Adherence to Guideline-Directed Medical Therapy in Hospitalized Older People with Heart Failure at Discharge and 3-Month Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
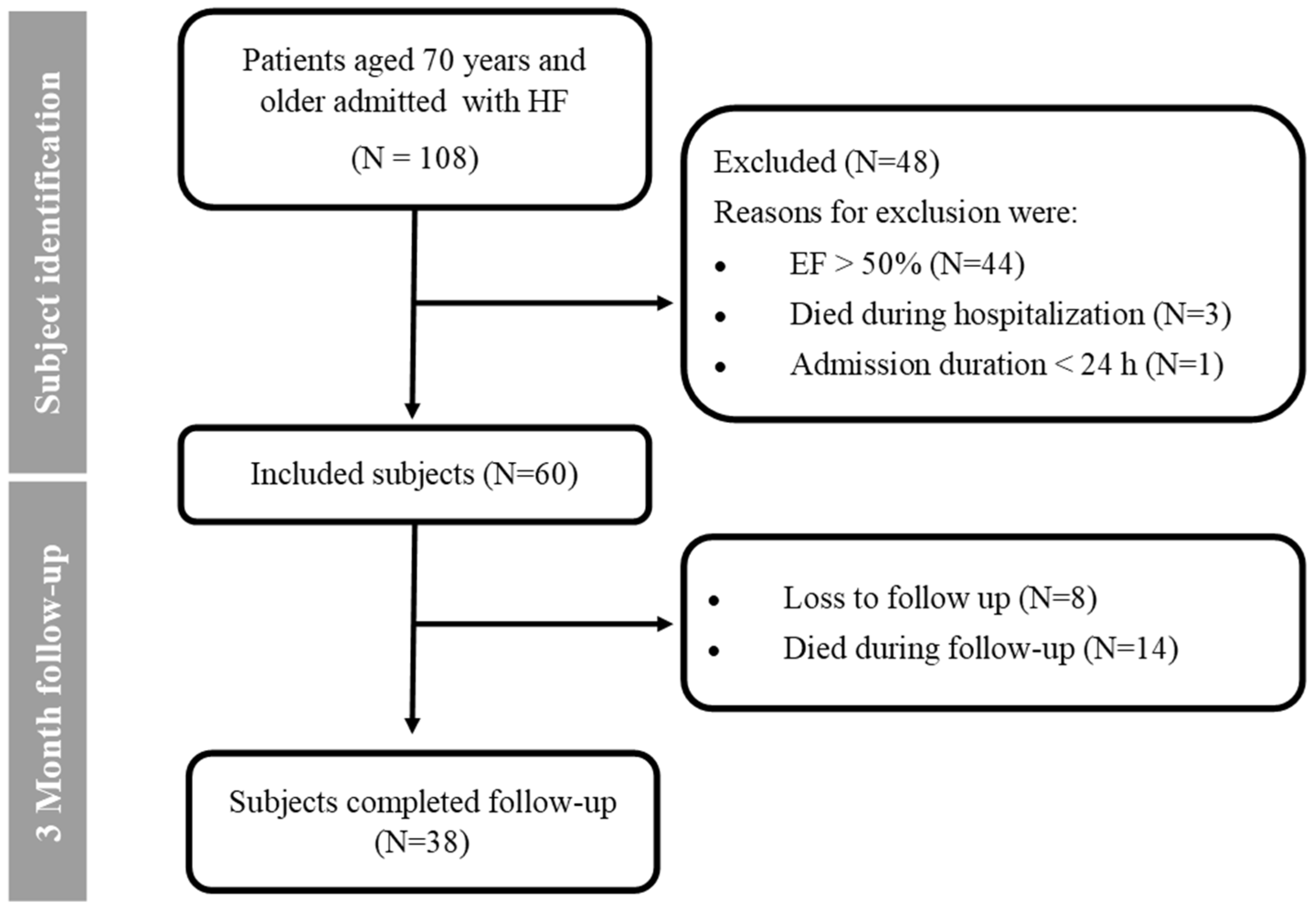
2.2. Study Population
2.3. Exposure
2.4. Ethics
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Medication Use
3.2.1. Discharge
3.2.2. Three-Month Follow-Up
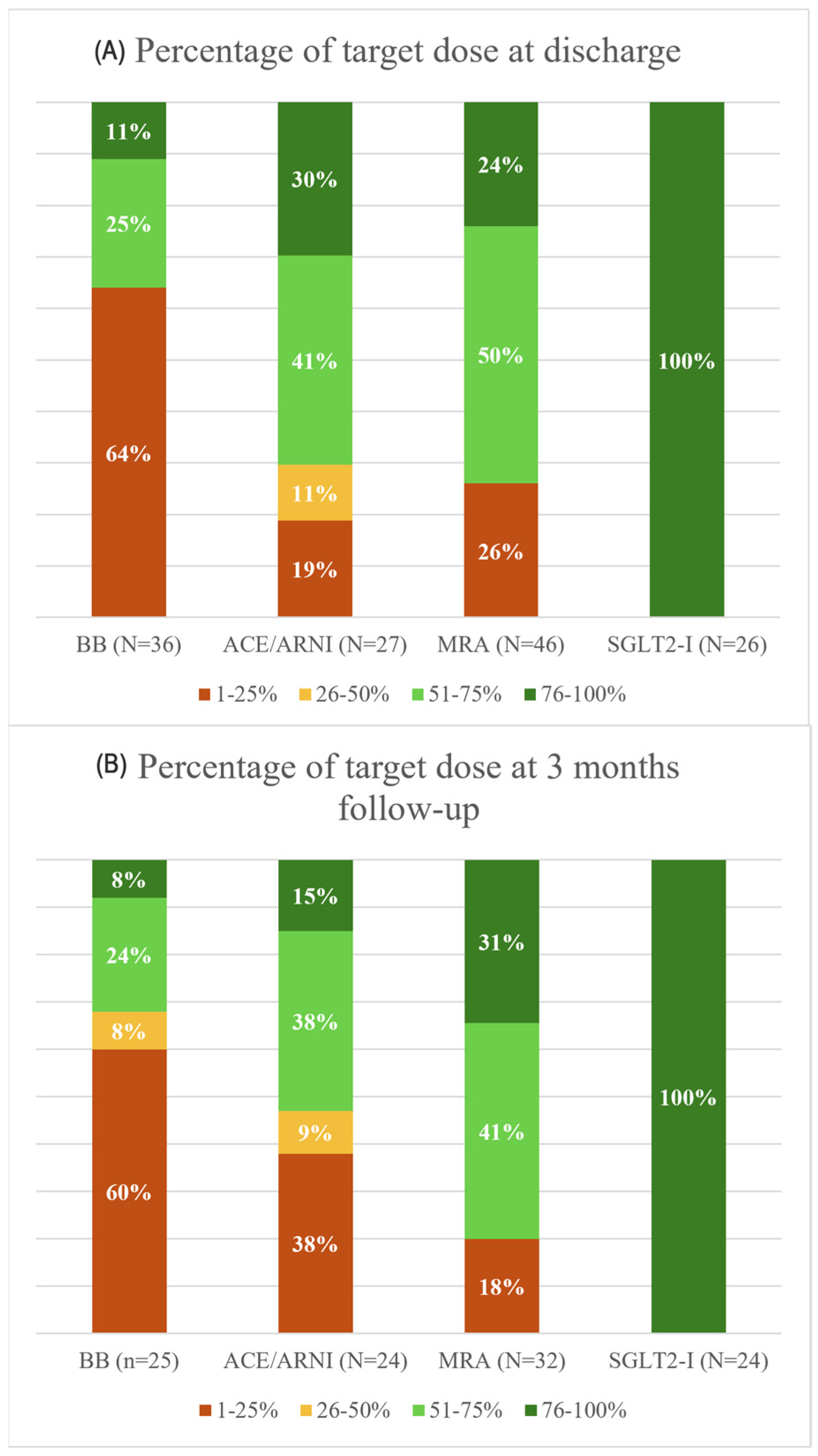
3.3. Optimal Medical Therapy: Target Doses
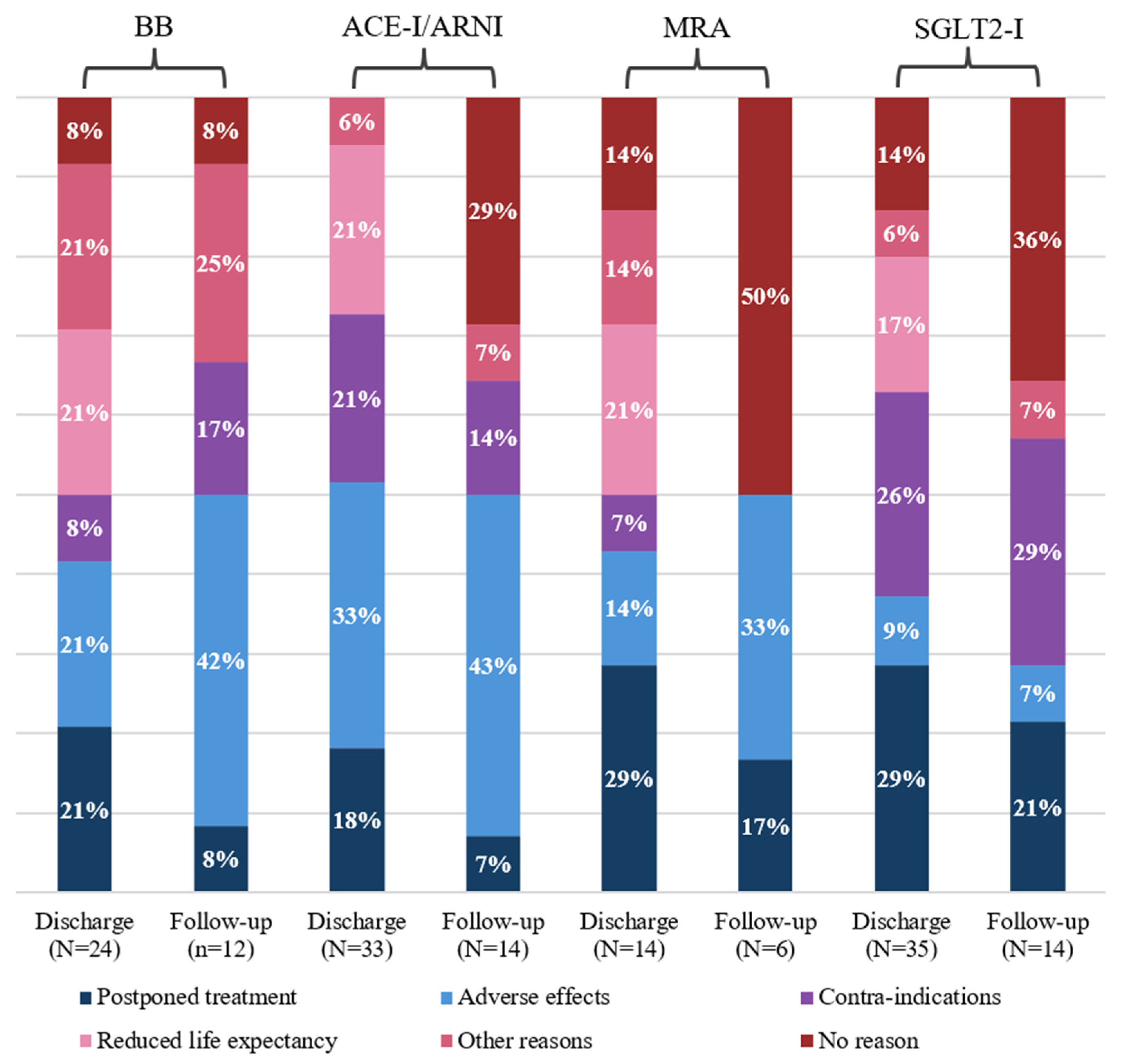
3.4. Reasons for Guideline Deviations
4. Discussion
4.1. Strengths and Limitations
4.2. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dharmarajan, K.; Rich, M.W. Epidemiology, Pathophysiology, and Prognosis of Heart Failurein Older Adults. Heart Fail. Clin. 2017, 13, 417–426. [Google Scholar] [CrossRef]
- Thomas, S.; Rich, M.W. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Clin. Geriatr. Med. 2007, 23, 1–10. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Malgie, J.; Clephas, P.R.D.; Brunner-La Rocca, H.-P.; de Boer, R.A.; Brugts, J.J. Guideline-directed medical therapy for HFrEF sequencing strategies and barriers for life-saving drug therapy. Heart Fail. Rev. 2023, 28, 1221–1234. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Kreutz, R.; Dräger, D.; Zwillich, C.; Hörter, S.; Kuhlmey, A.; Gellert, P. Lower Prescription Rates in Centenarians with Heart Failure and Heart Failure and Kidney Disease Combined: Findings from a Longitudinal Cohort Study of Very Old Patients. Drugs Aging 2018, 35, 907–916. [Google Scholar] [CrossRef]
- Stolfo, D.; Lund, L.H.; Becher, P.M.; Orsini, N.; Thorvaldsen, T.; Benson, L.; Hage, C.; Dahlström, U.; Sinagra, G.; Savarese, G. Use of evidence-based therapy in heart failure with reduced ejection fraction across age strata. Eur. J. Heart Fail. 2022, 24, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Jujo, K.; Maeda, D.; Saito, K.; Ogasahara, Y.; Saito, K.; Ogasahara, Y.; Saito, K.; Saito, H.; Iwata, K.; et al. The interaction between physical frailty and prognostic impact of heart failure medication in elderly patients. ESC Heart Fail. 2023, 10, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Kubo, T.; Kawai, K.; Nakaoka, Y.; Yabe, T.; Furuno, T.; Yamada, E.; Kitaoka, H.; Kochi YOSACOI study. Frailty interferes with the guideline-directed medical therapy in heart failure patients with reduced ejection fraction. ESC Heart Fail. 2023, 10, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Aghanya, V.; Sepehrvand, N.; Dover, D.C.; Kaul, P.; Ezekowitz, J. Use of Guideline-Directed Medical Therapy in Patients Aged ≥ 65 Years After the Diagnosis of Heart Failure: A Canadian Population-Based Study. CJC Open. 2022, 4, 1015–1023. [Google Scholar] [CrossRef]
- Cherubini, A.; Oristrell, J.; Pla, X.; Ruggiero, C.; Ferretti, R.; Diestre, G.; Clarfield, A.M.; Crome, P.; Hertogh, C.; Lesauskaite, V.; et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch. Intern. Med. 2011, 171, 550–556. [Google Scholar] [CrossRef]
- Ye, L.; Nieboer, D.; Yang--Huang, J.; Borrás, T.A.; Garcés--Ferrer, J.; Verma, A.; van Grieken, A.; Raat, H. The association between frailty and the risk of medication-related problems among community dwelling older adults in Europe. J. Am. Geriatr. Soc. 2023, 71, 2485–2494. [Google Scholar] [CrossRef]
- Sukumar, S.; Orkaby, A.R.; Schwartz, J.B.; Marcum, Z.; Januzzi, J.L.; Vaduganathan, M.; Warraich, H.J. Polypharmacy in older heart failure patients: A multidisciplinary approach. Comorbidities 2022, 19, 290–302. [Google Scholar] [CrossRef]
- Jarjour, M.; Henri, C.; de Denus, S.; Fortier, A.; Bouabdallaoui, N.; Nigam, A. Care Gaps in adherence to heart failure guidelines: Clinical inertia or physiological limitations? Heart Fail. 2020, 8, 725–738. [Google Scholar] [CrossRef]
- Jefferies, J.L.; Ibrahim, N.E. Are Guidelines Merely Suggestions? J. Am. Coll. Cardiol. 2018, 72, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Linssen, G.C.M.; Veenis, J.F.; Brunner-La Rocca, H.P.; Van Pol, P.E.J.; Engelen, D.J.M.; Brugts, J.J.; CHECK-HF investigators. Differences in guideline-recommended heart failure medication between Dutch heart failure clinics: An analysis of the CHECK-HF registry. Neth. Heart J. 2020, 28, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, R.; Okahara, A.; Tokutome, M.; Itonaga, J.; Koga, E.; Hara, A.; Kisanuki, H.; Sada, M.; Okabe, K.; Kawai, S.; et al. A scoring evaluation for the practical introduction of guideline-directed medical therapy in heart failure patients. ESC Heart Fail. 2023, 10, 3352–3363. [Google Scholar] [CrossRef] [PubMed]
- Stille, K.; Temmel, N.; Hepp, J.; Herget-Rosenthal, S. Validation of the Clinical Frailty Scale for retrospective use in acute care. Eur. Geriatr. Med. 2020, 11, 1009–1015. [Google Scholar] [CrossRef]
- World Health Organization. Anatomical Therapeutic Chemical (ATC) Classification; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Poe, S.S.; Dawson, P.B.; Cvach, M.; Burnett, M.; Kumble, S.; Lewis, M.; Thompson, C.B.; Hill, E.E. The Johns Hopkins Fall Risk Assessment Tool: A Study of Reliability and Validity. J. Nurs. Care Qual. 2018, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mair, A.; Donaldson, L.; Kelley, E.; Hill, S.; Dhingra-Kumar, N.; Garner, S.; Cherian, J.J.; Etzel, D.; Hakkinen, M.; Paget, D.; et al. Medication Safety in Polypharmacy; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 29.0; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Malgie, J.; Wilde, M.I.; Clephas, P.R.D.; Emans, M.E.; Koudstaal, S.; Schaap, J.; Mosterd, A.; van Ramshorst, J.; Wardeh, A.J.; van Wijk, S.; et al. Contemporary guideline-directed medical therapy in de novo, chronic, and worsening heart failure patients: First data from the TITRATE-HF study. Eur. J. Heart Fail. 2024, 26, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.S.; Marco, I.; Restrepo-Cordoba, M.A.; Vila Costa, I.; Perez-Villacastin, J.; Goirigolzarri-Artaza, J. An observational study of evidence-based therapies in older patients with heart failure with reduced ejection fraction: Insights from a dedicated heart failure clinic. J. Clin. Med. 2024, 13, 7171. [Google Scholar] [CrossRef]
- Barry, A.R.; Grewal, M.; Blain, L. Use of Guideline-Directed Medical Therapy in Patients Aged 80 Years or Older with Heart Failure with Reduced Ejection Fraction. CJC Open 2023, 5, 303–309. [Google Scholar] [CrossRef]
- Linssen, G.C.M.; Veenis, J.F.; Kleberger, A.; Grosfeld, M.J.W.; Viergever, E.P.; van Dalen, B.M.; de Valk-Bedijn, W.; Langerveld, J.; Brunner-La Rocca, H.P.; Hoes, A.W.; et al. Medical treatment of octogenarians with chronic heart failure: Data from CHECK-HF. Clin. Res. Cardiol. 2020, 109, 1155–1164. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, J.K.; Ko, D.; Cheng, S.; Patorno, E.; Glynn, R.J.; Tsacogianis, T.; Kim, D.H. Frailty and uptake of angiotensin receptor neprilysin inhibitor for heart failure with reduced ejection fraction. J. Am. Geriatr. Soc. 2023, 71, 3110–3121. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Canepa, M.; Benson, L.; Hage, C.; Thorvaldsen, T.; Dahlström, U.; Savarese, G.; Lund, L.H. Non-cardiology vs. cardiology care of patients with heart failure and reduced ejection fraction is associated with lower use of guideline-based care and higher mortality: Observations from The Swedish Heart Failure Registry. Int. J. Cardiol. 2021, 343, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davson, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability, and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Aldafas, R.; Crabtree, T.; Alkharaiji, M.; Vinogradova, Y.; Idris, I. Sodium-glucose cotransporter-2 inhibitors (SGLT2) in frail or older people with type 2 diabetes and heart failure: A systematic review and meta-analysis. Age Ageing 2024, 53, afad254. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavelle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef]
| Total (N = 60) | Geriatric Ward (N = 32) | Cardiology Ward (N = 28) | |
|---|---|---|---|
| Demographics | |||
| Age, years | 79.5 (75.3–85.0) | 83.0 (78.0–87.0) * | 77.4 (73.3–81.0) * |
| Female, % | 25 (41.7) | 16.0 (50.0) | 9 (32.1) |
| Ejection fraction, % | 33.8 (28.5–42.0) | 36.0 (30.0–42.8) | 31.6 (21.3–39.5) |
| Body mass index, kg/m2 | 25.4 (22.0–28.0) | 24.9 (22.0–26.9) | 25.9(22.4–28.8) |
| Charlson comorbidity index | 6 (5–7) | 6.4 (5.3–7) * | 5.8 (4.3–7) * |
| Medical history | |||
| Hypertension, % | 31 (51.7) | 18 (56.3) | 13 (46.4) |
| Hypercholesterolemia, % | 33 (55.0) | 19 (59.4) | 14 (50.0) |
| Myocardial infarction, % | 30 (50.0) | 16 (50.0) | 14 (50.0) |
| Diabetes Mellitus, % | 18 (30.0) | 11 (34.4) | 7 (25.0) |
| Chronic kidney disease (eGFR < 30) | 6 (10.0) | 3 (9.4) | 3 (10.7) |
| Anemia, % | 7 (11.7) | 5 (15.6) | 2 (7.1) |
| Atrial fibrillation or flutter, % | 32 (53.3) | 18 (56.3) | 14 (50.0) |
| Heart valve disease, % | 15 (25.0) | 9 (28.1) | 6 (21.4) |
| HFrEF, % | 38 (63.3) | 16 (50.0) * | 22 (78.6) * |
| Heart failure etiology | |||
| Ischemic, % | 36 (60.0) | 21 (65.6) | 15 (53.6) |
| Valvular, % | 5 (8.3) | 2 (6.3) | 3 (10.7) |
| Arrhythmia, % | 4 (6.7) | 1 (3.1) | 3 (10.7) |
| Polypharmacy | |||
| Minor polypharmacy (5–9), % | 42 (70.0) | 21 (65.6) | 21 (75.0) |
| Hyper polypharmacy (≥10), % | 14 (23.3) | 8 (25.0) | 6 (21.4) |
| Cardiovascular drugs | |||
| Diuretics, % | 50 (83.3) | 26 (81.3) | 24 (85.7) |
| Anti-arrhythmics, % | 16 (26.7) | 6 (18.8) | 10 (35.7) |
| Antihypertensives, % | 7 (11.7) | 3 (6.4) | 4 (14.3) |
| Anticoagulants, % | 34 (56.7) | 18 (56.3) | 16 (57.1) |
| Thrombocyte aggregation inhibitors, % | 21 (35.0) | 10 (31.3) | 11 (39.3) |
| Clinical frailty scale ‡ | |||
| Non-frail (1–2) | 6 (10.2) | 2 (6.3) | 4 (14.8) |
| Mildly frail (3–4) | 19 (32.2) | 7 (21.9) | 12 (44.4) |
| Frail (>5) | 34 (57.6) | 23 (71.9) * | 11 (39.3) * |
| Fall risk ‡ | |||
| Low risk, % | 10 (16.7) | 2 (6.3) * | 8 (28.6) * |
| Medium risk, % | 32 (53.3) | 16 (50.0) | 16 (57.1) |
| High risk, % | 17 (28.3) | 14 (43.8) * | 3 (10.7) * |
| Residential status ‡ | |||
| Home without care, % | 45 (75.0) | 23 (71.9) | 22 (81.5) |
| Home with care, % | 11 (18.3) | 6 (18.8) | 5 (18.5) |
| Residential home, % | 3 (5.0) | 3 (9.4) | 0 (0) |
| Discharge | Three-Month Follow-Up | |||||
| All (n = 60) | HFrEF (n = 38) | HFmrEF (n = 22) | All (n = 38) | HFrEF (n = 26) | HFmrEF (n = 12) | |
| GDMT, per drug group | % | % | % | % | % | % |
| ACE-I | 45 | 45 | 46 | 63 | 65 | 58 |
| ARNI | 8 | 5 | 0 | 11 | 15 | 0 |
| BB | 60 | 22 | 14 | 68 | 69 | 67 |
| MRA | 77 | 29 | 17 | 84 | 89 | 75 |
| SGLT2-I | 42 | 20 * | 5 * | 63 | 73 | 41 |
| GDMT, number of drugs | ||||||
| All 4 | 15 | 18 | 9 | 26 | 31 | 17 |
| 3/4 | 30 | 32 | 27 | 34 | 38 | 25 |
| 2/4 | 27 | 24 | 32 | 32 | 27 | 42 |
| 1/4 | 20 | 16 | 27 | 8 | 4 | 17 |
| 0/4 | 8 | 11 | 1 | - | - | - |
| Discharge | 3-Month Follow-Up | |||||
| Characteristics | Number of Patients | Use of All 4 GDMT Drugs (%) | p-Value | Number of Patients | Use of All 4 GDMT Drugs (%) | p-Value |
| Age | ||||||
| 70–79 | 30 | 27 | - | 22 | 32 | - |
| 80+ | 30 | 3 | 0.03 * | 16 | 19 | 0.47 |
| Sex | ||||||
| Female | 25 | 12 | - | 14 | 14 | - |
| Male | 29 | 17 | 0.72 | 24 | 33 | 0.27 |
| CCI | ||||||
| <4 | 3 | 33 | - | 3 | 67 | - |
| ≥4 | 57 | 14 | 0.39 | 35 | 23 | 0.16 |
| History of HF | ||||||
| Newly diagnosed HF | 22 | 18 | - | 17 | 29 | - |
| History of HF | 38 | 13 | 0.71 | 31 | 24 | 0.73 |
| John Hopkins fall assessment score | ||||||
| Low–medium risk | 42 | 17 | - | 27 | 22 | - |
| High risk | 17 | 12 | 1.00 | 10 | 30 | 0.68 |
| Polypharmacy | ||||||
| 0–9 drugs | 46 | 15 | - | 29 | 28 | - |
| ≥10 drugs | 14 | 14 | 1.00 | 9 | 22 | 1.00 |
| Clinical frailty score | ||||||
| CSF < 5 | 25 | 20 | - | 22 | 23 | - |
| CSF > 5 | 34 | 12 | 0.47 | 15 | 33 | 0.71 |
| Admission specialty | ||||||
| Cardiology | 28 | 18 | - | 22 | 23 | - |
| Geriatrics | 32 | 13 | 0.72 | 16 | 31 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raijmann, R.C.M.A.; Haverkamp, M.; van der Meer, M.G.; Knol, W.; Tseng, C.C.S.; Keijsers, C.J.P.W.; Emmelot-Vonk, M.H.; Koek, H.L. Adherence to Guideline-Directed Medical Therapy in Hospitalized Older People with Heart Failure at Discharge and 3-Month Follow-Up. J. Clin. Med. 2025, 14, 6928. https://doi.org/10.3390/jcm14196928
Raijmann RCMA, Haverkamp M, van der Meer MG, Knol W, Tseng CCS, Keijsers CJPW, Emmelot-Vonk MH, Koek HL. Adherence to Guideline-Directed Medical Therapy in Hospitalized Older People with Heart Failure at Discharge and 3-Month Follow-Up. Journal of Clinical Medicine. 2025; 14(19):6928. https://doi.org/10.3390/jcm14196928
Chicago/Turabian StyleRaijmann, Renee C. M. A., Melanie Haverkamp, Manon G. van der Meer, Wilma Knol, Cheyenne C. S. Tseng, Carolina J. P. W. Keijsers, Marielle H. Emmelot-Vonk, and Huiberdina L. Koek. 2025. "Adherence to Guideline-Directed Medical Therapy in Hospitalized Older People with Heart Failure at Discharge and 3-Month Follow-Up" Journal of Clinical Medicine 14, no. 19: 6928. https://doi.org/10.3390/jcm14196928
APA StyleRaijmann, R. C. M. A., Haverkamp, M., van der Meer, M. G., Knol, W., Tseng, C. C. S., Keijsers, C. J. P. W., Emmelot-Vonk, M. H., & Koek, H. L. (2025). Adherence to Guideline-Directed Medical Therapy in Hospitalized Older People with Heart Failure at Discharge and 3-Month Follow-Up. Journal of Clinical Medicine, 14(19), 6928. https://doi.org/10.3390/jcm14196928







