Comparative Ultrasonographic Evaluation of Morphology and Vascularization in Endometriomas and Ovarian Mature Cystic Teratomas †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ultrasonographic Examination and Data Analysis
- 1.
- Morphological Parameters: The morphological assessment included the largest tumor diameter, volume (calculated using the prolate ellipsoid formula: length × height × width × 0.523), laterality, capsule thickness, and internal echogenicity (categorized as anechoic, homogeneous low-level “ground-glass”, hyperechoic, or mixed/heterogeneous). The presence of septa, papillary projections, solid components, acoustic shadowing, free fluid in the pouch of Douglas, and signs of tumor fixation were also recorded.
- 2.
- Hemodynamic Parameters: Assessed using color and pulsed Doppler techniques. The location of blood flow (pericystic, intracystic, in septa) was noted. The peak systolic velocity (Vmax), end-diastolic velocity (Vmin), and Resistance Index (RI) were measured from spectral Doppler waveforms. Measurements were also taken from the ipsilateral uterine artery to obtain its Resistance Index (AURI).
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANDEX | Adnexal Mass Evaluation System |
| AU | Uterine Artery |
| AURI | Resistance Index in Uterine Arteries |
| ΔAURI | Difference in AURI between Tumor and Contralateral Sides |
| ΔVmaxAU | Difference in Uterine Artery Vmax between Tumor and Contralateral Sides |
| Doppler RI | Doppler Resistance Index |
| FGF | Fibroblast Growth Factor |
| HIF-1A | Hypoxia-Inducible Factor 1 Alpha |
| IOTA | International Ovarian Tumor Analysis |
| MCT | Mature Cystic Teratoma |
| MIF | Macrophage Migration Inhibitory Factor |
| O-RADS | Ovarian-Adnexal Reporting and Data System |
| RI | Resistance Index |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| sVEGFR-2 | Soluble Vascular Endothelial Growth Factor Receptor-2 |
| TVUS | Transvaginal Ultrasonography |
| VEGF | Vascular Endothelial Growth Factor |
| Vmax | Maximal Systolic Flow Velocity |
| Vmin | Minimal Diastolic Flow Velocity |
References
- Carvalho, J.P.; Moretti-Marques, R.; Filho, A.L.D.S. Adnexal mass: Diagnosis and management. Rev. Bras. Ginecol. Obstet. 2020, 42, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Hermans, A.J.; Kluivers, K.B.; Janssen, L.M.; Siebers, A.G.; Wijnen, M.H.; Bulten, J.; Massuger, L.F.; Coppus, S.F. Adnexal masses in children, adolescents and women of reproductive age in the Netherlands: A nationwide population-based cohort study. Gynecol. Oncol. 2016, 143, 93–97. [Google Scholar] [CrossRef]
- Cathcart, A.M.; Nezhat, F.R.; Emerson, J.; Pejovic, T.; Nezhat, C.H.; Nezhat, C.R. Adnexal masses during pregnancy: Diagnosis, treatment, and prognosis. Am. J. Obstet. Gynecol. 2023, 228, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, V.; Umstead, B.; Chadwick, C. Adnexal masses: Diagnosis and management. Am. Fam. Physician 2023, 108, 580–587. [Google Scholar]
- Hiett, A.K.; Sonek, J.D.; Guy, M.; Reid, T.J. Performance of IOTA simple rules, simple rules risk assessment, ADNEX model and O-RADS in differentiating between benign and malignant adnexal lesions in North American women. Ultrasound Obstet. Gynecol. 2022, 59, 668–676. [Google Scholar] [CrossRef]
- Strachowski, L.M.; Jha, P.; Phillips, C.H.; Porter, M.M.B.; Froyman, W.; Glanc, P.; Guo, Y.; Patel, M.D.; Reinhold, C.; Suh-Burgmann, E.J.; et al. O-RADS US v2022: An update from the American College of Radiology’s ovarian-adnexal reporting and data system US committee. Radiology 2023, 308, e230685. [Google Scholar] [CrossRef] [PubMed]
- Sandler, M.A.; Karo, J.J. The spectrum of ultrasonic findings in endometriosis. Radiology 1978, 127, 229–231. [Google Scholar] [CrossRef]
- Daniilidis, A.; Grigoriadis, G.; Dalakoura, D.; D’Alterio, M.N.; Angioni, S.; Roman, H. Transvaginal ultrasound in the diagnosis and assessment of endometriosis—An overview: How, why, and when. Diagnostics 2022, 12, 2912. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Van Calster, B.; Guerriero, S.; Savelli, L.; Paladini, D.; Lissoni, A.A.; Czekierdowski, A.; Fischerova, D.; Zhang, J.; Mestdagh, G.; et al. Endometriomas: Their ultrasound characteristics. Ultrasound Obstet. Gynecol. 2010, 35, 730–740. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Scarpelli, E.; dell’Omo, S.; Rolla, M.; Pezzani, A.; Morganelli, G.; Gaiano, M.; Ghi, T.; Berretta, R. Atypical Endometriosis: A comprehensive systematic review of pathological patterns and diagnostic challenges. Biomedicines 2024, 12, 1209. [Google Scholar] [CrossRef]
- Cong, L.; Wang, S.; Yeung, S.Y.; Lee, J.H.S.; Chung, J.P.W.; Chan, D.Y.L. Mature cystic teratoma: An integrated review. Int. J. Mol. Sci. 2023, 24, 6141. [Google Scholar] [CrossRef]
- Brown, D.L.; Frates, M.C.; Laing, F.C.; DiSalvo, D.N.; Doubilet, P.M.; Benson, C.B.; Waitzkin, E.D.; Muto, M.G. Ovarian masses: Can benign and malignant lesions be differentiated with color and pulsed Doppler US? Radiology 1994, 190, 333–336. [Google Scholar] [CrossRef]
- Kurtz, A.B.; Tsimikas, J.V.; Tempany, C.M.C.; Hamper, U.M.; Arger, P.H.; Bree, R.L.; Wechsler, R.J.; Francis, I.R.; Kuhlman, J.E.; Siegelman, E.S.; et al. Diagnosis and staging of ovarian cancer: Comparative values of Doppler and conventional US, CT, and MR imaging correlated with surgery and histopathologic analysis—Report of the Radiology Diagnostic Oncology Group. Radiology 1999, 212, 19–27. [Google Scholar] [CrossRef]
- Heremans, R.; Valentin, L.; Sladkevicius, P.; Timmerman, S.; Moro, F.; Van Holsbeke, C.; Epstein, E.; Testa, A.C.; Timmerman, D.; Froyman, W. Imaging in gynecological disease (24): Clinical and ultrasound characteristics of ovarian mature cystic teratomas. Ultrasound Obstet. Gynecol. 2022, 60, 549–558. [Google Scholar] [CrossRef]
- Sayasneh, A.; Ekechi, C.; Ferrara, L.; Kaijser, J.; Stalder, C.; Sur, S.; Timmerman, D.; Bourne, T. The characteristic ultrasound features of specific types of ovarian pathology (review). Int. J. Oncol. 2015, 46, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Kocakoc, E.; Bhatt, S.; Dogra, V.S. Endometriosis. Ultrasound Clin. 2008, 3, 399–414. [Google Scholar] [CrossRef]
- Gareeballah, A.; Gameraddin, M.; Alshoabi, S.A.; Alsaedi, A.; Elzaki, M.; Alsharif, W.; Daoud, I.M.; Aldahery, S.; Alelyani, M.; AbdElrahim, E.; et al. The diagnostic performance of International Ovarian Tumor Analysis: Simple Rules for diagnosing ovarian tumors—A systematic review and meta-analysis. Front. Oncol. 2025, 14, 1474930. [Google Scholar] [CrossRef]
- Kidron, D.; Bernheim, J.; Aviram, R.; Cohen, I.; Fishman, A.; Beyth, Y.; Tepper, R. Resistance to blood flow in ovarian tumors: Correlation between resistance index and histological pattern of vascularization. Ultrasound Obstet. Gynecol. 1999, 13, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kurjak, A.; Jukic, S.; Kupesic, S.; Babic, D. A combined Doppler and morphopathological study of ovarian tumors. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 147–150. [Google Scholar] [CrossRef]
- Kurjak, A.; Kupesic, S.; Sparac, V.; Prka, M.; Bekavac, I. The detection of stage I ovarian cancer by three-dimensional sonography and power Doppler. Gynecol. Oncol. 2003, 90, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xuan, Z.; Wang, Y. Diagnostic value of ultrasound score, color Doppler ultrasound RI and spiral CT for ovarian tumors. Oncol. Lett. 2019, 17, 5499–5504. [Google Scholar] [CrossRef]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation—The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77, e12641. [Google Scholar] [CrossRef]
- Wu, M.H.; Hsiao, K.Y.; Tsai, S.J. Endometriosis and possible inflammation markers. Gynecol. Minim. Invasive Ther. 2015, 4, 61–67. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Xiong, Y.; Li, N.; He, H.; Du, Y.; Liu, Y. Hypoxia-inducible factor-1α promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 2017, 153, 809–820. [Google Scholar] [CrossRef]
- Salzillo, C.; Imparato, A.; Fortarezza, F.; Maniglio, S.; Lucà, S.; La Verde, M.; Serio, G.; Marzullo, A. Gonadal Teratomas: A State-of-the-Art Review in Pathology. Cancers 2024, 16, 2412. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.M.; Rosai, J.; Young, R.H. Ovarian teratomas with florid benign vascular proliferation: A distinctive finding associated with the neural component of teratomas that may be confused with a vascular neoplasm. Int. J. Gynecol. Pathol. 2002, 21, 16–21. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum. Reprod. Update 2018, 24, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Tomao, F.; Manganaro, L.; Yazdanian, D.; Fuggetta, E.; Piccioni, M.; Panici, P.B.; Benagiano, G. Impaired uterine artery flow associated with the presence of ovarian endometrioma: Preliminary results of a prospective study. J. Ovarian Res. 2014, 7, 1. [Google Scholar] [CrossRef]
- Laschke, M.W.; Giebels, C.; Menger, M.D. Vasculogenesis: A new piece of the endometriosis puzzle. Hum. Reprod. Update 2011, 17, 628–636. [Google Scholar] [CrossRef]
- Asch, E.; Levine, D. Variations in appearance of endometriomas. J. Ultrasound Med. 2007, 26, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, A.; Brakus, J.; Rakic, A.; Jurisic, Z.; Djakovic, E.; Cabunac, P. EP23.62: Ovarian teratomas and endometriomas: Is there any difference in ultrasonographic morphology and vascularisation? Ultrasound Obstet. Gynecol. 2024, 64 (Suppl. S1), 343. [Google Scholar] [CrossRef]
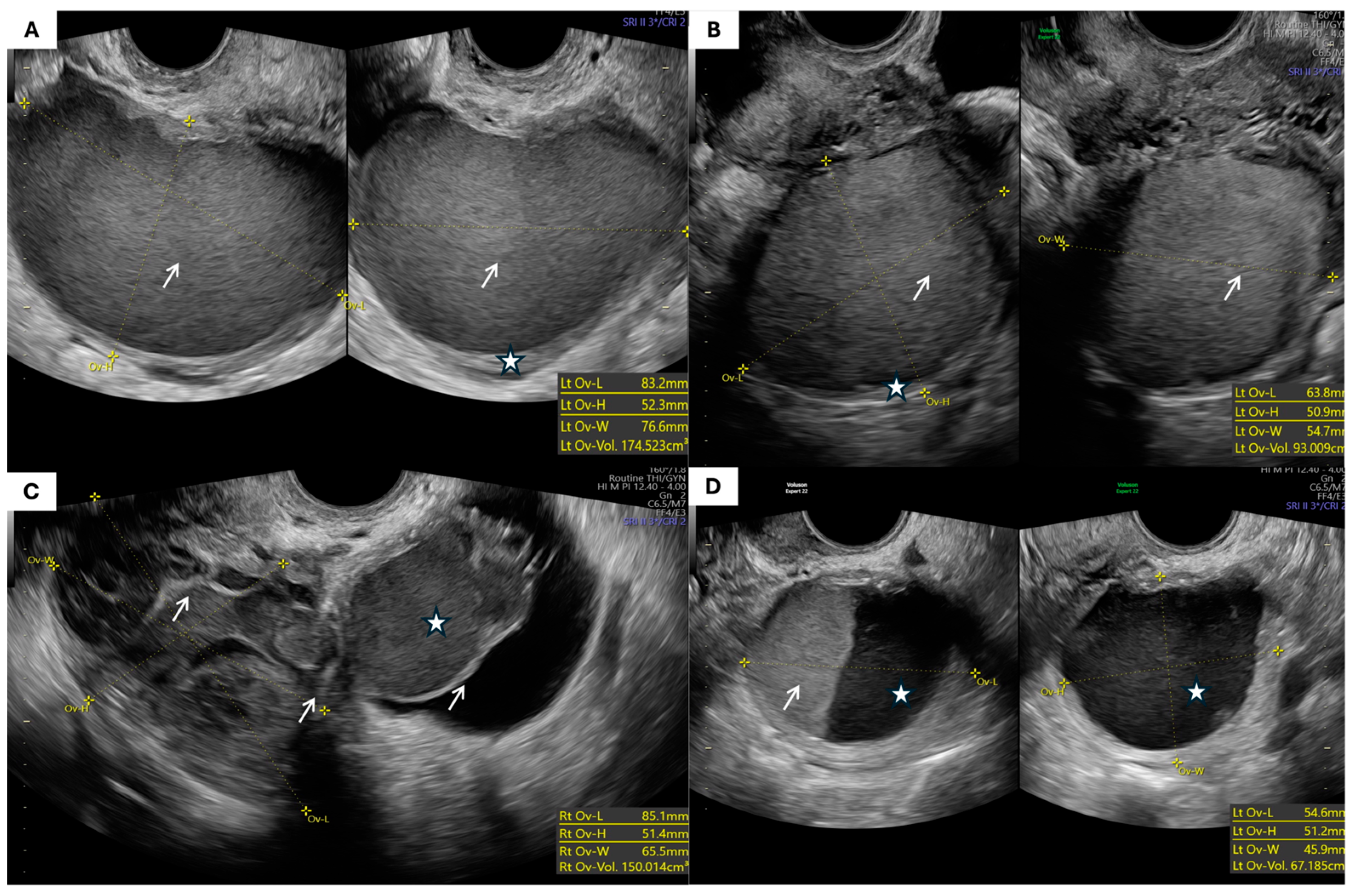

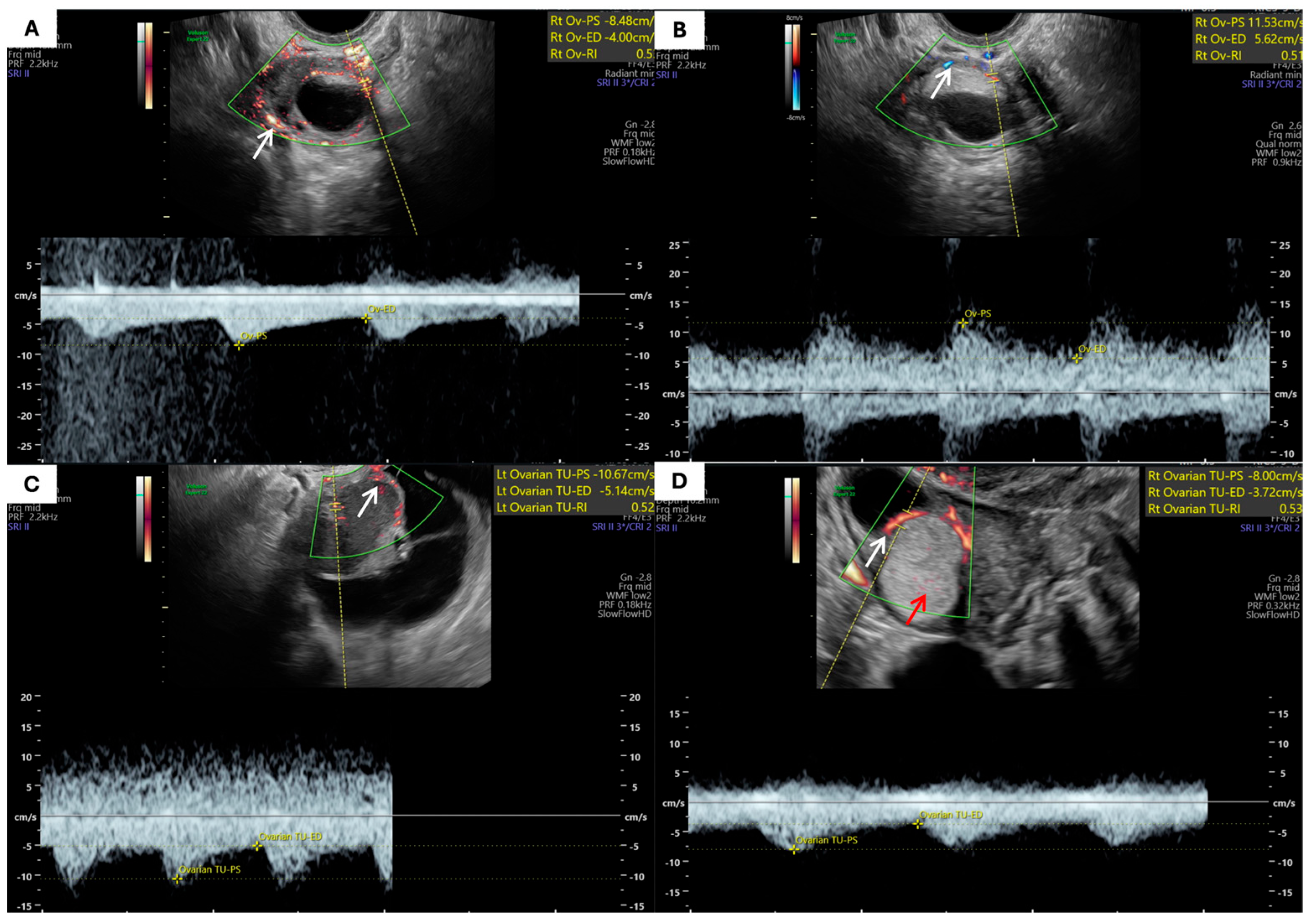
| Parameter | Patients with Endometrioma (n = 47) | Patients with MCT (n = 47) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 37.8 ± 10.2 | 38.4 ± 13.3 | 0.8 |
| Menopausal status, n (%) | 0.2 | ||
Premenopausal | 41 (87.2%) | 36 (76.6%) | |
Menopausal | 6 (12.8%) | 11 (23.4%) | |
| Duration of menopause (years), mean ± SD | 2.0 ± 2.9 * | 8.1 ± 9.5 ** | 0.1 |
| Parity, median [IQR] | 0 [0–1] | 1 [0–2] | 0.02 *** |
| Parameter | Endometriomas (n = 47) | MCTs (n = 47) | p-Value |
|---|---|---|---|
| The largest tumor dimension (mm), mean ± SD | 78.2 ± 3.3 | 81.9 ± 4.3 | 0.6 |
| Tumor volume (cm3), mean ± SD | 247.9 ± 65.2 | 254.3 ± 75.3 | 0.7 |
| Tumor capsule thickness (mm), mean ± SD | 2.87 ± 1.1 | 2.9 ± 1.1 | 0.9 |
| Septal thickness (mm), mean ± SD | 2.6 ± 0.7 | 2.7 ± 1.4 | 0.7 |
| Free fluid in the pouch of Douglas, n (%) | 1 (2.1%) | 2 (4.3%) | 0.500 |
| Tumor fixation **, n (%) | 7 (14.9%) | 3 (6.4%) | 0.200 |
| Tumor localization, n (%) | 0.042 * | ||
| Unilateral | 39 (83.0%) | 45 (95.7%) | |
| Bilateral | 8 (17.0%) | 2 (4.3%) |
| Morphological Type (IOTA Terminology) | Endometriomas (n = 55) | % | Teratomas (n = 49) | % | p-Value |
|---|---|---|---|---|---|
| Unilocular cyst with ground-glass echogenicity | 25 | 45.5% | 0 | 0% | <0.001 * |
| Unilocular solid cyst with hyperechoic content | 1 | 1.8% | 10 | 20.4% | |
| Multilocular cyst | 8 | 14.5% | 5 | 10.2% | |
| Multilocular solid cyst | 9 | 16.4% | 25 | 51.0% | |
| Cyst with papillary projections (unilocular or bilocular) | 1 | 1.8% | 1 | 2.0% | |
| Solid tumor | 3 | 5.5% | 4 | 8.2% | |
| Other/not otherwise specified | 8 | 14.5% | 4 | 8.2% |
| Parameter | Endometriomas | MCTs | p-Value |
|---|---|---|---|
| Total number of analyzed blood vessels (across all tumors) | 168 | 149 | — |
| Mean number of blood vessels (n ± SD) | 3.03 ± 1.67 | 3.00 ± 2.30 | 0.5 |
| Localization of blood vessels (n) | 0.5 | ||
| – Pericystic | 125 | 111 | |
| – In septa | 24 | 20 | |
| – Within the papillary projection | 6 | 2 | |
| – Within the solid-appearing components * | 13 | 16 |
| Parameter (n ± SD) | Endometriomas | Ovarian Teratomas | p-Value |
|---|---|---|---|
| RI | 0.57 ± 0.11 | 0.54 ± 0.13 | 0.04 * |
| Vmax | 10.72 ± 5.56 | 10.28 ± 5.96 | 0.5 |
| Vmin | 4.43 ± 2.24 | 4.34 ± 2.20 | 0.7 |
| AURI tumor | 0.81 ± 0.14 | 0.83 ± 0.07 | 0.02 * |
| AURI contralateral | 0.84 ± 0.07 | 0.85 ± 0.07 | 0.7 |
| ΔAURI | 0.03 ± 0.11 | 0.01 ± 0.07 | 0.8 |
| AUVmax tumor | 42.62 ± 21.73 | 38.10 ± 15.0 | 0.2 |
| AUVmax contralateral | 36.80 ± 17.27 | 34.50 ± 13.45 | 0.5 |
| ΔVmaxAU | 4.73 ± 24.28 | 3.23 ± 14.88 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakić, A.; Đaković, E.; Milovanović, Z.; Ristić, A.; Nejković, L.; Đorđević, A.; Brakus, J.; Štulić, J.; Jurišić, Ž.; Jurišić, A. Comparative Ultrasonographic Evaluation of Morphology and Vascularization in Endometriomas and Ovarian Mature Cystic Teratomas. J. Clin. Med. 2025, 14, 6912. https://doi.org/10.3390/jcm14196912
Rakić A, Đaković E, Milovanović Z, Ristić A, Nejković L, Đorđević A, Brakus J, Štulić J, Jurišić Ž, Jurišić A. Comparative Ultrasonographic Evaluation of Morphology and Vascularization in Endometriomas and Ovarian Mature Cystic Teratomas. Journal of Clinical Medicine. 2025; 14(19):6912. https://doi.org/10.3390/jcm14196912
Chicago/Turabian StyleRakić, Aleksandar, Elena Đaković, Zagorka Milovanović, Aleksandar Ristić, Lazar Nejković, Ana Đorđević, Jelena Brakus, Jelena Štulić, Žaklina Jurišić, and Aleksandar Jurišić. 2025. "Comparative Ultrasonographic Evaluation of Morphology and Vascularization in Endometriomas and Ovarian Mature Cystic Teratomas" Journal of Clinical Medicine 14, no. 19: 6912. https://doi.org/10.3390/jcm14196912
APA StyleRakić, A., Đaković, E., Milovanović, Z., Ristić, A., Nejković, L., Đorđević, A., Brakus, J., Štulić, J., Jurišić, Ž., & Jurišić, A. (2025). Comparative Ultrasonographic Evaluation of Morphology and Vascularization in Endometriomas and Ovarian Mature Cystic Teratomas. Journal of Clinical Medicine, 14(19), 6912. https://doi.org/10.3390/jcm14196912







