Two-Year Outcome of Selective Laser Trabeculoplasty for Normal-Tension Glaucoma in Japan: First-Line or Second-Line Selective Laser Trabeculoplasty (FSS) Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Outcome Measures
2.3. Statistical Analyses
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Treatment Outcomes
3.3. Complications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, R.; Azuara-Blanco, A.; Ramsay, C.R. Variation of clinical outcomes used in glaucoma randomised controlled trials: A systematic review. Br. J. Ophthalmol. 2014, 98, 464–468. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.G.; Liebmann, J.M.; Levin, L.A. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog. Retin. Eye Res. 2017, 56, 107–147. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary openangle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef]
- Bengtsson, B.; Leske, M.C.; Hyman, L.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology 2007, 114, 205–209. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Mikelberg, F.S.; Balaszi, A.G.; LeBlanc, R.P.; Lesk, M.R.; Trope, G.E.; Canadian Glaucoma Study Group. Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma. Arch. Ophthalmol. 2008, 126, 1030–1036. [Google Scholar] [CrossRef]
- Garway-Heath, D.F.; Lascaratos, G.; Bunce, C.; Crabb, D.P.; Russell, R.A.; Shah, A.; United Kingdom Glaucoma Treatment Study Investigators. The United Kingdom Glaucoma Treatment Study: A multicenter, randomized, placebo-controlled clinical trial: Design and methodology. Ophthalmology 2013, 120, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Garg, A.; Vickerstaff, V.; Hunter, R.; Ambler, G.; Bunce, C.; Wormald, R.; Nathwani, N.; et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre randomised controlled trial. Lancet 2019, 393, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Vickerstaff, V.; Nathwani, N.; Garway-Heath, D.; Konstantakopoulou, E.; Ambler, G.; Bunce, C.; Wormald, R.; Barton, K.; Gazzard, G.; et al. Primary selective laser trabeculoplasty for open-angle glaucoma and ocular hypertension: Clinical outcomes, predictors of success, and safety from the laser in glaucoma and ocular hypertension trial. Ophthalmology 2019, 126, 1238–1248. [Google Scholar] [CrossRef]
- Garg, A.; Vickerstaff, V.; Nathwani, N.; Garway-Heath, D.; Konstantakopoulou, E.; Ambler, G.; Bunce, C.; Wormald, R.; Barton, K.; Gazzard, G.; et al. Efficacy of repeat selective laser trabeculoplasty in medication-naïve open-angle glaucoma and ocular hypertension during the LiGHT trial. Ophthalmology 2020, 127, 467–476. [Google Scholar] [CrossRef]
- Wright, D.M.; Konstantakopoulou, E.; Montesano, G.; Nathwani, N.; Garg, A.; Garway-Heath, D.; Crabb, D.P.; Gazzard, G.; on behalf of the Laser in Glaucoma and Ocular Hypertension Trial (LiGHT) Study Group. Visual field outcomes from the multicenter, randomized controlled laser in glaucoma and ocular hypertension trial (LiGHT). Ophthalmology 2020, 127, 1313–1321. [Google Scholar] [CrossRef]
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Adeleke, M.; Vickerstaff, V.; Ambler, G.; Hunter, R.; Bunce, C.; Nathwani, N.; Barton, K.; et al. Laser in glaucoma and ocular hypertension (LiGHT) trial: Six-year results of primary selective laser trabeculoplasty versus eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology 2023, 130, 139–151. [Google Scholar] [CrossRef]
- Ang, G.S.; Fenwick, E.K.; Constantinou, M.; Gan, A.T.L.; Man, R.E.K.; Casson, R.J.; Finkelstein, E.A.; Goldberg, I.; Healey, P.R.; Pesudovs, K.; et al. Selective laser trabeculoplasty versus topical medication as initial glaucoma treatment: The glaucoma initial treatment study randomised clinical trial. Br. J. Ophthalmol. 2020, 104, 813–821. [Google Scholar] [CrossRef]
- Wong, M.O.; Lee, J.W.; Choy, B.N.; Chan, J.C.; Lai, J.S. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv. Ophthalmol. 2015, 60, 36–50. [Google Scholar] [CrossRef]
- Tzimis, V.; Tze, L.; Ganesh, J.; Muhsen, S.; Kiss, A.; Kranemann, C.; Birt, C.M. Laser trabeculoplasty: An investigation into factors that might influence outcomes. Can. J. Ophthalmol. 2011, 46, 305–309. [Google Scholar] [CrossRef]
- King, D.; Drance, S.M.; Douglas, G.; Schulzer, M.; Wijsman, K. Comprison of visual field defects in normal-tension glaucoma and high-tension glaucoma. Am. J. Ophthalmol. 1986, 101, 204–207. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Seong, G.J.; Lee, N.H.; Song, K.C.; Namil Study Group, Korean Glaucoma Society. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 2011, 118, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Sugiyama, K.; Mawatari, Y.; Tanahashi, T. Results of selective laser trabeculoplasty (SLT) as initial treatment for normal tension glaucoma. Nippon Ganka Gakkai Zasshi 2013, 117, 335–343. (In Japanese) [Google Scholar] [PubMed]
- Lee, J.W.Y.; Shum, J.J.W.; Chan, J.C.H.; Lai, J.S.M. Two-year clinical results after selective laser trabeculoplasty for normal tension glaucoma. Medicine 2015, 94, e984. [Google Scholar] [CrossRef]
- Nitta, K.; Sugihara, K.; Narita, A.; Naito, T.; Miki, T.; Katai, M.; Mizoue, S.; Yoshikawa, K.; Tanito, M.; Sugiyama, K.; et al. Efficacy and safety of first-line or second-line selective laser trabeculoplasty for normal-tension glaucoma: A multicentre cohort study. BMJ Open Ophthalmol. 2024, 9, e001563. [Google Scholar] [CrossRef]
- Fahy, E.T.; Montesano, G.; Garg, A.; Vickerstaff, V.; Konstantakopoulou, E.; Gazzard, G.; LiGHT Trial Study Group. The Impact of Baseline Intraocular Pressure on Initial Treatment Response in the LiGHT Trial: Selective Laser Trabeculoplasty versus Medication. Ophthalmology 2024, 131, 1366–1376. [Google Scholar] [CrossRef]
- Schulzer, M. Intraocular pressure reduction in normal-tension glaucoma patients. Ophthalmology 1992, 99, 1468–1470. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Tsumura, T.; Tsukahara, S. Long-term effects of latanoprost monotherapy on intraocular pressure in Japanese glaucoma patients. J. Glaucoma 2008, 17, 662–666. [Google Scholar] [CrossRef]
- El Mallah, M.K.; Walsh, M.M.; Stinnett, S.S.; Asrani, S.G. Selective laser trabeculoplasty reduces mean IOP and IOP variation in normal tension glaucoma patients. Clin. Ophthalmol. 2010, 4, 889–893. [Google Scholar] [CrossRef]
- Tojo, N.; Oka, M.; Miyakoshi, A.; Ozaki, H.; Hayashi, A. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J. Glaucoma 2014, 23, e138–e143. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Fu, L.; Chan, J.C.H.; Lai, J.S.M. Twenty-four-Hour intraocular pressure related changes following adjuvant selective laser trabeculoplasty for normal tension glaucoma. Medicine 2014, 93, e238. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Tan, Q.; Xia, X.; Jiang, H.; Jiang, J. Outcome of selective laser trabeculoplasty in young patients with primary open-angle glaucoma and ocular hypertension. J. Ophthalmol. 2020, 2020, 5742832. [Google Scholar] [CrossRef]
- Pillunat, K.R.; Kocket, G.A.; Herber, R.; Jasper, C.S.; Lenk, J.; Pillunat, L.E. Efficacy of selective laser trabeculoplasty on lowering intraocular pressure fluctuations and nocturnal peak intraocular pressure in treated primary open-angle glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1979–1985. [Google Scholar] [CrossRef]
- Robin, A.L.; Muir, K.W. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev. Ophthalmol. 2019, 14, 199–210. [Google Scholar] [CrossRef]
- Cabinet Office, Japan: Annual Report on the Ageing Society (Data of 2024, in Japanese). (Data of 2021, in English). Available online: https://www8.cao.go.jp/kourei/english/annualreport/2021/pdf/2021.pdf (accessed on 22 December 2024).
- Broadway, D.C.; Grierson, I.; O’Brien, C.; Hitchings, R.A. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch. Ophthalmol. 1994, 112, 1446–1454. [Google Scholar] [CrossRef]
- Gulati, V.; Fan, S.; Gardner, B.J.; Havens, S.J.; Schaaf, M.T.; Neely, D.G.; Toris, C.B. Mechanism of Action of Selective Laser Trabeculoplasty and Predictors of Response. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1462–1468. [Google Scholar] [CrossRef]
- Hirabayashi, M.; Ponnusamy, V.; An, J. Predictive factors for outcomes of selective laser trabeculoplasty. Sci. Rep. 2020, 10, 9428. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Campbell, J.H.; Kirby, N.; Chandwani, H.S.; Keyzor, I.; Parekh, M.; McNaught, A.I.; UK Glaucoma Real-World Data Consortium. Real-world outcomes of selective laser trabeculoplasty in the United Kingdom. Ophthalmology 2020, 127, 748–757. [Google Scholar] [CrossRef]
- Ayala, M.; Chen, E. Predictive factors of success in selective laser trabeculoplasty (SLT) treatment. Clin. Ophthalmol. 2011, 5, 573–576. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Liu, C.C.L.; Chan, J.C.H.; Lai, J.S.M. Predictors of success in selective laser trabeculoplasty for normal tension glaucoma. Medicine 2014, 93, e236. [Google Scholar] [CrossRef]
- Whitacre, M.M.; Stein, R.A.; Hassanein, K. The effect of corneal thickness on applanation tonometry. Am. J. Ophthalmol. 1993, 115, 592–596. [Google Scholar] [CrossRef]
- Feltgen, N.; Leifert, D.; Funk, J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br. J. Ophthalmol. 2001, 85, 85–87. [Google Scholar] [CrossRef]
- Regina, M.; Bunya, V.Y.; Orlin, S.E.; Ansari, H. Corneal edema and haze after selective laser trabeculoplasty. J. Glaucoma 2011, 20, 327–329. [Google Scholar] [CrossRef]
- Moubayed, S.P.; Hamid, M.; Choremis, J.; Li, G. An unusual finding of corneal edema complicating selective laser trabeculoplasty. Can. J. Ophthalmol. 2009, 44, 337–338. [Google Scholar] [CrossRef]
- Ong, K.; Ong, L.; Ong, L.B. Corneal endothelial abnormalities after selective laser trabeculoplasty. J. Glaucoma 2015, 24, 286–290. [Google Scholar] [CrossRef]
- Rhee, D.J.; Krad, O.; Pasquale, L.R. Hyphema following selective laser trabeculoplasty. Ophthalmic Surg. Lasers Imaging Retin. 2009, 40, 493–494. [Google Scholar] [CrossRef]
- Shihadeh, W.A.; Ritch, R.; Liebmann, J.M. Hyphema occurring during selective laser trabeculoplasty. Ophthalmic Surg. Lasers Imaging Retin. 2006, 37, 432–433. [Google Scholar] [CrossRef]
- Kim, D.Y.; Singh, A. Severe Iritis and choroidal effusion following selective laser trabeculoplasty. Ophthalmic Surg. Lasers Imaging Retin. 2008, 39, 409–411. [Google Scholar] [CrossRef]
- Wechsler, D.Z.; Wechsler, I.B. Cystoid macular oedema after selective laser trabeculoplasty. Eye 2010, 24, 1113. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Chan, J.C.H.; Chang, R.T.; Singh, K.; Liu, C.C.L.; Gangwani, R.; Wong, M.O.M.; Lai, J.S.M. Corneal changes after a single session of selective laser trabeculoplasty for open-angle glaucoma. Eye 2014, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
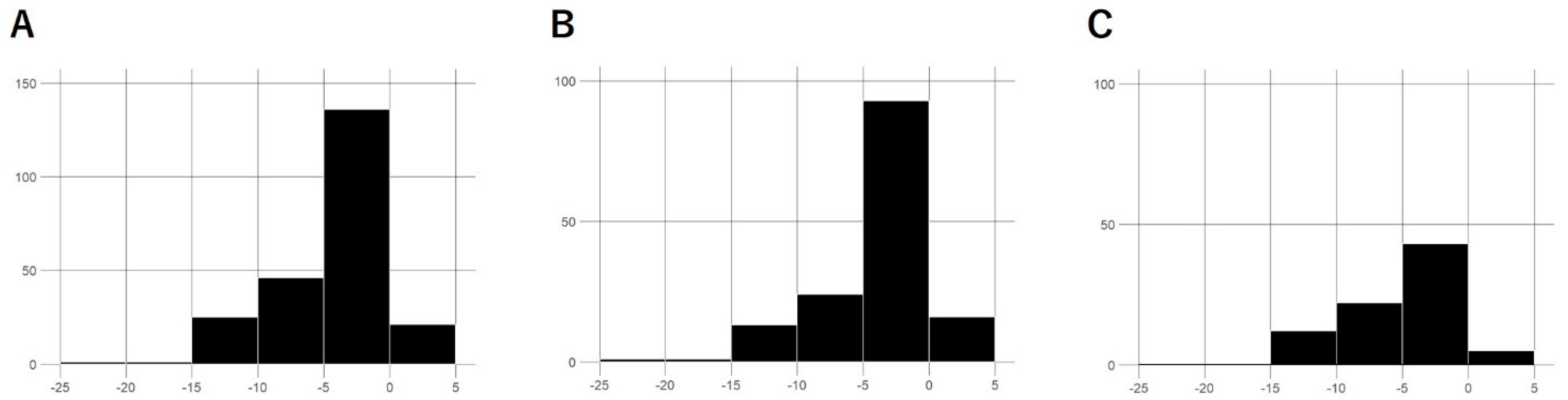
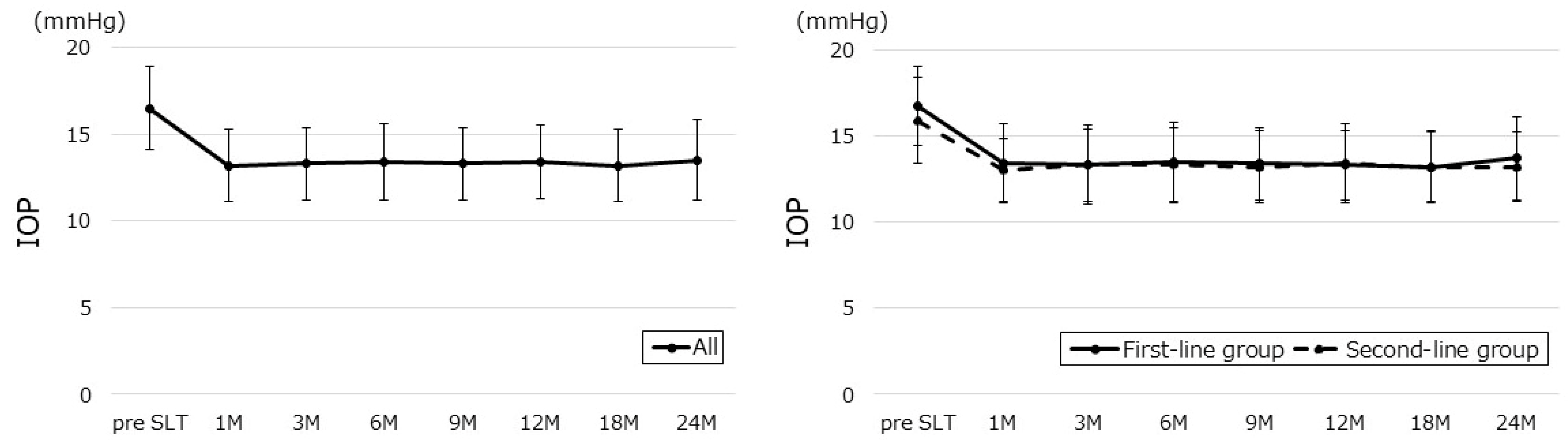
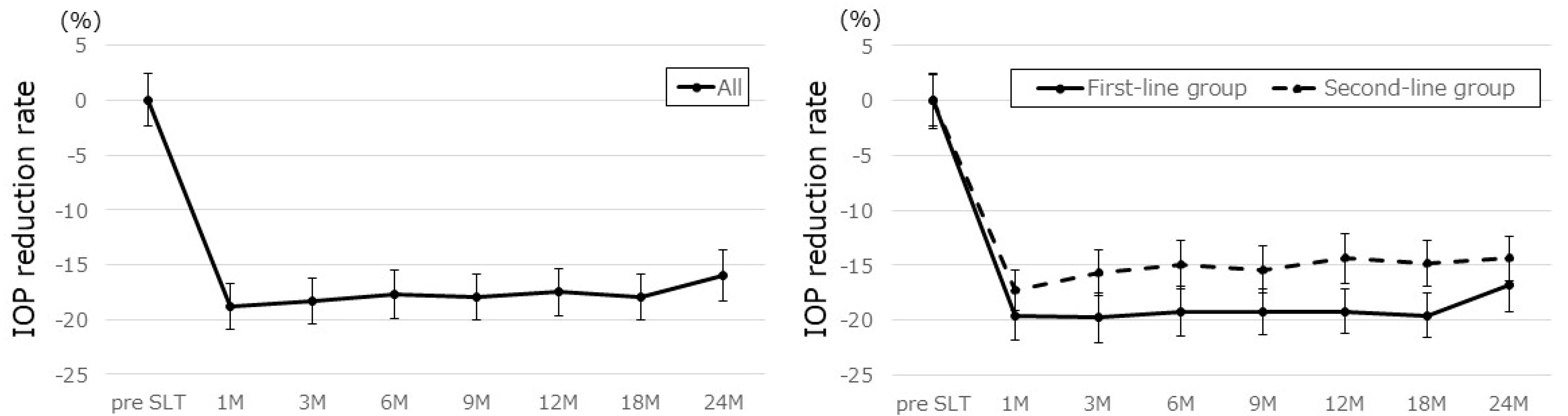
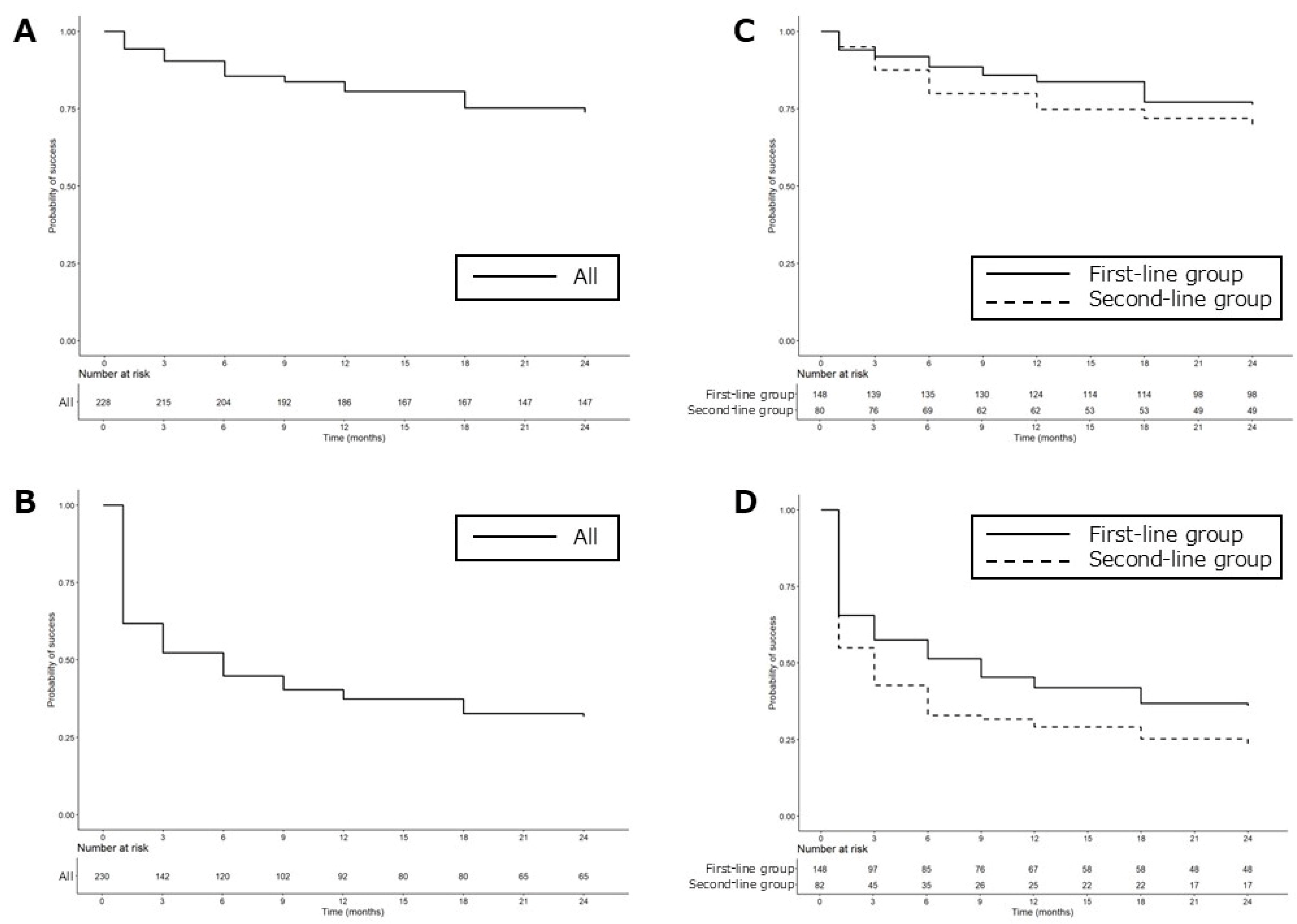
| Parameter | All | First-Line | Second-Line | p-Value |
|---|---|---|---|---|
| Eyes (n) | 230 | 148 | 82 | |
| Age (years) | 60.8 ± 11.9 | 59.1 ± 11.7 | 64.0 ± 11.7 | 0.003 * |
| Gender (female/male) | 143/87 | 96/52 | 47/35 | 0.261 † |
| Pretreatment IOP (mmHg) | 16.5 ± 2.4 | 16.7 ± 2.3 | 15.9 ± 2.5 | 0.016 * |
| Visual field, MD (dB) | −4.3 ± 4.2 | −3.8 ± 4.2 | −5.0 ± 4.0 | 0.035 * |
| Refractive error (spherical D) | −3.91 ± 3.66 | −4.04 ± 3.75 | −3.68 ± 3.51 | 0.468 * |
| Decimal visual acuity | 1.18 ± 0.26 | 1.19 ± 0.26 | 1.18 ± 0.27 | 0.804 ‡ |
| CCT (μm) | 531.6 ± 31.7 | 533.0 ± 33.5 | 529.0 ± 28.3 | 0.331 * |
| ECD (/mm2) | 2635.2 ± 295.6 | 2653.1 ± 296.0 | 2603.2 ± 293.9 | 0.222 * |
| β | S.E. | t Value | p-Value | |
|---|---|---|---|---|
| Time (months) | −0.2872 | 0.0400 | −7.1800 | 0.0000 * |
| Group (second-line) | 1.0163 | 1.1707 | 0.8680 | 0.3859 |
| Age | 0.0840 | 0.0413 | 2.0310 | 0.0435 * |
| Pretreatment IOP | −1.9169 | 0.2126 | −9.0140 | 0.0000 * |
| CCT | 0.0846 | 0.0159 | 5.3210 | 0.0000 * |
| Time*Group (second-line) | 0.0706 | 0.0679 | 1.0400 | 0.2984 |
| Univariate Model | HR | 95% CI | p-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Group | ||||
| Definition A | 1.408 | 0.824 | 2.405 | 0.211 |
| Definition B | 1.534 | 1.047 | 2.246 | 0.028 * |
| Age | ||||
| Definition A | 1.007 | 0.985 | 1.029 | 0.547 |
| Definition B | 1.018 | 1.002 | 1.035 | 0.031 * |
| Pretreatment IOP | ||||
| Definition A | 1.043 | 0.932 | 1.168 | 0.465 |
| Definition B | 0.825 | 0.759 | 0.896 | 0.000 * |
| Visual field MD | ||||
| Definition A | 0.980 | 0.922 | 1.043 | 0.531 |
| Definition B | 0.998 | 0.955 | 1.043 | 0.931 |
| CCT | ||||
| Definition A | 1.012 | 1.004 | 1.021 | 0.006 * |
| Definition B | 1.005 | 0.999 | 1.011 | 0.090 |
| Multivariate model | Adjusted HR | 95% CI | p-value | |
| Lower limit | Upper limit | |||
| Group | ||||
| Definition A | 1.451 | 0.829 | 2.538 | 0.192 |
| Definition B | 1.458 | 0.969 | 2.195 | 0.071 |
| Age | ||||
| Definition A | 1.007 | 0.984 | 1.030 | 0.567 |
| Definition B | 1.013 | 0.995 | 1.031 | 0.149 |
| Pretreatment IOP | ||||
| Definition A | 1.004 | 0.887 | 1.137 | 0.952 |
| Definition B | 0.788 | 0.719 | 0.864 | 0.000 * |
| Visual field MD | ||||
| Definition A | 0.967 | 0.907 | 1.030 | 0.295 |
| Definition B | 0.996 | 0.949 | 1.046 | 0.876 |
| CCT | ||||
| Definition A | 1.015 | 1.005 | 1.024 | 0.003 * |
| Definition B | 1.013 | 1.006 | 1.020 | 0.000 * |
| Adverse Events During SLT | Eyes (n) | Incidence Rate (%) |
|---|---|---|
| Anterior chamber inflammation | 141 | 61.3 |
| Conjunctival hyperemia | 37 | 16.1 |
| Adverse events after SLT | Eyes (n) | Incidence rate (%) |
| Conjunctival hyperemia | 19 | 8.3 |
| Discomfort (ocular or headache) | 19 | 8.3 |
| Anterior chamber inflammation | 18 | 7.8 |
| Blurred/altered vision | 14 | 6.1 |
| Photophobia | 5 | 2.2 |
| Macular edema | 1 (Due to BRVO) | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, T.; Nitta, K.; Miki, T.; Narita, A.; Kimura, T.; Ikuno, Y.; Mizoue, S.; Katai, M.; Saito, Y.; Nanno, M.; et al. Two-Year Outcome of Selective Laser Trabeculoplasty for Normal-Tension Glaucoma in Japan: First-Line or Second-Line Selective Laser Trabeculoplasty (FSS) Study. J. Clin. Med. 2025, 14, 3459. https://doi.org/10.3390/jcm14103459
Naito T, Nitta K, Miki T, Narita A, Kimura T, Ikuno Y, Mizoue S, Katai M, Saito Y, Nanno M, et al. Two-Year Outcome of Selective Laser Trabeculoplasty for Normal-Tension Glaucoma in Japan: First-Line or Second-Line Selective Laser Trabeculoplasty (FSS) Study. Journal of Clinical Medicine. 2025; 14(10):3459. https://doi.org/10.3390/jcm14103459
Chicago/Turabian StyleNaito, Tomoko, Koji Nitta, Takako Miki, Akiko Narita, Tairo Kimura, Yasushi Ikuno, Shiro Mizoue, Maki Katai, Yoshiaki Saito, Mami Nanno, and et al. 2025. "Two-Year Outcome of Selective Laser Trabeculoplasty for Normal-Tension Glaucoma in Japan: First-Line or Second-Line Selective Laser Trabeculoplasty (FSS) Study" Journal of Clinical Medicine 14, no. 10: 3459. https://doi.org/10.3390/jcm14103459
APA StyleNaito, T., Nitta, K., Miki, T., Narita, A., Kimura, T., Ikuno, Y., Mizoue, S., Katai, M., Saito, Y., Nanno, M., Tojo, N., Tokuda, N., Yamabayashi, S., Suzuki, K., Konno, K., Ozaki, H., Nakazawa, T., Nakano, T., Nakamoto, K., ... Tanito, M. (2025). Two-Year Outcome of Selective Laser Trabeculoplasty for Normal-Tension Glaucoma in Japan: First-Line or Second-Line Selective Laser Trabeculoplasty (FSS) Study. Journal of Clinical Medicine, 14(10), 3459. https://doi.org/10.3390/jcm14103459






