3. Results
A total of 60 subjects were prospectively enrolled, including 30 patients diagnosed with open-angle glaucoma and 30 healthy volunteers. Six participants, two glaucoma patients, and four healthy subjects were excluded due to the insufficient quality of OCTA imaging for evaluation. The final statistical analysis included 87 eyes: 41 eyes from 29 patients with established OAG and 46 eyes from 26 healthy controls.
No significant differences were found between groups regarding gender distribution. In the glaucoma group, 60.7% (17/28) were female, compared to 61.5% (16/26) in the control group (p = 0.951). The proportion of pseudophakic patients was similar between groups (61.5% in controls vs. 60.7% in glaucoma patients, p = 0.951). Hypercholesterolemia prevalence was comparable (19.2% in controls vs. 21.4% in glaucoma patients, p = 0.555), whereas hypertension was significantly more frequent in the glaucoma group (57.1% vs. 19.2%, p = 0.005). No participants with diabetes were included.
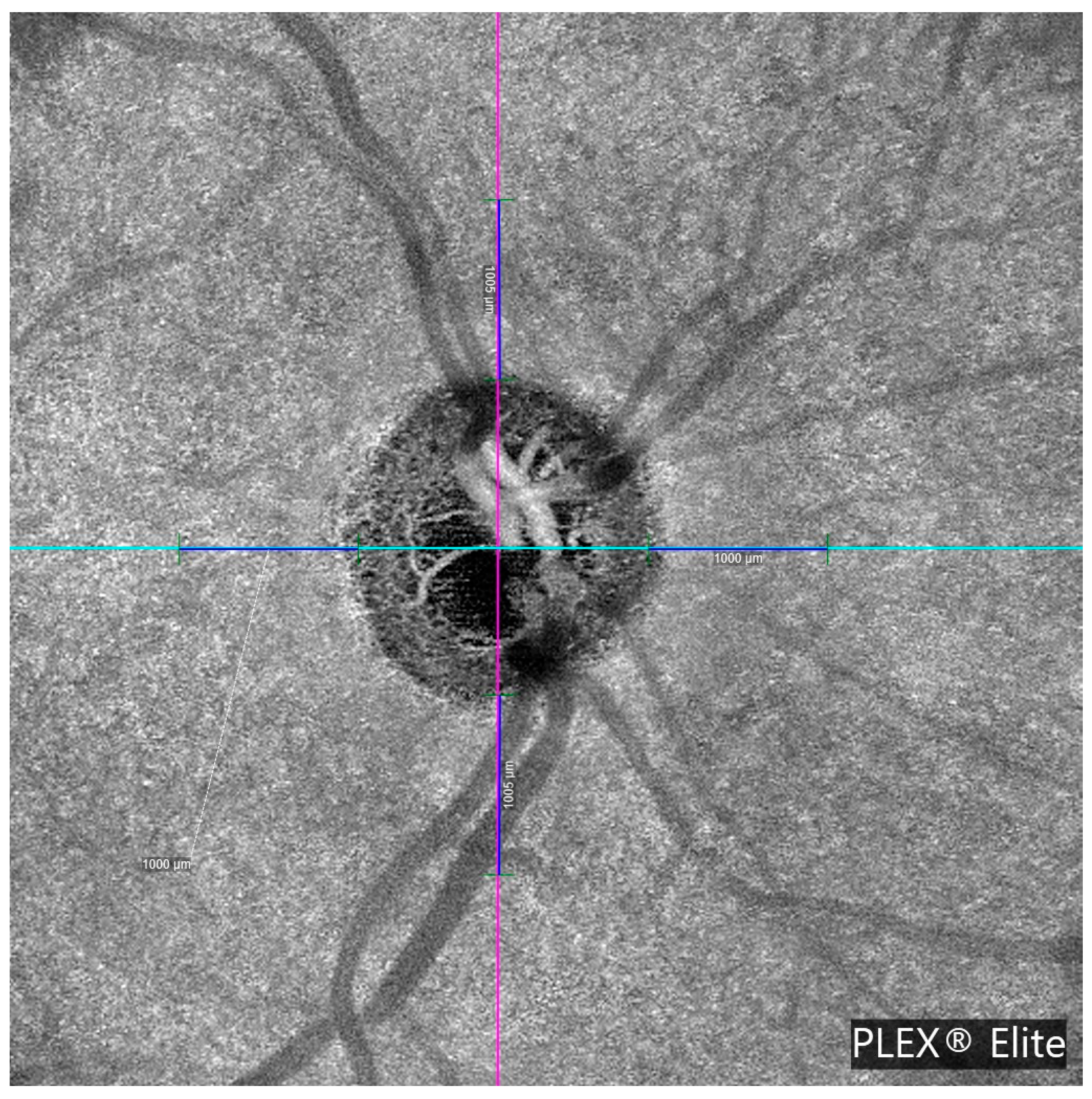
Table 1 summarizes demographic characteristics and the retinal nerve fiber layer (RNFL) thickness values obtained via OCTA PLEX Elite 9000. No significant differences were observed in age, refractive error, visual acuity, or central corneal thickness. Glaucoma patients had higher treated IOP (18.3 ± 4.7 vs. 16.2 ± 2.6 mmHg,
p = 0.008), greater mean defects (MDs) (2.75 ± 4.0 vs. 1.14 ± 2.18 dB,
p = 0.0013), and lower peripapillary RNFL thickness (74.7 vs. 81.5 μm,
p < 0.001).
Among patients with glaucoma, 10.7% (3/28) had undergone filtering surgery, and 60.7% (17/28) were receiving topical antiglaucoma treatment. The mean number of medications per patient was 0.85 ± 0.16.
Table 2 presents peripapillary vascular density parameters, β-PPA measurements, PCT values across four peripapillary quadrants, and choroidal vascular indices (CVI, CLA, and CSA) in the temporal and nasal peripapillary sectors. Glaucoma patients exhibited significantly lower superficial vessel density (pVD 0.52 vs. 0.53,
p = 0.006) and flow indices (pFI 0.34 vs. 0.37,
p < 0.001).
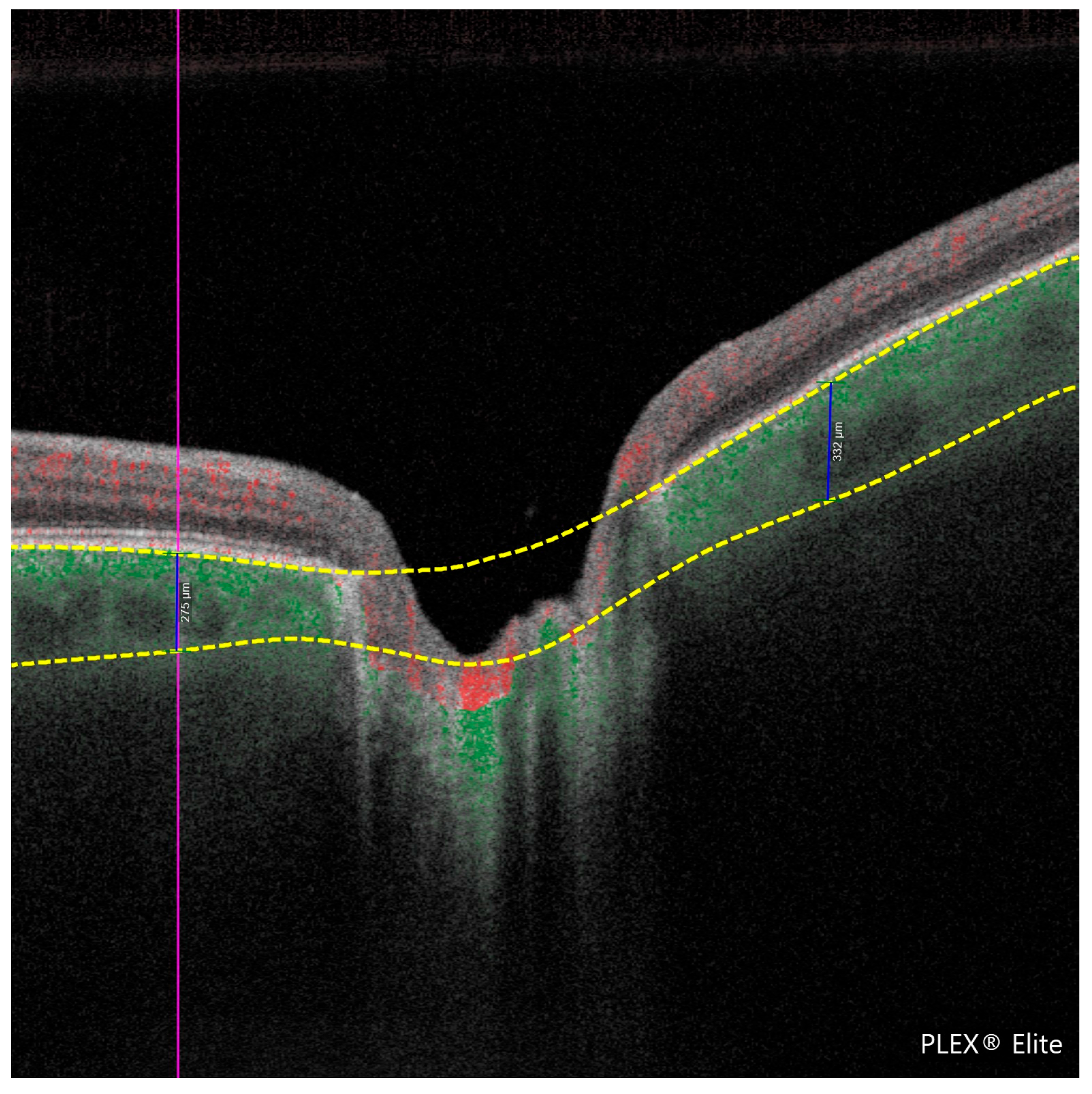
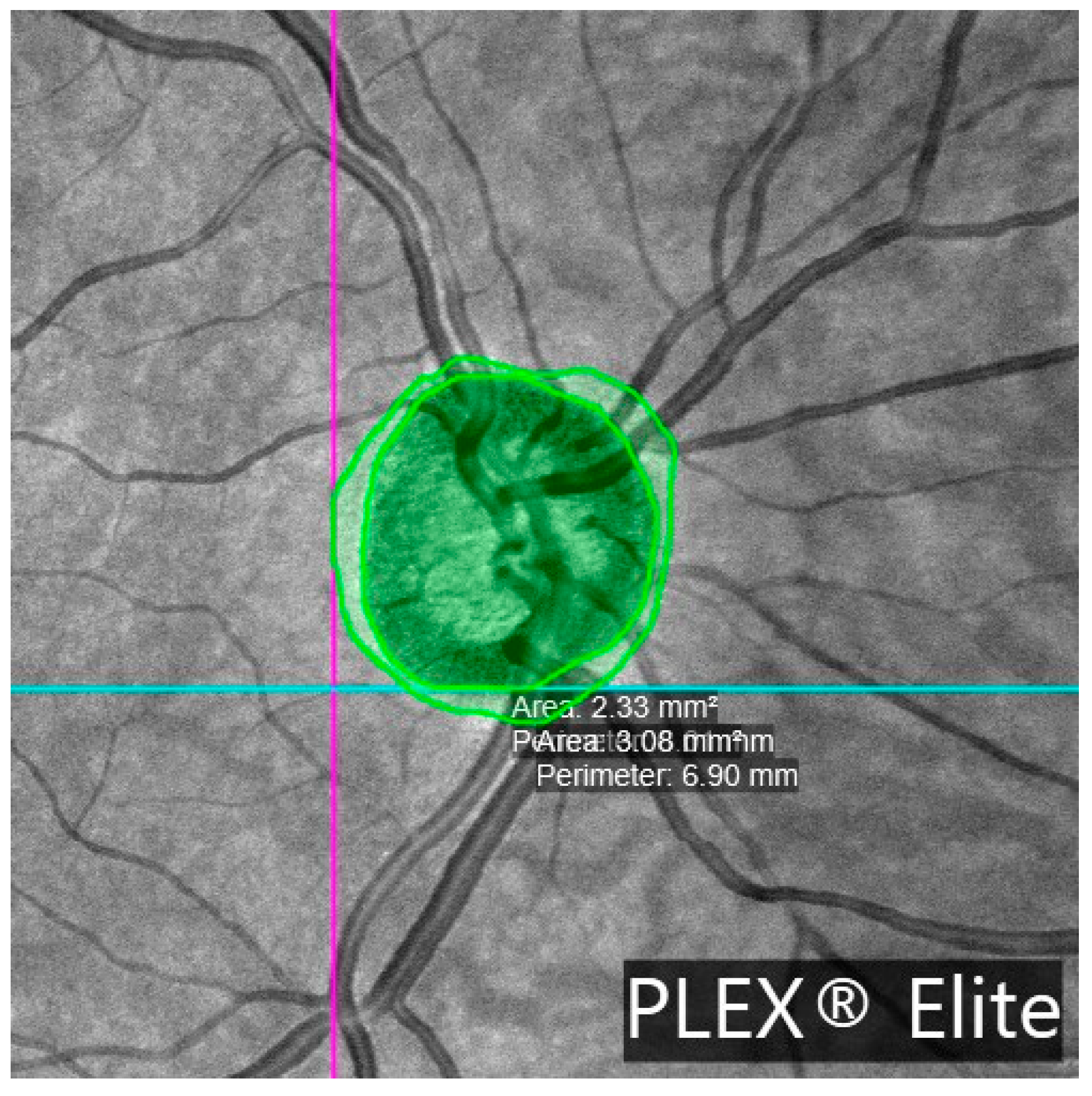
β-PPA was present in 95.1% of glaucomatous eyes (39/41) and 78.3% of control eyes (36/46), p = 0.030. The β-PPA area was significantly larger in glaucoma patients (1.44 ± 1.36 vs. 0.88 ± 0.61 mm2, p = 0.039). Choroidal thickness was significantly reduced in glaucoma patients (162.99 ± 49.80 vs. 221.94 ± 76.79 μm, p = 0.007), along with smaller choroidal luminal areas (CLA: 0.26 ± 0.06 vs. 0.31 ± 0.07 mm2, p = 0.003) and stromal areas (CSA: 0.43 ± 0.12 vs. 0.50 ± 0.14 mm2, p = 0.014).
MvD was detected in 7 of the 29 OAG patients, with bilateral involvement in three cases, resulting in 10 affected eyes. No MvD was observed in healthy controls. Both examiners agreed on the determination of the presence of MvD in all cases. The interexaminer ICC for the MvD perimeter measurement was 0.979 (95% CI: 0.917–0.995;
p < 0.001).
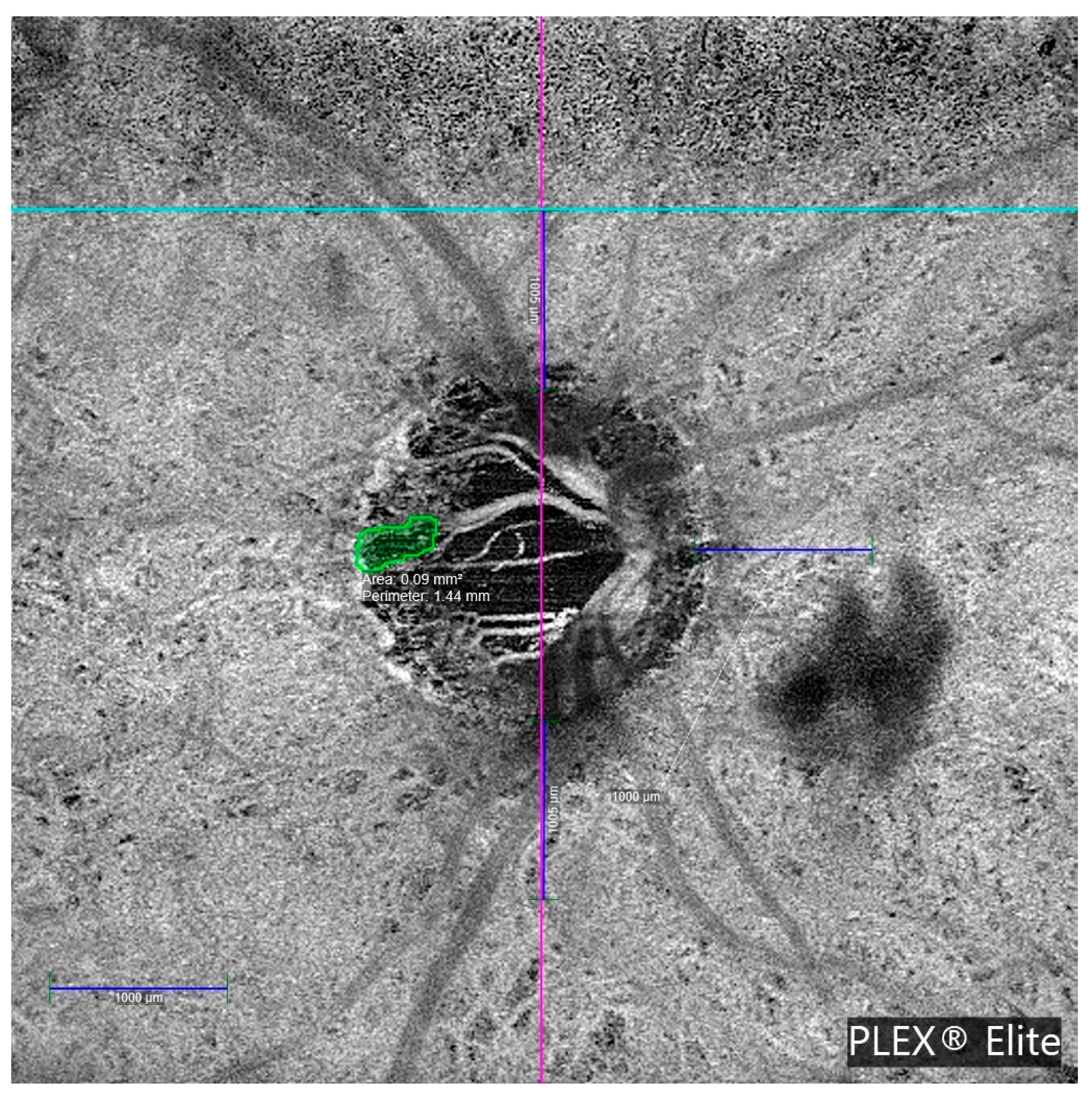
Table 3 details the clinical characteristics of glaucoma patients with MvD. Most MvDs were located in deep vascular layers, specifically, the choriocapillaris and choroid. Only 2 of the 10 cases showed MvD in the superficial vascular plexus. All MvD-positive eyes exhibited β-PPA.
Figure 4 illustrates a representative en face OCTA image showing MvD in a glaucomatous eye.
No significant differences in gender distribution were found between glaucoma patients with and without MvD (60% vs. 58%, p = 0.606). Similarly, pseudophakia rates were comparable (60% vs. 63%, p = 0.208. Two of the seven MvD-positive patients (28.6%) had undergone glaucoma surgery.
Table 4 summarizes demographic data for glaucoma patients with and without MvD, and
Table 5 presents differences in the peripapillary vascular density parameters. MvD-positive eyes had significantly larger β-PPA areas (median [IQR]: 1.62 (1.17–1.91) vs. 0.87 (0.67–1.26) mm
2,
p = 0.011), smaller choroidal luminal areas (0.22 [0.17–0.26] vs. 0.27 [0.23–0.31] mm
2,
p = 0.015, and reduced choroidal stromal areas (0.30 [0.26–0.45] vs. 0.47 [0.35–0.55] mm
2,
p = 0.012.
Peripapillary vessel density showed a strong correlation with RNFL thickness (r = 0.715, p < 0.001). Sectorial analysis revealed the strongest correlation in the inferior peripapillary quadrant (r = 0.749, p < 0.001), followed by the superior (r = 0.700, p < 0.001), temporal (r = 0.654, p < 0.001), and nasal sectors (r = 0.419, p < 0.001). No correlation was found between pVD and choroidal thickness.
Univariate linear regression showed a significant negative association between the number of glaucoma medications and PCT (β = −24.94; 95% CI: −46.27 to −3.62;
p = 0.022). StructureIndex showed a trend toward a positive association (
p = 0.059). In the final multivariate model, both the number of medications (β = −23.37; 95% CI: −46.50 to −0.24;
p = 0.048) and StructureIndex (β = 14.88; 95% CI: 0.32 to 29.45;
p = 0.045) remained as independent variables significantly associated with PCT. The model explained 14% of the variability in global choroidal thickness (adjusted R
2 = 0.14) (
Table 6).
Binary logistic regression analysis revealed that the visual field MD index was significantly associated with the presence of MvD (OR = 1.283; 95% CI: 1.076–1.529; p = 0.005). The slope of MD progression was also significantly associated (OR = 3.198; 95% CI: 1.058–9.666; p = 0.039). β-PPA area showed a significant association (OR = 1.662; 95% CI: 1.012–2.729; p = 0.045). Other variables (systemic arterial hypertension, age, number of ocular hypotensive treatments, IOP, pachymetry, RNFL progression slope, StructureIndex, PCT, and CVI) did not show significant associations.
Due to the limited number of MvD events (
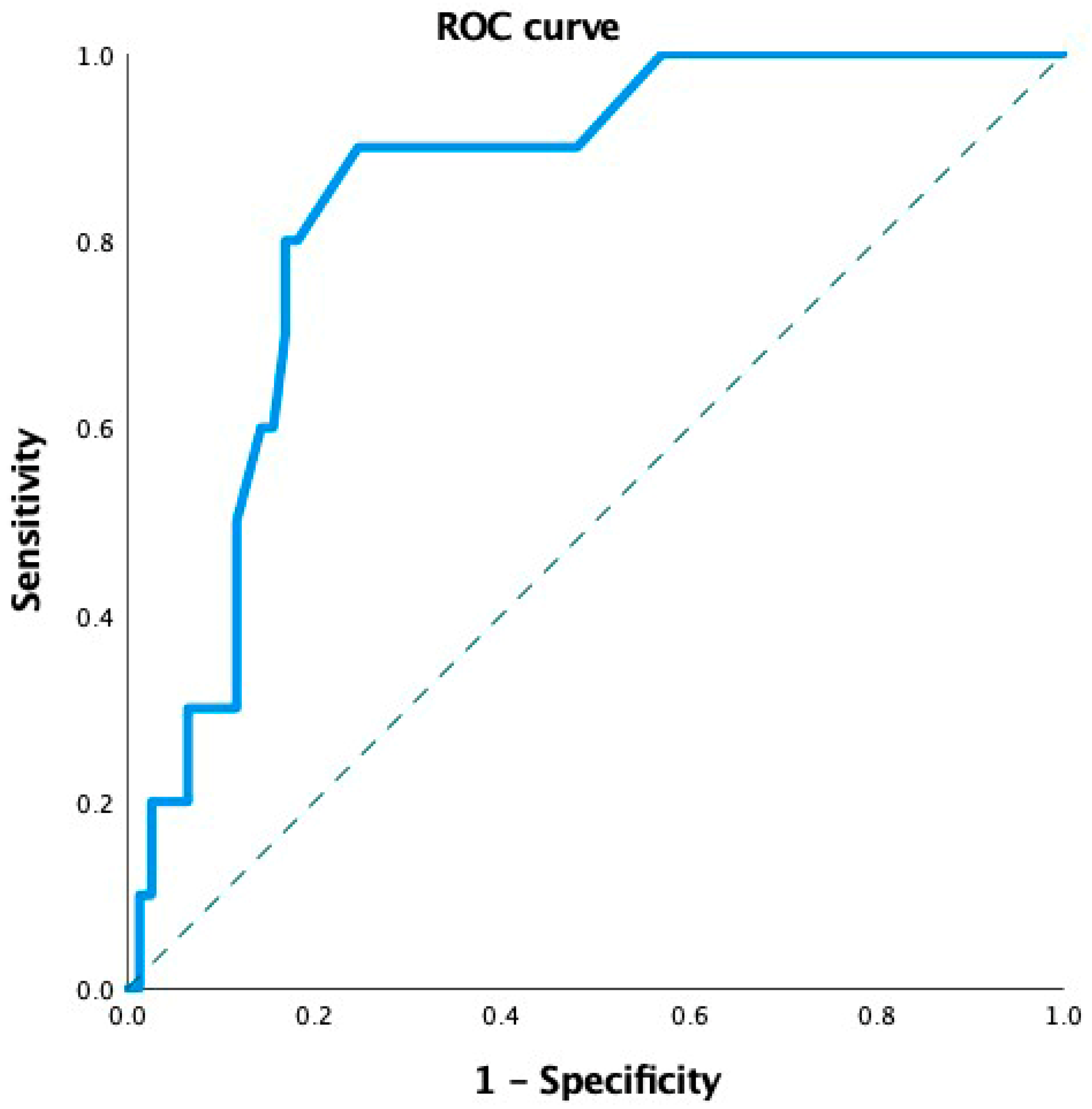
n = 10), a robust multivariate logistic regression model could not be constructed. Therefore, we assessed the ability of MvD to discriminate between different levels of MD impairment using Receiver Operating Characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was 0.77 (95% CI: 0.69–0.87;
p = 0.005), indicating good discriminatory capacity (
Figure 5).
4. Discussion
This study identified parapapillary MvD in a subset of eyes with OAG, accounting for 24.4% of cases. The localization of MvD varied across retinal vascular layers, with a predominance in the choroid and choriocapillaris, suggesting deeper vascular compromise in glaucomatous pathology. These findings are consistent with previous reports indicating that MvD typically occurs within β-zone parapapillary atrophy and preferentially affects deeper microvascular layers [
16].
Although dropout in the superficial and deep plexuses was less consistently observed, its absence does not exclude the presence of parapapillary MvD in other layers.
Secondary analysis revealed significant reductions in PCT, choroidal stromal and luminal areas, and pVD in glaucoma patients compared to healthy controls. These results support the hypothesis of vascular involvement in glaucomatous damage and align with prior studies emphasizing the diagnostic relevance of peripapillary vascular density [
17,
18,
19].
The observed increase in β-PPA area and its association with parapapillary MvD further suggest a link between microvascular dropout and localized structural degeneration. Our results are consistent with previous studies that have reported a reduction in PCT in glaucomatous eyes, which may reflect vascular support dysfunction at the choroidal level, particularly in areas adjacent to the optic nerve head [
20,
21].
The inverse relationship between the number of ocular antiglaucomatous treatments and PCT, both in univariate and multivariate models, may reflect the impact of chronic intraocular pressure management on choroidal vascular dynamics. Additionally, the StructureIndex was positively associated with PCT in the multivariate model, indicating that structural integrity may contribute to maintaining choroidal architecture. Although variables such as β-PPA, choroidal luminal area, and visual field mean defect did not reach statistical significance, their inclusion highlights the multifactorial nature of choroidal changes in glaucoma. The adjusted R2 value (0.14) suggests that other unmeasured factors may contribute to the observed variability. Given that the sample size of the study was limited and that the multivariate linear regression models had insufficient statistical power to reliably detect real effects, it was decided to present the results individually using univariate linear regression analysis in addition to multivariate analysis.
A strong correlation between pVD and peripapillary RNFL thickness, particularly in the inferior and superior sectors, reinforces the interdependence between structural integrity and vascular perfusion in the optic nerve head [
22,
23,
24].
The comparative analysis between glaucoma patients with and without parapapillary MvD revealed significant differences in specific clinical parameters. MvD-positive eyes exhibited more pathological MD values and greater visual field progression, suggesting a potential link between microvascular compromise and functional deterioration. Lower GAT IOP values in the parapapillary MvD group may reflect more advanced disease stages or treatment effects. No significant differences were found in RNFL thickness or its progressive thinning between groups, indicating that MvD is not independently associated with structural loss.
Further comparison of peripapillary superficial vessel and choroidal measurements revealed significantly lower pVD in the temporal and nasal sectors of MvD-positive eyes. These eyes also showed greater β-PPA, reduced choroidal luminal and stromal areas, and a trend toward thinner PCT. While not all differences reached statistical significance, the findings suggest localized microvascular compromise. Unlike previous studies that reported a direct relationship between MvD extent and reduced PCT or IOP [
25,
26,
27,
28], our study did not find significant differences in these variables, possibly due to the topographic focus of our analysis. On the other hand, we found that patients with MvD had significantly smaller choroidal luminal and stromal areas, suggesting that, although total choroidal thickness may not differ significantly, there are quantitative alterations in choroidal vascular perfusion in glaucoma eyes with MvD. Studies such as that by Lee et al. have already reported that MvD is associated with lower flow density and a pattern of localized choroidal thinning [
27].
Importantly, all MvD-positive patients exhibited associated β-PPA, supporting the hypothesis that parapapillary atrophy represents a structurally vulnerable environment for vascular dropout. This observation is consistent with prior studies reporting an association between β-PPA extent and the presence of MvD [
16,
29]. Our results demonstrate that MvD is associated with worse MD values and greater MD progression, supporting its role as a structural marker of disease severity [
13]. However, we did not observe a relationship between RNFL thickness changes and MvD, suggesting that choroidal MvD may not be independently associated with structural deterioration [
30,
31]. Our finding of no significant correlation between RNFL thinning and the presence of MvD is noteworthy, as it suggests that vascular dropout may represent a mechanism of glaucomatous functional progression that could be, at least in part, independent of structural damage. These findings support the hypothesis that vascular dysfunction, as reflected by MvD, may play a distinct and clinically relevant role in the progression of glaucomatous functional deterioration, independently of RNFL thinning.
Although superficial vascular density parameters (pVD and pFI) were lower in glaucoma patients, they did not differ significantly between those with and without parapapillary MvD. This indicates that OCTA-detected perfusion reduction may not reliably reflect deep microvascular compromise. The presence of parapapillary MvD was assessed in the superficial and deep vascular plexuses, choriocapillaris, and choroid. This strategy was chosen to provide a comprehensive assessment of microvascular compromise in glaucoma, as vascular dropout may occur at different depths and anatomical levels within the peripapillary region. Superficial and deep retinal vascular plexuses, choriocapillaris, and choroids are interconnected through a complex vascular network supplying the optic nerve head and peripapillary region mainly through the branches of the short posterior ciliary arteries. The pathological processes of glaucoma can affect these layers simultaneously or sequentially, as ischemic or degenerative processes affecting one layer may extend to or coexist in adjacent layers due to a shared or closely related vascular supply, as well as anatomical and functional interconnections. Therefore, simultaneous assessment of all these layers allows for a more accurate characterization of the extent and pattern of microvascular dropout and may help to elucidate whether vascular compromise is focal or diffuse.
Our findings support the theory that the combined involvement of the retinal and choroidal microvasculature is associated with more severe functional impairment in glaucoma. Nevertheless, the association between MvD and worse MD values and greater visual field progression suggests that deeper vascular involvement may be more pronounced in advanced disease stages, as previously described [
32].
To explore the possible influence of systemic arterial hypertension on peripapillary choroidal thickness (PCT) and the presence of MvD in glaucoma patients, we performed a stratified analysis according to systemic arterial hypertension status, as presented in
Supplementary Table S1. This table shows the PCT measurement and the presence or absence of MvD in both the control and glaucoma groups, separated by the presence or absence of systemic arterial hypertension. No statistically significant differences in PCT values were observed between hypertensive and non-hypertensive subjects, either in the control group (
p = 0.285) or in glaucoma patients (
p = 0.202). Similarly, the prevalence of MvD did not differ significantly between hypertensive and non-hypertensive patients with glaucoma (22% vs. 28%,
p = 0.936).
Furthermore, univariate linear regression analysis revealed that systemic arterial hypertension was not significantly associated with PCT (B = −4.24, 95% CI: −37.15 to 28.67, p = 0.798). Similarly, binary logistic regression analysis showed no significant association between systemic arterial hypertension and the presence of MvD (B = −0.325, 95% CI: 0.173–3.019, p = 0.722). These findings suggest that, within the limitations of our sample size, systemic arterial hypertension does not have a relevant impact on PCT or the presence of MvD in our study group.
Limitations of this study include the relatively small sample size, particularly in the MvD subgroup, which may have limited the statistical power to detect significant differences in some comparisons.
Regarding statistical power and sample size limitations, although a significant association between the MD index and the presence of microvasculature dropout (MvD) was found, we acknowledge that the number of glaucomatous eyes with MvD included in the analysis (n = 10) is limited. A post hoc power analysis was conducted to address the possibility of overfitting and false-positive results in both the regression models and the ROC curve analysis. Using the observed odds ratio (OR = 1.283), a significance level of α = 0.05, and the proportion of MvD-positive cases (11.5%), the estimated statistical power was about 71%, indicating a moderate ability to detect true effects. Ideally, an a priori power analysis would have required a sample size of at least 150–180 eyes to achieve 80% power under similar assumptions. This could be considered a methodological limitation and underscores the need for future studies with larger samples to validate our findings.
The cross-sectional design precludes causal inference. Manual quantification of PCT and β-PPA may be subject to interobserver variability, although all measurements were performed by a single examiner using high-resolution en face OCTA images.
Strengths include the simultaneous sectoral evaluation of multiple vascular and structural parameters using a single high-resolution OCTA platform. The use of a composite index (StructureIndex) improved model robustness and reduced collinearity.
The StructureIndex is a new combined variable that integrates structural and vascular information from RNFL thickness and pVD. Both measures are correlated and show the complementary characteristics of optic nerve head morphology and vascularization assessed by SD-OCT and OCTA imaging. Its inclusion in this study was mainly methodological, addressing collinearity and sample size limitations.
This index did not demonstrate a statistically significant advantage over pVD or RNFL thickness in discriminating between glaucoma patients with or without MvD.
The StructureIndex had an AUROC value of 0.681 (SE 0.080; 95% CI: 0.524–0.838), compared with 0.519 (SE 0.101; 95% CI: 0.321–0.717) for pVD and 0.560 (SE 0.109; 95% CI: 0.347–0.773) for peripapillary RNFL thickness. A statistical comparison of the ROC curves using a z-test showed no significant differences between the StructureIndex and pVD (z = 1.257, p = 0.21) or between the StructureIndex and RNFL thickness (z = 0.895, p = 0.37).
For this reason, although this combined parameter showed a statistically significant association with PCT in our multivariate model, we acknowledge that this index has not been validated in previous studies, so these results should be interpreted with caution.
Its validation in future studies and longitudinal designs would be necessary to verify its usefulness.
This study was conducted at a single center with a relatively homogeneous population, which may limit the generalizability of our findings. It is possible that the association observed between MvD and glaucomatous progression may differ in populations with different ethnic, genetic, or systemic risk profiles. For example, vascular autoregulation, susceptibility to microvascular compromise, and optic nerve head structural characteristics may vary among different demographic groups. Multicenter studies involving more diverse populations are needed to validate our results and to determine whether MvD performs similarly as a marker of functional progression in different clinical contexts.
Prospective recruitment under uniform imaging and clinical protocols minimized bias, and manual validation of OCTA-derived parameters by a blinded examiner enhanced internal consistency. These methodological aspects strengthen the reliability of our findings and provide a basis for future longitudinal studies aimed at confirming the prognostic value of choroidal MvD in glaucoma progression.
In conclusion, parapapillary MvD in deep vascular layers is associated with reduced choroidal vascular indices, greater β-parapapillary atrophy, and visual field deterioration.
Peripapillary choroidal thickness and vascularization are lower in patients with glaucoma. Glaucomatous eyes with OCTA-conformed parapapillary choroidal dropout show lower choroidal vascular indices than those without dropout. MvD is associated with the worsening of mean defects, suggesting its possible role as a marker of increased risk of functional impairment.