D levels in patients with rheumatoid arthritis. Osteoporos. Int. 2017, 28 (Suppl. S1), S435–S436. [Google Scholar]
- Vasiliu, O. Impact of SGLT2 inhibitors on metabolic status in patients with psychiatric disorders undergoing treatment with second-generation antipsychotics (Review). Exp. Ther. Med. 2023, 25, 125. [Google Scholar] [CrossRef]
- Htoo, S.T.; Cusano, N.E. Management of Primary Hyperparathyroidism: Historical and Contemporary Perspectives. Endocr. Pract. 2025. [Google Scholar] [CrossRef]
- Lauricella, E.; Chaoul, N.; D’aNgelo, G.; Giglio, A.; Cafiero, C.; Porta, C.; Palmirotta, R. Neuroendocrine Tumors: Germline Genetics and Hereditary Syndromes. Curr. Treat. Options Oncol. 2025, 26, 55–71. [Google Scholar] [CrossRef]
- Balachandra, S.; Fazendin, J.; Chen, H. Complex Primary Hyperparathyroidism: Hereditary and Recurrent Disease. Surg. Clin. N. Am. 2024, 104, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Pieterman, C.R.C.; English, K.A.; Lines, K.E.; Shariq, O.A.; Marini, F.; Cuny, T.; Lewis, M.A.; Stratakis, C.A.; Perrier, N.D.; et al. Multiple endocrine neoplasia type 1 (MEN1): Recommendations and guidelines for best practice. Lancet Diabetes Endocrinol. 2025, 13, 699–721. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Benevento, E.; De Cicco, F.; Grossrubatscher, E.M.; Hasballa, I.; Tarsitano, M.G.; Centello, R.; Isidori, A.M.; Colao, A.; Pellegata, N.S.; et al. Multiple endocrine neoplasia type 4 (MEN4): A thorough update on the latest and least known men syndrome. Endocrine 2023, 82, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Cristina, E.V.; Alberto, F. Management of familial hyperparathyroidism syndromes: MEN1, MEN2, MEN4, HPT-Jaw tumour, Familial isolated hyperparathyroidism, FHH, and neonatal severe hyperparathyroidism. Best Pccat. Res. Clin. Endocrinol. Metab. 2018, 32, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Gioia, A.; Nappi, C.J.; Socin, M.; Vallon, G.; Naviglio, S.; Bernardi, S.; Boscarelli, A.; Dobrinja, C. Primary Hyperparathyroidism: A Case Series, Patient-Centered Approach to Diagnosis and Management Review. Ann. Ital. Chir. 2025, 96, 878–893. [Google Scholar] [CrossRef]
- Al-Salameh, A.; Haissaguerre, M.; Tresallet, C.; Kuczma, P.; Marciniak, C.; Cardot-Bauters, C. Chapter 6: Syndromic primary hyperparathyroidism. Ann. Endocrinol. 2025, 86, 101695. [Google Scholar] [CrossRef]
- Magnabosco, F.F.; Brescia, M.D.G.; Nascimento Júnior, C.P.; Massoni Neto, L.M.; Arap, S.S.; de Castro Junior, G.; Ledesma, F.L.; Ferreira Alves, V.A.; Kowalski, L.P.; Martin, R.M.; et al. Time to Recurrence as a Prognostic Factor in Parathyroid Carcinoma. J. Endocr. Soc. 2023, 7, bvad067. [Google Scholar] [CrossRef]
- Traini, E.; Lanzafame, A.; Carnassale, G.; Daloiso, G.; Borghesan, N.; Sanchez, A.M.; Mattia, A. Synchronous Multiple Parathyroid Carcinoma: A Challenging Diagnosis Influencing Optimal Primary Treatment-A Literature Review to Guide Clinical Decision-Making. J. Clin. Med. 2025, 14, 5228. [Google Scholar] [CrossRef]
- Simescu, R.; Piciu, A.; Muntean, V.; Mester, A.; Leucuta, D.C.; Piciu, D. Diagnostic and Surgical Challenges in Parathyroid Neoplasia: An Extensive Analysis of a Single Endocrine Surgery Center Cohort of Patients. Cancers 2025, 17, 1783. [Google Scholar] [CrossRef]
- Jentus, M.M.; Corver, W.E.; Snel, M.; van Haalen, F.M.; van Wezel, T.; Ruano, D.; Kapiteijn, E.; Crobach, S.; Appelman-Dijkstra, N.M.; Schepers, A.; et al. Diagnosing the silent: The molecular landscape of non-functional parathyroid carcinoma. Virchows Arch. 2025. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Xie, X.; Li, X.; Gié, M.-L.M.; Bouvet, M.; Manyalich-Blasi, M.; Wu, Y. Potential higher malignancy of nonfunctional parathyroid carcinoma: A case series study. Gland. Surg. 2025, 14, 947–957. [Google Scholar] [CrossRef]
- Fuenzalida, L.; Indo, S.; Contreras, H.R.; Rappoport, D.; Cabané, P. Basic-Clinical Analysis of Parathyroid Cancer. Biomedicines 2025, 13, 687. [Google Scholar] [CrossRef]
- Gubbiotti, M.; Livolsi, V.A. Late Recurrence of Hyperparathyroidism: Parathyromatosis or Recurrent Parathyroid Carcinoma? Mayo Clin. Proc. 2022, 97, 2161–2163. [Google Scholar] [CrossRef]
- Kaszczewska, M.; Popow, M.; Chudziński, W.; Kaszczewska, J.; Bogdańska, M.; Podgórska, J.; Czarniecka, A.; Gałązka, Z. A Woman with a 27-Year History of Hyperparathyroidism and Hypercalcemia Who Was Diagnosed with Low-Grade Parathyroid Carcinoma. Am. J. Case Rep. 2021, 22, e930301. [Google Scholar] [CrossRef] [PubMed]
- Bartoňová, L.; Campr, V.; Chmelová, R.; Taudy, M.; Kodet, R. Nonfunctioning parathyroid carcinoma associated with parathyromatosis. A case report. Cesk. Patol. 2018, 54, 37–42. [Google Scholar] [PubMed]
- Schulte, J.J.; Pease, G.; Taxy, J.B.; Hall, C.; Cipriani, N.A. Distinguishing Parathyromatosis, Atypical Parathyroid Adenomas, and Parathyroid Carcinomas Utilizing Histologic and Clinical Features. Head Neck Pathol. 2021, 15, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kitahara, A.; Koike, T.; Hashimoto, T.; Ohashi, R.; Motoi, N.; Tsuchida, M. Resection of a large ectopic parathyroid adenoma: A case report. Int. J. Surg. Case Rep. 2016, 23, 8–11. [Google Scholar] [CrossRef][Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Bellorin-Font, E.; Rojas, E.; Martin, K.J. Bone Disease in Chronic Kidney Disease and Kidney Transplant. Nutrients 2022, 15, 167. [Google Scholar] [CrossRef]
- Magagnoli, L.; Ciceri, P.; Cozzolino, M. Secondary hyperparathyroidism in chronic kidney disease: Pathophysiology, current treatments and investigational drugs. Expert Opin. Investig. Drugs 2024, 33, 775–789. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Zhang, B.; Liu, X.-Y.; Wang, Z.-M.; Qi, P.; Zhang, T.-Y.; Zhang, Q. Advances in the treatment of secondary and tertiary hyperparathyroidism. Front. Endocrinol. 2022, 13, 1059828. [Google Scholar] [CrossRef]
- Hu, L.; Napoletano, A.; Provenzano, M.; Garofalo, C.; Bini, C.; Comai, G.; La Manna, G. Mineral Bone Disorders in Kidney Disease Patients: The Ever-Current Topic. Int. J. Mol. Sci. 2022, 23, 12223. [Google Scholar] [CrossRef]
- Tsai, S.-H.; Kan, W.-C.; Jhen, R.-N.; Chang, Y.-M.; Kao, J.-L.; Lai, H.-Y.; Liou, H.-H.; Shiao, C.-C. Secondary hyperparathyroidism in chronic kidney disease: A narrative review focus on therapeutic strategy. Clin. Med. 2024, 24, 100238. [Google Scholar] [CrossRef]
- Stack, B.C. Secondary Hyperparathyroidism. Otolaryngol. Clin. N. Am. 2024, 57, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Dream, S.; Kuo, L.E.M.; Kuo, J.H.; Sprague, S.M.D.; Nwariaku, F.E.; Wolf, M.M.; Olson, J.A.J.; Moe, S.M.; Lindeman, B.M.; Chen, H. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Secondary and Tertiary Renal Hyperparathyroidism. Ann. Surg. 2022, 276, e141–e176. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.; Yabanoglu, H.; Hargura, A.; Kus, M.; Arer, I.M. Outcomes of Total Parathyroidectomy with Autotransplantation versus Subtotal Parathyroidectomy Techniques for Secondary Hyperparathyroidism in Chronic Renal Failure. J. Coll. Physicians Surg. Pak. 2020, 30, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, P.V.; Staloff, J.A.; Wozniak, M.J.; Mazzaglia, P.J. Subtotal Parathyroidectomy vs Total Parathyroidectomy with Autotransplantation for Secondary Hyperparathyroidism in Dialysis Patients: Short- and Long-Term Outcomes. J. Am. Coll. Surg. 2019, 228, 831–838. [Google Scholar] [CrossRef]
- Hiramitsu, T.; Hasegawa, Y.; Futamura, K.; Okada, M.; Goto, N.; Narumi, S.; Watarai, Y.; Tominaga, Y.; Ichimori, T. Treatment for secondary hyperparathyroidism focusing on parathyroidectomy. Front. Endocrinol. 2023, 14, 1169793. [Google Scholar] [CrossRef]
- Reitz, R.J.; Dreimiller, A.; Khil, A.; Horwitz, E.; McHenry, C.R. Ectopic and supernumerary parathyroid glands in patients with refractory renal hyperparathyroidism. Surgery 2021, 169, 513–518. [Google Scholar] [CrossRef]
- Kwak, S.; Fishman, E.K. Ectopic mediastinal parathyroid: A case report of recurrent secondary hyperparathyroidism. Radiol. Case Rep. 2023, 18, 2229–2231. [Google Scholar] [CrossRef]
- Lentsch, E.J.; Withrow, K.P.; Ackermann, D.; Bumpous, J.M. Parathyromatosis and recurrent hyperparathyroidism. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Haimi, M.; Yang, J.W.; Kremer, R. Refractory hypercalcemia caused by parathyroid-hormone-related peptide secretion from a metastatic pancreatic neuroendocrine tumor: A case report. J. Med. Case Rep. 2025, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Gaudenzi, G.; Oldani, M.; Pandozzi, C.; Filice, A.; Jaafar, S.; Barrea, L.; Colao, A.; Faggiano, A.; Nike Group. Nutritional status and gastroenteropancreatic neuroendocrine neoplasms: Lights and shadows with a clinical guide from the NIKE Group. Rev. Endocr. Metab. Disord. 2025, 26, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Tavares, L.B.; Tauchmanova, L.; Milone, F.; Mansueto, G.; Ramundo, V.; De Caro, M.L.D.B.; Lombardi, G.; De Rosa, G.; Colao, A. Effect of treatment with depot somatostatin analogue octreotide on primary hyperparathyroidism (PHP) in multiple endocrine neoplasia type 1 (MEN1) patients. Clin. Endocrinol. 2008, 69, 756–762. [Google Scholar] [CrossRef]
- Storvall, S.; Leijon, H.; Ryhänen, E.; Louhimo, J.; Haglund, C.; Schalin-Jäntti, C.; Arola, J. Somatostatin receptor expression in parathyroid neoplasms. Endocr. Connect. 2019, 8, 1213–1223. [Google Scholar] [CrossRef]
- Lee, P.C.; Mateo, R.B.; Clarke, M.R.; Brown, M.L.; Carty, S.E. Parathyromatosis: A cause for recurrent hyperparathyroidism. Endocr. Pract. 2001, 7, 189–192. [Google Scholar] [CrossRef]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union 2016, 51, 476–480. [Google Scholar]
- Kamenicky, P.; Mirallie, E.; Hindie, E.; Vantyghem, M.-C.; Brunaud, L. Chapter 0: Introduction to the consensus on primary hyperparathyroidism from the French Society of Endocrinology, French speaking Association of Endocrine Surgery and French Society of Nuclear Medicine. Ann. Endocrinol. 2025, 86, 101689. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Zhu, Z.; Li, W.; Shi, B.; Deng, Y.; Li, C.; Sha, O. Fn1 Regulates the Third Pharyngeal Pouch Patterning and Morphogenesis. J. Dent. Res. 2022, 101, 1082–1091. [Google Scholar] [CrossRef]
- Valea, A.; Ghervan, C.; Morar, A.; Pop, D.D.; Carsote, M.; Albu, S.E.; Georgescu, C.E.; Chiorean, A. Hashimoto’s thyroiditis and breast cancer: Coincidence or correlation? Arch. Balk. Med. Union 2016, 51, 129–132. [Google Scholar]
- Peissig, K.; Condie, B.G.; Manley, N.R. Embryology of the Parathyroid Glands. Endocrinol. Metab. Clin. N. Am. 2018, 47, 733–742. [Google Scholar] [CrossRef]
- Parvari, R.; Diaz, G.A.; Hershkovitz, E. Parathyroid development and the role of tubulin chaperone E. Horm. Res. 2007, 67, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zajac, J.D.; Danks, J.A. The development of the parathyroid gland: From fish to human. Curr. Opin. Nephrol. Hypertens. 2008, 17, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O. Therapeutic management of atypical antipsychotic-related metabolic dysfunctions using GLP-1 receptor agonists: A systematic review. Exper. Ther. Med. 2023, 26, 355. [Google Scholar] [CrossRef] [PubMed]
- di Masi, A.; Leboffe, L.; Sodo, A.; Tabacco, G.; Cesareo, R.; Sbroscia, M.; Giovannoni, I.; Taffon, C.; Crucitti, P.; Longo, F.; et al. Metabolic profile of human parathyroid adenoma. Endocrine 2020, 67, 699–707. [Google Scholar] [CrossRef]
- Xu, Q.; La, T.; Ye, K.; Wang, L.; Wang, S.; Hu, Y.; Teng, L.; Yan, L.; Li, J.; Zhang, Z.; et al. KMT2A and chronic inflammation as potential drivers of sporadic parathyroid adenoma. Clin. Transl. Med. 2024, 14, e1734. [Google Scholar] [CrossRef]
- Klein, P.; Alsleibi, S.; Cohen, O.; Ilany, J.; Hemi, R.; Barhod, E.; Vered, I.; Winder, O.; Avior, G.; Tripto-Shklonik, L. Parathyroid fine-needle aspiration with parathyroid hormone washout as a preoperative localisation of parathyroid adenoma-A retrospective study. Clin. Endocrinol. 2023, 99, 246–252. [Google Scholar] [CrossRef]
- Kendrick, M.L.; Charboneau, J.W.; Curlee, K.J.; Van Heerden, J.A.; Farley, D.R. Risk of parathyromatosis after fine-needle aspiration. Am. Surg. 2001, 67, 290–293; discussion 293–294. [Google Scholar] [CrossRef]
- Agarwal, G.; Dhingra, S.; Mishra, S.K.; Krishnani, N. Implantation of parathyroid carcinoma along fine needle aspiration track. Langenbecks Arch. Surg. 2006, 391, 623–626. [Google Scholar] [CrossRef]
- Spinelli, C.; Bonadio, A.G.; Berti, P.; Materazzi, G.; Miccoli, P. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J. Endocrinol. Investig. 2000, 23, 255–257. [Google Scholar] [CrossRef]
- Balbaloglu, H.; Deniz, O.; Ozaydin, R.Y.; Tasdoven, I.; Karadeniz Cakmak, G. Parathyroid fine needle aspiration with PTH washout: Can it lead to parathyroid cell seeding in primary hyperparathyroidism? Medicine 2024, 103, e37754. [Google Scholar] [CrossRef]
- Lou, X.; Wang, X.; Wang, Z.; Mao, G.; Zhu, W.; Wang, Y.; Pan, X.; Chen, Z.; Mo, Z. The Effect of Iodine Status on the Risk of Thyroid Nodules: A Cross-Sectional Study in Zhejiang, China. Int. J. Endocrinol. 2020, 2020, 3760375. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Y.; Chen, X.; Wei, C.; Yang, P.; Xu, W. The Effect of Inflammation on the Formation of Thyroid Nodules. Int. J. Endocrinol. 2020, 2020, 9827349. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Kozłowski, T.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Makarewicz, K.; Zalewska, A.; et al. Cross-Talk Between Nitrosative Stress, Inflammation and Hypoxia-Inducible Factor in Patients with Adrenal Masses. J. Inflamm. Res. 2021, 14, 6317–6330. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Román, Á.R.; Corrales, E.P.; Idrobo, C.; Ramírez, P.P.; Rojas, P.M.; Lázaro, C.R.; Araujo-Castro, M. Adrenal incidentalomas, cortisol secretion and cancer: Is there a real crosstalk? Front. Endocrinol. 2024, 14, 1335202. [Google Scholar] [CrossRef]
- Pitsava, G.; Stratakis, C.A. Genetic Alterations in Benign Adrenal Tumors. Biomedicines 2022, 10, 1041. [Google Scholar] [CrossRef]
- Wiersinga, W.M.; Poppe, K.G.; Effraimidis, G. Hyperthyroidism: Aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. 2023, 11, 282–298. [Google Scholar] [CrossRef]
- Prete, A.; Bancos, I. Mild autonomous cortisol secretion: Pathophysiology, comorbidities and management approaches. Nat. Rev. Endocrinol. 2024, 20, 460–473. [Google Scholar] [CrossRef]
- Hasan, A.; Deyab, A.; Monazea, K.; Salem, A.; Futooh, Z.; Mostafa, M.A.; Youssef, A.; Nasr, M.; Omar, N.; Rabaan, A.A.; et al. Clinico-pathological assessment of surgically removed abdominal wall endometriomas following cesarean section. Ann. Med. Surg. 2021, 62, 219–224. [Google Scholar] [CrossRef]
- Yıldırım, D.; Tatar, C.; Doğan, O.; Hut, A.; Dönmez, T.; Akıncı, M.; Toptaş, M.; Bayık, R.N. Post-cesarean scar endometriosis. Turk. J. Obstet. Gynecol. 2018, 15, 33–38. [Google Scholar] [CrossRef]
- Touleimat, S.; Darwish, B.; Vassilieff, M.; Stochino Loi, E.; Hennetier, C.; Roman, H. Abdominal wall endometriosis following cesarean section: A study of the growth rate of parietal endometriosis implants. Minerva Ginecol. 2017, 69, 440–446. [Google Scholar] [CrossRef]
| Reference Number | First Author | Year of Publication | Age of Patient (Years) | Female/Male |
|---|---|---|---|---|
| [24] | Bashantoof | 2024 | 38 | female |
| [25] | Garg | 2024 | 42 | female |
| [26] | Spillane | 2024 | 59 | female |
| [27] | Sapuppo | 2023 | 52 | female |
| [28] | Saleh | 2023 | 36 | male |
| [29] | Yang | 2023 | 46 | female |
| [30] | Li | 2023 | 53 | male |
| [31] | Tzotzas | 2022 | 48 | male |
| [32] | Kaur | 2022 | 68 | male |
| [33] | Latgé | 2022 | 60 | male |
| [34] | Ilyicheva | 2021 | 57 | female |
| [35] | Altin | 2020 | 58 | female |
| [36] | Yang | 2020 | 53 | female |
| [37] | Haciyanli | 2019 | 63 | female |
| [38] | Cao | 2019 | 61 | female |
| [39] | Miller | 2019 | 45 | female |
| [40] | Wei | 2019 | 33 | male |
| [41] | Aggarwal | 2017 | 55 | male |
| [42] | Jain | 2017 | 55 | male |
| [43] | Nakamura | 2017 | 51 | female |
| [44] | Sharma | 2016 | 37 | female |
| [45] | Scorza | 2014 | 68 | female |
| Reference | Surgical History | Clinical Presentation That Led to Parathyromatosis Discovery |
|---|---|---|
| [24] | PTx for parathyroid cyst at 18 y, ruptured with spillage | Symptomatic (cervical swelling, bone pain, kidney stones) |
| [25] | Left PTx and hemi-thyroidectomy 1 y prior | Hypercalcemic crisis 1 y after left PTx and hemi-thyroidectomy for PC |
| [26] | 3.5 PTx for SHPT due to CKD | Asymptomatic recurrent hyperparathyroidism 13 y after 3.5 gland PTx, incidentally found (CT surveillance for colorectal cancer) |
| [27] | Inferior right PTx at 36 y, left and right PTx at 50 and 52 y for PHPT | Recurrent hyperparathyroidism 16 years following initial surgery |
| [28] | Total PTx and forearm auto-transplantation for secondary HPTH due to CKD 12 y prior | Symptomatic (bone pain) recurrent HPTH |
| [29] | Total PTx and forearm auto-transplantation for SHPT due to CKD 5 y prior | Symptomatic (back pain) recurrent hyperparathyroidism |
| [30] | Total PTx and forearm auto-transplantation for SHPT due to CKD 17 y prior | Symptomatic (bone pain, skin itching) recurrent HPTH |
| [31] | Partial left-thyroid lobectomy and selective PTx 6 y prior to HPTH recurrence | Symptomatic recurrent HPTH (hypercalcemia, fatigue, myalgia, bilateral nephrolithiasis, later hypercalcemic crisis) |
| [32] | Video-assisted thoracoscopic resection of a mediastinal parathyroid adenoma 12 y prior Wedge resection of the upper-right lobe of the right lung 3 years after the initial procedure | Recurrent HPTH |
| [33] | Partial thyroidectomy Upper-left parathyroidectomy Excision of a retrosternal ectopic parathyroid adenoma | Recurrent PHPT |
| [34] | Right inferior PTx 12 y prior | Symptomatic (bone pain) recurrent PHPT |
| [35] | No surgical history | Symptomatic PHPT (bone and joint pain) |
| [36] | Total PTx for SHPT with AT Percutaneous ethanol injection therapy | Symptomatic (bone pain) recurrent HPTH |
| [37] | Subtotal thyroidectomy 20 y prior and upper-left PTx for PHPT 2 y prior | Recurrent HPTH |
| [38] | PTx with auto-transplantation in the forearm, removal of auto-transplanted graft, percutaneous ethanol injection | Recurrent SHPT |
| [39] | PTx for PHPT 10 y prior | Symptomatic (polyuria, constipation, abdominal pain) recurrent PHPT |
| [40] | Upper-right PTx due to PHPT 5 y prior | Severe hypercalcemia, recurrent PHPT |
| [41] | Endoscopic left superior PTx 2 y prior | Symptomatic (bone pain, fatigue) recurrent PHPT |
| [42] | Right inferior PTx for PHPT 17 y prior | Asymptomatic recurrent PHPT |
| [43] | Percutaneous ethanol injection therapy, total PTx and AT for SHPT 12 y prior | Recurrent HPTH |
| [44] | Endoscopic PTx 5 y prior | Symptomatic recurrent HPTH |
| [45] | Left inferior PTx 3 y prior | Symptomatic (nausea, abdominal pain, muscle weakness) recurrent HPT |
| Reference | Imaging Evaluation | Pathology Report |
|---|---|---|
| [24] | US and 99m-Tc sestamibi scintigraphy: adenoma anterior to sternocleidomastoid muscle CT and 99m-Tc sestamibi scintigraphy: parathyroid lesion Positive 99m-Tc sestamibi scintigraphy or SPECT before every surgery | Parathyroid tissue: In platysma muscle Sternocleidomastoid Subcutaneous tissue Suprasternal tissue |
| [25] | SPECT: subcutaneous cystic solid mass (PC) 11-Choline PET-CT: subcutaneous nodule and cervical and supraclavicular lymph nodes | Parathyromatosis and PC |
| [26] | CT: lesion in the pre-tracheal region of 1.5 × 1.3 cm PET-CT: left-thyroid mass and pre-tracheal node Sestamibi scan: mediastinal parathyroid nodule | NA |
| [27] | CT: multiple nodules in neck/upper mediastinum of <1 cm US: hypoechoic nodules with blending blurred margins Gallium-68 DOTATATE: increased uptake | Hyperplastic parathyroid tissue |
| [28] | 99m-Tc sestamibi scintigraphy: retrosternal hyper-functioning parathyroid tissue | Nodules of hyper-cellular parathyroid tissue within thymus tissue |
| [29] | SPECT/CT, US, and CT: hyperactivity at inferior pole of left thyroid lobe CT: right subcutaneous nodule | PC (left nodule) Parathyromatosis: nodular proliferation of chief cells |
| [30] | US: two hypoechoic nodules posterior to left thyroid lobe SPECT/CT: no tracer uptake in neck, mediastinal nodule | Diffuse nodular hyperplasia and hyperplasia around suture material (cervical nodules) Nodular hyperplasia of parathyroid tissue (mediastinal nodule) |
| [31] | US: cervical lesion, nodular foci under sternocleidomastoid muscle 99m-Tc sestamibi scintigraphy: multiple uptakes MRI: lesions in left para-tracheal area and carotid region | Multiple hyperplastic parathyroid nodules with no signs of malignancy |
| [32] | 99m-Tc sestamibi scintigraphy: increased uptake in posterior aspect of right lung CT: nodule of 14 × 5 mm | Hyper-cellular parathyroid tissue |
| [33] | US: uninformative PET-CT: focal uptake ahead of cricoid cartilage | Multiple millimetric benign islets of parathyroid tissue juxtaposed with each other |
| [34] | US: two hypoechoic formations behind right thyroid lobe Scintigraphy: no increased uptake | Diffuse-nodular hyperplasia, without capsule, no trabecular growth or vascular invasion |
| [35] | US: no lesion CT: well-defined retrosternal lesion of 1 cm in pre-vascular region 99m-Tc sestamibi scintigraphy: retrosternal hyper-functioning tissue | Hyperplastic parathyroid tissue foci scattered within the pericardial adipose tissue |
| [36] | SPECT/CT, US, 4D CT: nodule in left inferior clavicle head 4D CT: nodule located subcutaneously in anterior sternocleidomastoid muscle 99m-Tc sestamibi scintigraphy: uptake in autografted site | Hyperplasia, small nodular foci of atypical hyperplastic parathyroid tissue in fat surrounding remnant parathyroid |
| [37] | US: six nodules of 5 mm to 16 mm located subcutaneously anterior of right sternocleidomastoid muscle 99m-Tc sestamibi scintigraphy: no hyper-functional foci CT: similar to US | Parathyromatosis |
| [38] | 99m-Tc-MIBI imaging (SPECT/CT): three foci of elevated uptake on early phase with slow washout on delayed phase | Hyperplastic parathyroid tissues |
| [39] | US and sestamibi scan: no localization CT: 2 nodules in mediastinum | Multifocal parathyroid tissue with papillary architecture |
| [40] | US, CT, PET-CT, sestamibi: unremarkable 4D CT: increased signal enhancement and hyper-vascularity in posterior right thyroid region | Small nodules of parathyroid tissue |
| [41] | US, sestamibi, PET-CT: no localization | Multiple, small, hyper-cellular nodules of parathyroid tissue |
| [42] | US: two bilateral nodules SPECT: bilateral increased uptake 4D CT: nodule in right thyrothymic tract | Nests of hyper-cellular parathyroid tissue |
| [43] | 99m-Tc sestamibi scintigraphy: uptake in cervical region, mediastinum and right lung | Parathyroid cells without nuclear atypia or vascular or capsular invasion |
| [44] | PET-CT: uptake along endoscopic tract Chest US: nodules along endoscopic tract | FNAC: parathyroid tissue without atypia or mitosis Immunohistochemistry: positive for PTH |
| [45] | Negative localization studies before second surgery Before third surgery: 99m-Tc sestamibi scintigraphy: retrosternal uptake CT: enhancing lesion Before forth surgery: Negative 99m-Tc sestamibi scintigraphy and CT Positive venous sampling | Parathyroid nests without atypia or mitosis |
| Reference | Management of Parathyromatosis | Outcome After Surgery for Parathyromatosis |
|---|---|---|
| [24] | Seven surgical procedures Medical treatment (cinacalcet and denosumab) | Serum calcium control |
| [25] | Surgical excision and medical treatment | Recurrent hypercalcemic crises, with remission following denosumab treatment |
| [26] | Surveillance | NA |
| [27] | Two additional surgeries and thyroidectomy Medical management with bisphosphonates, cinacalcet, and lanreotide | Persistent hypercalcemia |
| [28] | Surgical: thoracoscopy with fluoroscopy and lymph node biopsy | Symptom remission, but high PTH levels (350 pg/mL) |
| [29] | Surgical removal | Improvement in symptoms PTH = 190–320 pg/mL Ca = 2.23–2.48 mmol/L |
| [30] | Surgical removal: cervicotomy and thoracoscopy | Improvement in symptoms PTH = 108–195 pg/mL Ca = 2.09–2.27 mmol/L |
| [31] | Multiple surgeries Medical treatment with cinacalcet, bisphosphonates, and denosumab | Hypercalcemia control |
| [32] | Video-assisted thoracoscopic resection of pleural parathyroid adenoma | Recurrent HPTH, without localization |
| [33] | Excision of a pre-tracheal nodule of 7 mm | NA |
| [34] | Resection of 3 fragments in central tissue of neck | Normal serum calcium after 2 months, remission of symptoms |
| [35] | Pericardial adipose tissue excision and thymectomy | No recurrence for 4 years of follow-up |
| [36] | Excision of subcutaneous nodule and right inferior and left inferior clavicle head; removal of nodules from autografted site | Normalization of serum calcium and remission of symptoms |
| [37] | Excision of nodules and surrounding tissue | Normalization of PTH and serum calcium 10 mo after surgery: recurrence treated by bilateral neck exploration with excision of parathyroid tissue foci and completion of thyroidectomy |
| [38] | Excision of three nodules | Normalization of serum calcium and PTH |
| [39] | Neck exploration revealing nodules covering left thyroid lobe | Lost to follow-up |
| [40] | Right hemi-thyroidectomy and central compartment neck dissection | Decrease in serum calcium and PTH levels |
| [41] | Neck exploration with left hemi-thyroidectomy and excision of multiple nodules in left-side neck compartment | Normalization of serum calcium |
| [42] | Neck exploration and bilateral neck exploration with subtotal PTx and cervical thymectomy | Normalization of serum calcium and PTH under further medical management |
| [43] | Thoracoscopic excision of pulmonary nodules, followed by excision of cervical nodules and auto-transplanted graft after 2 y | Normalization of serum calcium and PTH under medical management |
| [44] | Thoracoscopic exploration 1 y later: FNAC | NA |
| [45] | Three further surgeries and medical management (cinacalcet, alendronate) | Normalization of serum calcium and PTH after second surgery Lost to follow-up for 5 years Symptomatic hypercalcemia 5 years later Symptomatic (weakness, abdominal pain, myalgia) after 4 surgeries |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, A.-M.; Nistor, C.; Carsote, M. Parathyromatosis: The Pathogenic Background (Post-Parathyroidectomy Seeding or Exceptional Embryologic Remnant) and the Importance of a Fine Clinical Index for Recurrent Primary Hyperparathyroidism (a Narrative Review). J. Clin. Med. 2025, 14, 6937. https://doi.org/10.3390/jcm14196937
Gheorghe A-M, Nistor C, Carsote M. Parathyromatosis: The Pathogenic Background (Post-Parathyroidectomy Seeding or Exceptional Embryologic Remnant) and the Importance of a Fine Clinical Index for Recurrent Primary Hyperparathyroidism (a Narrative Review). Journal of Clinical Medicine. 2025; 14(19):6937. https://doi.org/10.3390/jcm14196937
Chicago/Turabian StyleGheorghe, Ana-Maria, Claudiu Nistor, and Mara Carsote. 2025. "Parathyromatosis: The Pathogenic Background (Post-Parathyroidectomy Seeding or Exceptional Embryologic Remnant) and the Importance of a Fine Clinical Index for Recurrent Primary Hyperparathyroidism (a Narrative Review)" Journal of Clinical Medicine 14, no. 19: 6937. https://doi.org/10.3390/jcm14196937
APA StyleGheorghe, A.-M., Nistor, C., & Carsote, M. (2025). Parathyromatosis: The Pathogenic Background (Post-Parathyroidectomy Seeding or Exceptional Embryologic Remnant) and the Importance of a Fine Clinical Index for Recurrent Primary Hyperparathyroidism (a Narrative Review). Journal of Clinical Medicine, 14(19), 6937. https://doi.org/10.3390/jcm14196937








