Evidence-Based Perioperative Prevention of Postoperative Nausea and Vomiting (PONV) in Patients Undergoing Laparoscopic Bariatric Surgery: A Scoping Review
Abstract
1. Introduction
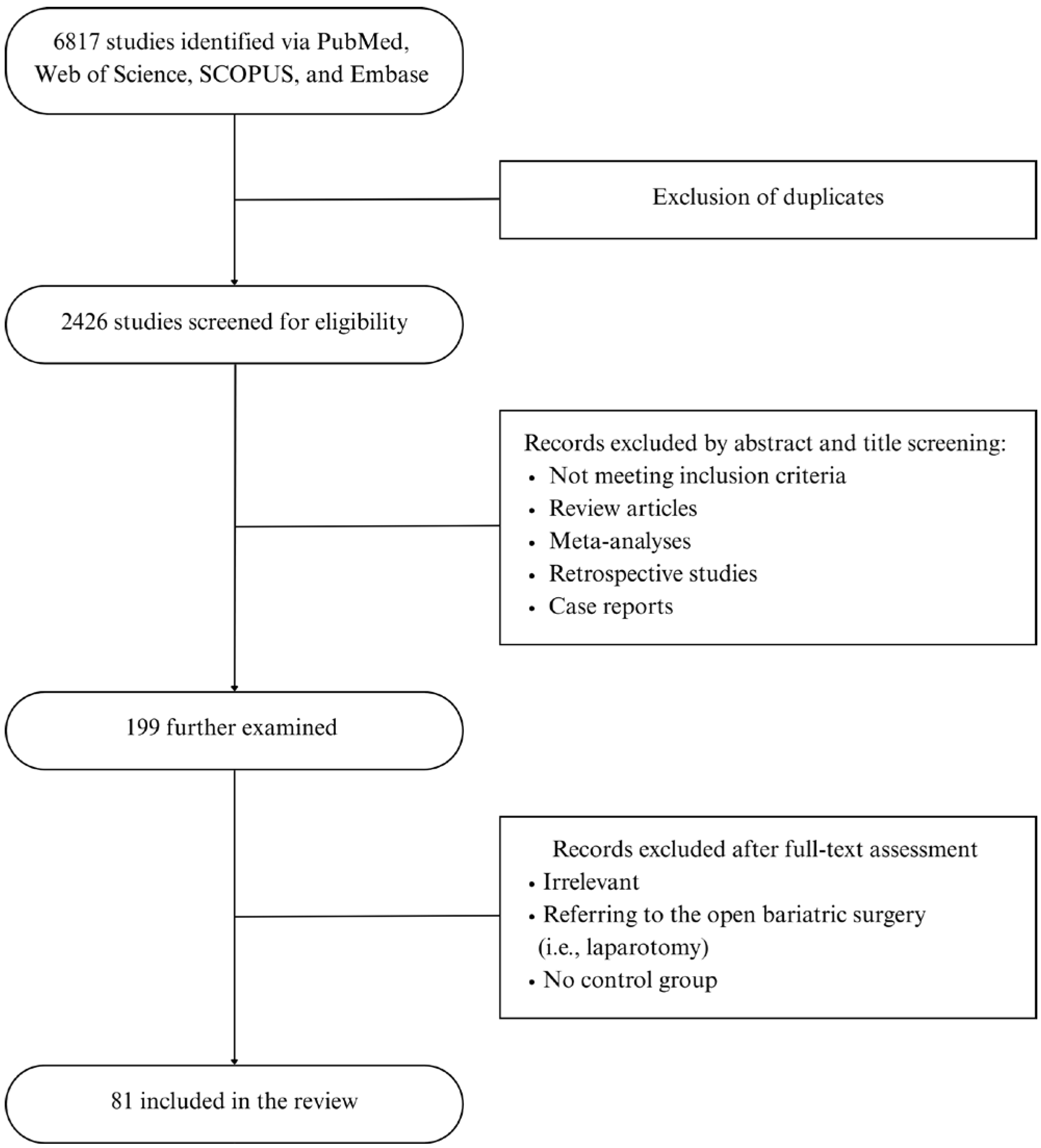
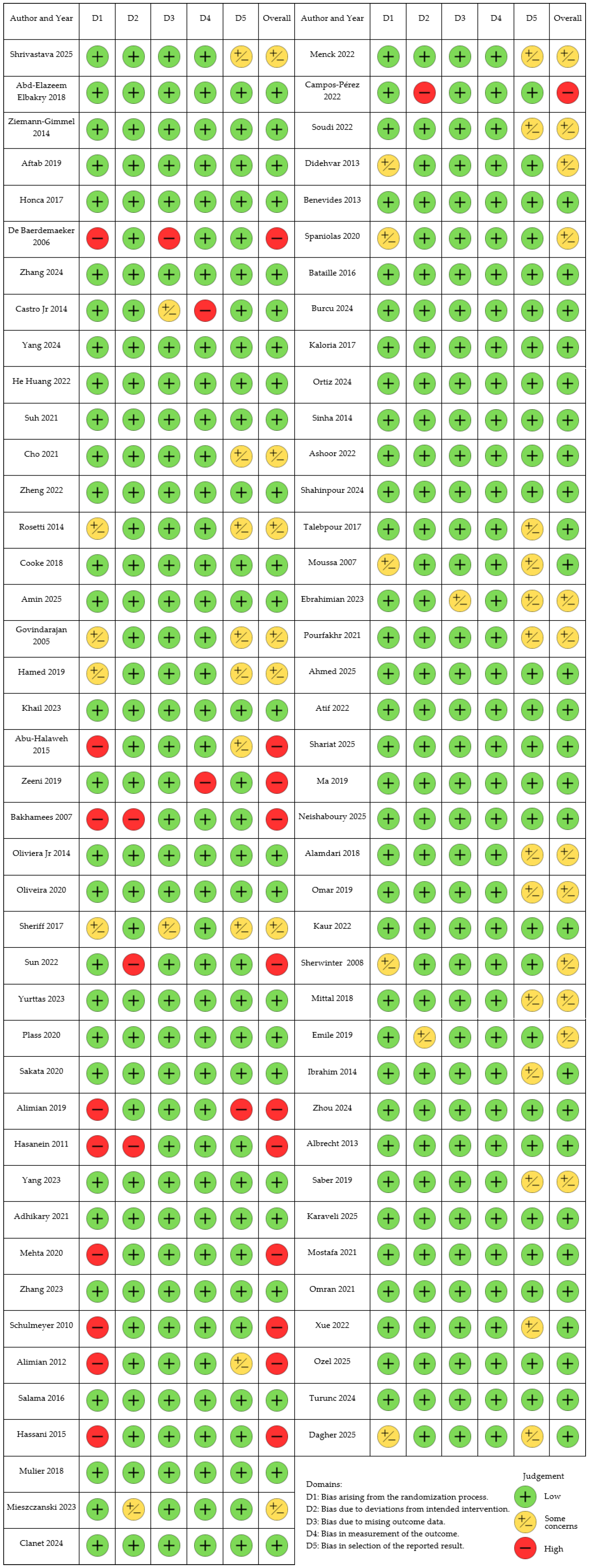
2. Materials and Methods
3. Results
3.1. Anesthetic Factors in PONV Prophylaxis
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|
| Shrivastava [19] | 2025 | 168 | LSG | Propofol TIVA, GDFT vs. control | Dexamethasone, azasetron, metoclopramide | 4-point NRS within 24 h postoperatively | Propofol TIVA with GDFT decreased the incidence of PONV at 3–24 h postoperatively by 27.51%. |
| Elbakry [20] | 2018 | 100 | LSG | Propofol TIVA with dexmedetomidine vs. desflurane | Ondansetron | PONV score within 24 h postoperatively | Lower PONV incidence in the study group, 10% vs. 30% compared to desflurane |
| Ziemann-Gimmel [21] | 2014 | 119 | LYGB, LSG, LGB, revisional surgeries | Propofol TIVA, OFA vs. volatile anesthesia with opioids | Dexamethasone, droperidol, or promethazine | 4-point Verbal Rating Scale in the morning after surgery | PONV Risk reduction of 46.4% in the TIVA group |
| Spaniolas [22] | 2020 | 83 | LSG | Propofol TIVA, aprepitant, and transdermal scopolamine vs. volatile anesthesia | Dexamethasone, ondansetron, metoclopramide, compazine | 10-point Verbal Rating Scale at 1, 4, 12, 24 h postoperatively | PONV scores lower at all the time points in the study group |
| Aftab [23] | 2019 | 183 | LSG, LYGB | Propofol TIVA vs. desflurane | Ondansetron, metoclopramide | Visual Analog Scale at PACU, surgical ward, and 24–48 h postoperatively | No difference in PONV incidence compared with desflurane |
| Honca [24] | 2017 | 61 | LSG | Propofol TIVA vs. desflurane | Unspecified | PONV incidence at admission to PACU and 5, 10, 20 min postoperatively | No difference in PONV incidence compared with desflurane |
| De Baerdemaeker [25] | 2006 | 50 | LGB | Desflurane vs. sevoflurane | Ondansetron | Number of PONV episodes at admission to PACU and 30, 60, 120 min postoperatively | No difference in PONV incidence between desflurane and sevoflurane |
| Zhang [26] | 2024 | 90 | LSG | Light (BIS 50) vs. deep (BIS 35) anesthesia | Dexamethasone, palonosetron | 4-point PONV grade 24 h postoperatively | No difference in PONV incidence |
| Castro Jr [27] | 2014 | 88 | LBS | Sugammadex vs. neostigmine | Dexamethasone, ondansetron | PONV incidence before discharge from PACU | Less PONV in the sugammadex group |
| Yang [28] | 2024 | 80 | LSG | Deep (PTC 1–3) neuromuscular block vs. moderate | Dexamethasone, ondansetron | PONV incidence 40 min, 24 h, 48 h postoperatively | No difference between the groups |
| He Huang [29] | 2022 | 150 | LSG | Deep (PTC 1–2) neuromuscular block vs. moderate | Unspecified | PONV incidence at an unspecified period | No difference between the groups |
| Suh [30] | 2021 | 134 | LSG, LYGB | Preoperative carbohydrate loading vs. control | Unspecified | PONV duration | Shorter duration of nausea in the study group |
| Cho [31] | 2021 | 75 | LSG | GDFT, SVV-guided vs. control | Prochlorperazine | 11-point NRS at 0 min, 30 min, 1 h, 24 h, 48 h postoperatively | No difference between the groups |
| Zheng [32] | 2022 | 137 | LSG | GDFT, SVV-guided vs. control | Dexamethasone, tropisetron | 4-point PONV scale at 24 h and 48 h postoperatively | GDFT effective in decreasing the incidence of PONV, 47.1% vs. 71.6% in the control group |
| Rossetti [8] | 2014 | 145 | LSG | Nasogastric decompression | Unspecified | PONV incidence at an unspecified period | No difference between the groups |
3.1.1. Volatile Anesthesia vs. Total Intravenous Anesthesia (TIVA)
3.1.2. Neuromuscular Block
3.1.3. Perioperative Fluid Management
3.1.4. Nasogastric Tube Placement
3.2. Opioid-Sparing Agents
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Dosing | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|---|
| Cooke [40] | 2018 | 127 | LSG | Paracetamol vs. placebo | Paracetamol 1000 mg i.v. | Aprepitant or droperidol, dexamethasone, ondansetron | 11-point PONV scale until discharge | No difference between the groups |
| Amin [41] | 2025 | 106 | LSG, LYGB | Ketorolac vs. ibuprofen | Ketorolac 30 mg i.v. ibuprofen 800 mg i.v. | Dexamethasone, ondansetron | PONV incidence at an unspecified period | Ketorolac superior to ibuprofen |
| Govindarajan [42] | 2005 | 50 | LYGB | Ketorolac vs. remifentanil | Ketorolac infusion 6–9 mg/h i.v., postoperatively, infusion 4.5–9 mg/h i.v. | Ondansetron | PONV incidence at an unspecified period | PONV reduction 4% vs. 28% in the placebo group |
| Hamed [43] | 2019 | 132 | LSG | Dexmedetomidine vs. remifentanil | Dexmedetomidine 0.2–0.5 mcg/kg/h i.v. intraoperatively | Dexamethasone, droperidol | PONV incidence at an unspecified period | PONV reduction 3% vs. 10% in the placebo group |
| Ziemann-Gimmel [21] | 2014 | 124 | LSG, LYGB, LGB, revisional procedures | Propofol TIVA, OFA vs. volatile anesthesia with opioids | Dexmedetomidine loading dose 0.5 mcg/kg and infusion 0.1–0.3 mcg/kg/h i.v., ketamine 0.5 mg/kg i.v. | Dexamethasone, ondansetron, droperidol, promethazine | 4-point Verbal Rating Scale in the morning postoperatively | PONV absolute risk reduction of 17.3% in the OFA group |
| Khail [44] | 2023 | 90 | LSG | Ketamine vs. Dexmedetomidine vs. placebo | Ketamine loading dose 0.3 mg/kg IBW, infusion 0.3/kg/h i.v., Dexmedetomidine loading dose 0.5 mcg/kg IBW, infusion 0.5 mcg/kg/h i.v. | Ondansetron | 4-point PONV scale at 0, 6, 12, 24 h postoperatively | Less PONV in the dexmedetomidine group compared to ketamine and placebo |
| Abu-Halaweh [45] | 2015 | 60 | LBS | Dexmedetomidine vs. morphine | Dexmedetomidine 0.3 mcg/kg i.v. | Unspecified | PONV incidence within 24 h postoperatively | Less PONV in the dexmedetomidine group, 26.7% vs. 63.3% |
| Zeeni [46] | 2019 | 60 | LSG | Dexmedetomidine vs. morphine | Dexmedetomidine loading dose 1 mcg/kg, infusion 0.5 mcg/kg/h i.v. | Dexamethasone, ondansetron | NRS PONV score at 30 min intervals at PACU and 24 h postoperatively | No difference between the groups |
| Bakhamees [47] | 2007 | 80 | LYGB | Dexmedetomidine vs. placebo | Dexmedetomidine loading dose 0.8 mcg/kg, infusion 0.4 mcg/kg/h i.v. | Unspecified | PONV incidence within 24 h postoperatively | No difference between the groups |
| De Oliveira [48] | 2014 | 50 | LSG | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/h i.v. | Ondansetron | PONV incidence at PACU | No difference between the groups |
| De Oliveira [49] | 2020 | 60 | LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/h i.v. | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the lidocaine group |
| Sheriff [50] | 2017 | 150 | LSG | Lidocaine vs. dexmedetomidine vs. placebo | Lidocaine loading dose 2 mg/kg, infusion 1.5 mg/kg/h i.v., dexmedetomidine loading dose 1 mcg/kg, infusion 0.4 mcg/kg/h i.v. | Unspecified | PONV incidence within 6 h postoperatively | No difference between the groups |
| Sun [51] | 2022 | 99 | LSG, LYGB | Lidocaine vs. TAP Block vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/kg/h i.v. | Dexamethasone | PONV incidence within 24 h postoperatively | No difference between lidocaine and TAP Block |
| Yurttas [52] | 2023 | 137 | LSG, LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 1.5 mg/kg/h i.v. | Unspecified | 3-point PONV scale within 48 h postoperatively | No difference between the groups |
| Plass [53] | 2020 | 178 | LSG, LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/kg/h i.v. | Dexamethasone, droperidol | PONV incidence until discharge | No difference between the groups |
| Sakata [54] | 2020 | 58 | LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/kg/h i.v. | Ondansetron | PONV incidence until discharge from | No difference between the groups |
| Alimian [55] | 2019 | 42 | LYGB | Lidocaine 1 mg/kg/h vs. lidocaine 2 mg/kg/h | Lidocaine infusion 1 mg/kg/h vs. 2 mg/kg/h i.v. | Unspecified | PONV incidence at 0, 30 min, 1 h, 6 h, 12 h, 24 h postoperatively | No difference between the groups |
| Hasanein [56] | 2011 | 60 | LYGB | Ketamine and remifentanil vs. placebo | Ketamine 1 mcg/kg/min i.v. | Dexamethasone, metoclopramide | PONV incidence at an unspecified period | No difference between the groups |
| Yang [57] | 2023 | 68 | LSG | Esketamine | Esketamine loading dose 0.2 mg/kg, infusion 0.2 mg/kg/h i.v. | Dexamethasone | PONV score within 48 h postoperatively | No difference between the groups |
| Adhikary [58] | 2021 | 126 | LSG | Ketamine vs. Ketamine + Magnesium sulfate vs. placebo | Ketamine 0.5 mg/kg i.v., Magnesium sulfate 2 g i.v. | Dexamethasone, ondansetron | 4-point PONV scale every 4 h until 24 h postoperatively | No difference between the groups |
| Mehta [59] | 2020 | 54 | LYGB | Ketamine vs. control | Ketamine loading dose 20 mg i.v., infusion 5 mcg/kg/min i.v. | Dexamethasone | Need for antiemetics within 24 h postoperatively | No difference between the groups |
| Zhang [60] | 2023 | 74 | LBS | Esketamine vs. placebo | Esketamine infusion 0.5 mcg/kg/h i.v. | Dexamethasone, Palonosetron | PONV incidence during the 1-day postoperatively | No difference between the groups |
| Schulmeyer [61] | 2010 | 80 | LSG | Pregabalin vs. placebo | Pregabalin 150 mg p.o. | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the pregabalin group, 25.6% vs. 46.3% placebo |
| Alimian [62] | 2012 | 60 | LYGB | Pregabalin vs. placebo | Pregabalin 300 mg p.o. | Unspecified | PONV incidence within 24 h postoperatively | Absolute risk reductions of 36.7% for nausea and 30% for vomiting in the pregabalin group vs. placebo |
| Salama [63] | 2016 | 60 | LSG | Pregabalin and dexmedetomidine vs. placebo | Pregabalin 75 mg p.o., dexmedetomidine infusion 0.4 mcg/kg/h i.v. | Dexamethasone, ondansetron, metoclopramide | 11-point Verbal Rating Scale every 30 min at PACU, every 2 h on the first post-surgery day | Significantly less PONV within the first 18 h postoperatively |
| Hassani [64] | 2015 | 76 | LYGB | Gabapentin vs. placebo | Gabapentin 100 mg p.o. | Unspecified | Incidence of PONV within 6 h postoperatively | Less PONV in the gabapentin group 10% vs. 33% placebo |
| Mulier [65] | 2018 | 50 | LSG, LYGB, revisional procedures | OFA vs. sufentanil | Dexmedetomidine loading dose 0.5 mcg/kg, infusion 0.25–1 mcg/kg/h i.v., ketamine loading dose 0.25 mg/kg i.v., lidocaine loading dose 1.5 mg/kg, infusion 1.5–3 mg/kg/h i.v. | Ondansetron | Incidence of PONV at PACU | PONV reduction 13% vs. 63% in the sufentanil group |
| Mieszczanski [66] | 2023 | 59 | LSG | OFA vs. remifentanil | Dexmedetomidine loading dose 1 mcg/kg, infusion max 1 mcg/kg/h i.v., ketamine loading dose 0.5 mg/kg i.v., lidocaine loading dose 1.5 mg/kg, infusion max 3 mg/kg/h i.v., magnesium sulfate 40–50 mg/kg i.v. | Dexamethasone, ondansetron | PONV Impact scale within 24 h postoperatively | Less PONV in the OFA group, limited to the first postoperative hour |
| Clanet [67] | 2024 | 172 | LYGB | OFA vs. remifentanil | Dexmedetomidine loading dose 0.5 mcg/kg, infusion 0.4–0.8 mcg/kg/h | Dexamethasone, ondansetron | Incidence of PONV at PACU and 4 h, 24 h postoperatively | Less PONV in the OFA group in the first 4 h after the surgery |
| Dagher [68] | 2025 | 58 | LSG, LYGB | OFA vs. opioid-based anesthesia | Dexmedetomidine infusion 0.2–0.5 mcg/kg/h i.v., Dexamethasone 8 mg i.v., Ketamine loading dose 0.2 mg/kg, infusion 0.15 mg/kg/h i.v., lidocaine loading dose 1.5 mg/kg, infusion 1.5 mg/kg/h i.v., magnesium sulfate loading dose 50 mg/kg, infusion 8 mg/kg/h i.v. | Ondansetron | Incidence of PONV 4 h, 24 h postoperatively | No difference between the groups |
| Menck [69] | 2022 | 60 | LYGB | OFA vs. opioid-based anesthesia | Dexmedetomidine loading dose 0.5 mcg/kg i.v., Ketamine 25 mg i.v., Lidocaine loading dose 1.5–2 mg/kg, infusion 1 mg/kg/h i.v., Magnesium sulfate loading dose 40 mg/kg i.v., infusion 5 mg/kg/h i.v., clonidine infusion 0.15 mcg/kg/h i.v. | Dexamethasone, droperidol, ondansetron | PONV incidence at PACU and on the first post-surgery day | No difference between the groups |
| Campos-Pérez [70] | 2022 | 40 | LYGB | OFA vs. opioid-based anesthesia | Dexmedetomidine loading dose 1–1.5 mcg/kg, infusion 0.3–0.7 mcg/kg/min i.v., ketamine loading dose 0.12 mg/kg, infusion 0.15 mcg/kg/min i.v., lidocaine 1 mg/kg, infusion 1 mg/kg i.v., magnesium sulfate loading dose 30–50 mg/kg, infusion 10 mg/kg/min i.v. | Unspecified | PONV incidence within 24 h postoperatively | No difference between the groups |
| Soudi [71] | 2022 | 60 | LBS | OFA vs. opioid-based anesthesia | Dexmedetomidine loading 1 mcg/kg, infusion 0.5 mcg/kg/h i.v., ketamine loading 0.5 mg/kg, infusion 0.25 mg/kg/h i.v. | Unspecified | Number of PONV episodes within 24 h postoperatively | No difference between the groups |
3.2.1. Non-Opioid Analgesics
3.2.2. Dexmedetomidine
3.2.3. Lidocaine
3.2.4. NMDA Receptor Antagonists
Ketamine
Magnesium Sulfate
3.2.5. Gabapentinoids: Gabapentin and Pregabalin
3.2.6. Opioid-Free Anesthesia (OFA)
3.3. Pharmacological Prophylaxis
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Dosing Regimen | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|---|
| Didehvar [85] | 2013 | 76 | LBS | Dexamethasone vs. placebo | Dexamethasone 8 mg i.v. | Palonosetron | 4-point PONV scale at 2, 6, 12, 24, 72 h postoperatively | No difference between the groups |
| Benevides [86] | 2013 | 90 | LSG | Ondansetron vs. dexamethasone + ondansetron + haloperidol | Dexamethasone 8 mg i.v., Ondansetron 8 mg i.v., Haloperidol 2 mg i.v. | Unspecified | 11-point Verbal Rating Scale at 0–2, 2–12, 12–24, 24–36 h postoperatively | Less nausea in the dexamethasone, haloperidol, and dexamethasone group compared to the ondansetron-only group |
| Spaniolas [22] | 2020 | 83 | LSG | Propofol TIVA, aprepitant, and transdermal scopolamine vs. volatile anesthesia | Aprepitant 80 mg p.o., Dexamethasone 8 mg i.v., ondansetron 4 mg iv., scopolamine transdermal patch | Metoclopramide, compazine | 10-point Verbal Rating Scale at 1, 4, 12, 24 h postoperatively | PONV scores lower at all the time points in the study group |
| Bataille [4] | 2016 | 117 | LSG | Dexamethasone and ondansetron vs. placebo | Dexamethasone 4 mg i.v., ondansetron 4 mg i.v. | TIVA | PONV incidence within 24 h postoperatively and 11-point Verbal Rating Scale | Less PONV in the study group 45% vs. 54% in the control group |
| Burcu [87] | 2024 | 100 | LSG | Ondansetron vs. Palonosetron | Ondansetron 8 mg i.v., Palonosetron 75 mcg i.v. | Unspecified | PONV incidence during hospitalization | Less PONV in the palonosetron group |
| Kaloria [88] | 2017 | 22 | LSG | Ondansetron vs. palonosetron | Ondansetron max 8 mg i.v., palonosetron max 75 mcg | Dexamethasone | PONV incidence at 0, 0–6, 6–12, 12–24, 24–48, 48–72 h postoperatively | No difference between the groups |
| Ortiz [89] | 2024 | 400 | LSG | Aprepitant vs. placebo | Aprepitant 80 mg p.o. | Dexamethasone, ondansetron, metoclopramide | Rhodes index at 0, 6, 12, 24 h postoperatively | Less PONV in the aprepitant group in the first 24 h postoperatively |
| Sinha [90] | 2014 | 125 | LYGB, LGB | Aprepitant vs. placebo | Aprepitant 80 mg p.o. | Ondansetron | PONV incidence at 30 min, 1, 2, 6, 24, 48, 72 h postoperatively | Less vomiting in the aprepitant group, 3% vs. 15% in the placebo group |
| Ashoor [91] | 2022 | 90 | LSG | Aprepitant vs. mirtazapine vs. placebo | Aprepitant 80 mg p.o., mirtazapine 30 mg p.o. | Dexamethasone | 4-point Verbal Descriptive Scale every 4 h within 24 h postoperatively | Less PONV in the aprepitant group 34.5% vs. mirtazapine, 35.7% and placebo, 93.1% |
| Shahinpour [92] | 2024 | 83 | LSG, LYGB | Haloperidol vs. promethazine | Haloperidol 2 mg i.m., promethazine 25 mg i.m. | Dexamethasone, ondansetron | Numeric Verbal Rating Scale at 6 and 24 h postoperatively | Less PONV in the haloperidol group, 20% vs. promethazine 40% |
| Talebpour [93] | 2017 | 80 | Laparoscopic gastric plication | Metoclopramide vs. promethazine | Metoclopramide 10 mg i.v., promethazine 50 mg i.m. | Dexamethasone, ondansetron | 4-point PONV scale within 48 h postoperatively | Less PONV in the promethazine group 41% vs. 97.5% in the metoclopramide group |
| Moussa [94] | 2007 | 120 | LSG, LYGB, LGB | Granisetron vs. granisetron + droperidol vs. granisetron + dexamethasone vs. placebo | Dexamethasone 8 mg i.v., droperidol 1.25 mg i.v., granisetron 1 mg i.v. | Unspecified | PONV incidence within 24 h postoperatively | PONV incidence 30% in granisetron, 30% granisetron + droperidol, 20% granisetron + dexamethasone vs. 67% in the placebo group |
| Ebrahimian [95] | 2023 | 130 | LSG | Ondansetron vs. metoclopramide vs. granisetron vs. metoclopramide + ondansetron | Ondansetron max 8 mg i.v., metoclopramide max 10 mg i.v., granisetron 2 mg i.v. | Dexamethasone | PONV impact scale within 48 h postoperatively | No difference between the groups |
| Pourfakhr [96] | 2021 | 82 | LSG | Diphenhydramine vs. control | Diphenhydramine 0.4 mg/kg i.v. | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the diphendydramine group 30% vs. 56% in the control group |
| Ahmed [97] | 2025 | 210 | LSG | Cyclizine vs. metoclopramide vs. ondansetron | Cyclizine 50 mg i.v., metoclopramide 10 mg i.v., ondansetron 8 mg i.v. | Dexamethasone | 11-point Likert scale within 24 h postoperatively | No difference between the groups |
| Atif [98] | 2022 | 100 | LSG | Scopolamine vs. control | Scopolamine 10 mg i.v. | Metoclopramide | PONV intensity scale at 2 and 24 h postoperatively | No difference between the groups |
3.3.1. Dexamethasone
3.3.2. 5-HT3 Antagonists
3.3.3. NK-1 Receptor Antagonists
3.3.4. Dopamine Receptor Antagonists
3.3.5. Histamine Receptor Antagonists
3.3.6. Muscarinic Receptor Antagonists
3.4. Regional Anesthesia
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Control group | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|---|
| Shariat [111] | 2025 | 40 | LSG | QLB | Wound infiltration with local anesthetics | Ondansetron | PONV incidence within 24 h postoperatively | No difference between the groups |
| Ma [112] | 2019 | 179 | LYGB | Wound infiltration with liposomal bupivacaine | Wound infiltration with bupivacaine alone | Dexamethasone, transdermal scopolamine | PONV score every 4 h until discharge | No difference between the groups |
| Neishaboury [113] | 2025 | 72 | LSG | Intraperitoneal ropivacaine alone vs. intraperitoneal ropivacaine with dexmedetomidine | Intraperitoneal placebo | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the intraperitoneal ropivacaine with dexmedetomidine 8.3% vs. ropivacaine alone 29.2% vs. 50% placebo |
| Alamdari [114] | 2018 | 120 | LSG | Intraperitoneal bupivacaine | Control group | Unspecified | PONV incidence at unspecified time | Less PONV in the bupivacaine group 11.7% vs. 41.7% control |
| Omar [115] | 2019 | 100 | LBS | Intraperitoneal bupivacaine | Intraperitoneal placebo | Dexamethasone, ondansetron | PONV incidence within 24 h postoperatively | No difference between the groups |
| Kaur [116] | 2022 | 104 | LBS | Intraperitoneal ropivacaine | Intraperitoneal placebo | Dexamethasone, droperidol, ondansetron | Antiemetic use at 1, 2, 4, 6, 24, 48 h postoperatively | No difference between the groups |
| Sherwinter [117] | 2008 | 30 | Laparoscopic adjustable banding | Intraperitoneal bupivacaine | Intraperitoneal placebo | Dexamethasone, metoclopramide | PONV incidence at 30 min, 6, 12, 24, 48 h postoperatively | No difference between the groups |
| Mittal [118] | 2018 | 60 | LSG | TAP Block | No intervention | Unspecified | PONV incidence at 30 min, 3, 6, 12, 24, 48 h postoperatively | Less PONV in the TAP Block group |
| Emile [119] | 2019 | 92 | LBS | TAP Block | No intervention | Ondansetron | 4-point PONV scale within 24 h postoperatively | Less PONV in the TAP Block group |
| Ibrahim [120] | 2014 | 63 | LSG | Subcostal TAP Block | Wound infiltration with local anesthetics | Dexamethasone, ondansetron | PONV incidence at PACU and at 24 h postoperatively | No difference between the groups |
| Zhou [121] | 2024 | 71 | LSG | TAP Block | No intervention | Dexamethasone, ondansetron, droperidol | PONV score at 2, 6, 12, 24 h postoperatively | Less PONV in the TAP Block group |
| Albrecht [122] | 2013 | 70 | LYGB | Subcostal TAP Block + wound infiltration with local anesthetic | Wound infiltration with local anesthetic | Dexamethasone, ondansetron, granisetron | PONV incidence during phase I recovery, 0–24, 24–48 h postoperatively | No difference between the groups |
| Saber [123] | 2019 | 90 | LSG | TAP Block with bupivacaine, TAP Block with bupivacaine + adrenaline | No intervention | Dexamethasone, metoclopramide | 5-point PONV score every 6 h for 48 h | No difference between the groups |
| Karaveli [124] | 2025 | 40 | LSG | ESP Block | No intervention | Ondansetron | 4-point Likert scale within 24 h postoperatively | No difference between the groups |
| Mostafa [125] | 2021 | 60 | LBS | ESP Block | Sham block | Dexamethasone | PONV incidence within 24 h postoperatively | No difference between the groups |
| Omran [126] | 2021 | 30 | LSG, LYGB | QLB | Sham block | Unspecified | PONV incidence within 24 h postoperatively | Less PONV in the QLB group 13.3% vs. 46.7% |
| Xue [127] | 2022 | 225 | LSG | TAP Block, QLB | No intervention | Granisetron | PONV incidence | No difference between the groups |
| Ozel [128] | 2025 | 60 | LSG | EOIB | No intervention | Dexamethasone, ondansetron | 5-point Verbal Descriptive Scale within 24 h postoperatively | Fewer patients requiring antiemetics in the EOIB group, 16.7% vs. 40% |
| Turunc [129] | 2024 | 58 | LSG | EOIB | M-TAPA Block | Ondansetron | 5-point Verbal Descriptive Scale at 0, 3, 6, 12, 18, 24 h postoperatively | No difference between the groups |
3.4.1. Local Wound Infiltration
3.4.2. Intraperitoneal Local Anesthetic Installation
3.4.3. TAP Block
3.4.4. ESPB
3.4.5. QLB
3.4.6. EOIB
4. Discussion
4.1. Limitations of the Study
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PONV | Postoperative Nausea and Vomiting |
| RCTs | Randomized controlled trial |
| LBS | Laparoscopic bariatric surgery |
| LSG | Laparoscopic sleeve gastrectomy |
| ERAS | Enhanced Recovery After Surgery |
| TIVA | Total intravenous anesthesia |
| BIS | Bispectral index |
| GDFT | Goal-directed fluid therapy |
| SVV | Stroke Volume Variation |
| NMDA | N-methyl-d-aspartate |
| OFA | Opioid-free anesthesia |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PACU | Postoperative care unit |
| TAP | Transversus Abdominis Plane |
| LYGB | Laparoscopic Roux-en-Y gastric bypass |
| LGB | Laparoscopic gastric banding |
| PTC | Post-tetanic count |
| IBW | Ideal body weight |
| GABA | Gamma-aminobutyric acid |
| 5-HT3 | 5-hydroxytryptamine |
| NK-1 | Neurokinin-1 |
| ESPB TCI | Erectus Spinae Plane Block Target-Controlled Infusion |
| QLB | Quadratus Lumborum Block |
| EOIB | External Oblique Intercostal Fascial Plane Block |
| ABW | Adjusted body weight |
References
- Kushner, B.S.; Freeman, D.; Sparkman, J.; Salles, A.; Eagon, J.C.; Eckhouse, S.R. Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg. Obes. Relat. Dis. 2020, 16, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Groene, P.; Eisenlohr, J.; Zeuzem, C.; Dudok, S.; Karcz, K.; Hofmann-Kiefer, K. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Videosurg. Other Miniinvasive Tech. 2019, 14, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Halliday, T.A.; Sundqvist, J.; Hultin, M.; Walldén, J. Post-operative nausea and vomiting in bariatric surgery patients: An observational study. Acta Anaesthesiol. Scand. 2017, 61, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Bataille, A.; Letourneulx, J.-F.; Charmeau, A.; Lemedioni, P.; Léger, P.; Chazot, T.; Le Guen, M.; Diemunsch, P.; Fischler, M.; Liu, N. Impact of a prophylactic combination of dexamethasone–ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol–remifentanil anaesthesia. Eur. J. Anaesthesiol. 2016, 33, 898–905. [Google Scholar] [CrossRef]
- Liao, B.; Liao, W.; Wu, X.; Liu, S.; Li, Y.; Qin, R.; Yin, S. Analysis of influencing factors and construction of prediction model for postoperative nausea and vomiting in patients undergoing laparoscopic sleeve gastrectomy: A single-center retrospective cohort study. BMC Anesthesiol. 2024, 24, 131. [Google Scholar] [CrossRef]
- Scuderi, P.E.; Conlay, L.A. Postoperative Nausea and Vomiting and Outcome. Int. Anesthesiol. Clin. 2003, 41, 165–174. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Rossetti, G.; Fei, L.; Docimo, L.; Del Genio, G.; Micanti, F.; Belfiore, A.; Brusciano, L.; Moccia, F.; Cimmino, M.; Marra, T. Is Nasogastric Decompression Useful in Prevention of Leaks After Laparoscopic Sleeve Gastrectomy? A Randomized Trial. J. Investig. Surg. 2014, 27, 234–239. [Google Scholar] [CrossRef]
- Rashdan, M.; Al-Sabe, L.; Salameh, M.; Halaseh, S.; Al-Mikhi, B.; Sha’bIn, S.; Alqirem, L.; Alsaadi, T.; Ahmad, J.; Sabbagh, A.; et al. Predictive factors for readmission after bariatric surgery: Experience of an obesity center. Medicine 2024, 103, e39242. [Google Scholar] [CrossRef]
- Gress, K.; Urits, I.; Viswanath, O.; Urman, R.D. Clinical and economic burden of postoperative nausea and vomiting: Analysis of existing cost data. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 681–686. [Google Scholar] [CrossRef]
- Sizemore, D.C. Postoperative Nausea. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500029/ (accessed on 14 September 2025).
- Ruiz-Tovar, J.; Garcia, A.; Ferrigni, C.; Gonzalez, J.; Castellon, C.; Duran, M. Impact of implementation of an enhanced recovery after surgery (ERAS) program in laparoscopic Roux-en-Y gastric bypass: A prospective randomized clinical trial. Surg. Obes. Relat. Dis. 2019, 15, 228–235. [Google Scholar] [CrossRef]
- Elvir-Lazo, O.L.; White, P.F.; Yumul, R.; Eng, H.C. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: An updated review. F1000Research 2020, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wu, X.; Chen, Y.; Yuan, H. Effect of multimodal intervention on postoperative nausea and vomiting in patients undergoing gynecological laparoscopy. J. Int. Med. Res. 2019, 47, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; dos Reis Falcão, L.F.; O’KAne, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.M.; Greim, C.-A.; Roewer, N. A Simplified Risk Score for Predicting Postoperative Nausea and Vomiting. Anesthesiology 1999, 91, 693. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Pierre, S.; Whelan, R. Nausea and vomiting after surgery. Contin. Educ. Anaesth. Crit. Care Pain 2013, 13, 28–32. [Google Scholar] [CrossRef]
- Shrivastava, M.; Ugile, A.B.; Nam, P.B.; Bhalerao, J. A study of different anaesthetic methods on postoperative nausea and vomiting in laparoscopic sleeve gastrectomy in a tertiary hospital in Central India. Eur. J. Cardiovasc. Med. 2025, 15, 109–114. [Google Scholar] [CrossRef]
- Elbakry, A.-E.; Sultan, W.-E.; Ibrahim, E. A comparison between inhalational (Desflurane) and total intravenous anaesthesia (Propofol and dexmedetomidine) in improving postoperative recovery for morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A double-blinded randomised controlled trial. J. Clin. Anesth. 2018, 45, 6–11. [Google Scholar] [CrossRef]
- Ziemann-Gimmel, P.; Goldfarb, A.A.; Koppman, J.; Marema, R.T. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br. J. Anaesth. 2014, 112, 906–911. [Google Scholar] [CrossRef]
- Spaniolas, K.; Nie, L.; Moller, D.; Tatarian, T.; Hesketh, A.; Yang, J.; Docimo, S.; Bates, A.; Gan, T.J.; Pryor, A. A Comprehensive Approach for the Prevention of Nausea and Vomiting Following Sleeve Gastrectomy: A Randomized Controlled Trial. Obes. Surg. 2020, 30, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Aftab, H.; Fagerland, M.W.; Gondal, G.; Ghanima, W.; Olsen, M.K.; Nordby, T. Pain and nausea after bariatric surgery with total intravenous anesthesia versus desflurane anesthesia: A double blind, randomized, controlled trial. Surg. Obes. Relat. Dis. 2019, 15, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Honca, M.; Honca, T. Comparison of Propofol with Desflurane for Laparoscopic Sleeve Gastrectomy in Morbidly Obese patients: A Prospective Randomized Trial. Bariatr. Surg. Pract. Patient Care 2017, 12, 49–54. [Google Scholar] [CrossRef]
- De Baerdemaeker, L.; Jacobs, S.; Blauwen, N.M.M.D.; Pattyn, P.; Herregods, L.L.G.; Mortier, E.P.; Struys, M.M.R.F. Postoperative Results after Desflurane or Sevoflurane Combined with Remifentanil in Morbidly Obese Patients. Obes. Surg. 2006, 16, 728–733. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.-Y.; Gao, R.-J.; Huang, Y.; Mao, S.-M.; Feng, J.-Y. The Effect of Depth of Anesthesia on Postoperative Pain in Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Trial. Obes. Surg. 2024, 34, 1793–1800. [Google Scholar] [CrossRef]
- Castro, D.S.; Leão, P.; Borges, S.; Gomes, L.; Pacheco, M.; Figueiredo, P. Sugammadex Reduces Postoperative Pain After Laparoscopic Bariatric Surgery. Surg. Laparosc. Endosc. Percutaneous Tech. 2014, 24, 420–423. [Google Scholar] [CrossRef]
- Yang, W.-L.; Wen, Y.-L.; Xu, W.-M.; Xu, C.-L.; Yin, W.-Q.; Lin, J.-Y. Effect of deep neuromuscular block on the quality of early recovery after sleeve gastrectomy in obese patients: A randomized controlled trial. BMC Anesthesiol. 2024, 24, 101. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, L.; Yu, Y.; Liu, S.; Xu, H.; Xu, Z.; Yang, C.; Liu, C. Comparison of Deep and Moderate Neuromuscular Blockade on Intestinal Mucosal Barrier in Laparoscopic Gastrectomy: A Prospective, Randomized, Double-Blind Clinical Trial. Front. Med. 2022, 8, 789597. [Google Scholar] [CrossRef]
- Suh, S.; Hetzel, E.; Alter-Troilo, K.; Lak, K.; Gould, J.C.; Kindel, T.L.; Higgins, R.M. The influence of preoperative carbohydrate loading on postoperative outcomes in bariatric surgery patients: A randomized, controlled trial. Surg. Obes. Relat. Dis. 2021, 17, 1480–1488. [Google Scholar] [CrossRef]
- Cho, H.-J.; Huang, Y.-H.; Poon, K.-S.; Chen, K.-B.; Liao, K.H. Perioperative hemodynamic optimization in laparoscopic sleeve gastrectomy using stroke volume variation to reduce postoperative nausea and vomiting. Surg. Obes. Relat. Dis. 2021, 17, 1549–1557. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, K.; Liu, L.; Ma, J.; Liu, D.; Zhang, J. The Impact of Goal-Directed Fluid Therapy on Postoperative Nausea and Vomiting in High-Risk Patients Undergoing Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2022, 32, 3533–3540. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Tian, C.; Lu, J.; Lee, Y. Total Intravenous Anesthesia Versus Inhalation Anesthesia on Postoperative Analgesia and Nausea and Vomiting After Bariatric Surgery: A Systematic Review and Meta-Analysis. Asian J. Anesthesiol. 2021, 59, 135–151. [Google Scholar] [CrossRef]
- Oliveira, C.R.D.; Bernardo, W.M.; Nunes, V.M. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz. J. Anesthesiol. 2017, 67, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Tramèr, M.R.; Fuchs-Buder, T. Omitting antagonism of neuromuscular block: Effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. Br. J. Anaesth. 1999, 82, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Palomba, G.; Basile, R.; Capuano, M.; Pesce, M.; Rurgo, S.; Sarnelli, G.; De Palma, G.D.; Aprea, G. Nasogastric tube after laparoscopic Heller-Dor surgery: Do you really need it? Curr. Probl. Surg. 2024, 61, 101457. [Google Scholar] [CrossRef]
- Weijs, T.J.; Kumagai, K.; Berkelmans, G.H.K.; Nieuwenhuijzen, G.A.P.; Nilsson, M.; Luyer, M.D.P. Nasogastric decompression following esophagectomy: A systematic literature review and meta-analysis. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.; Varghese, C.; Thejasvin, K.; Prabakaran, V.; Robinson, S.; Pathak, S.; Dasari, B.V.M.; Pandanaboyana, S. Impact of routine nasogastric decompression versus no nasogastric decompression after pancreaticoduodenectomy on perioperative outcomes: Meta-analysis. BJS Open 2021, 5, zrab111. [Google Scholar] [CrossRef]
- Cooke, F.E.; Samuels, J.D.; Pomp, A.; Gadalla, F.; Wu, X.; Afaneh, C.; Dakin, G.F.; Goldstein, P.A. A Randomized, Double-Blind, Placebo-Controlled Trial of Intravenous Acetaminophen on Hospital Length of Stay in Obese Individuals Undergoing Sleeve Gastrectomy. Obes. Surg. 2018, 28, 2998–3006. [Google Scholar] [CrossRef]
- Amin, S.; Hasanin, A.; Soliman, S.; Mostafa, M.; Abdallah, A.S.; Zakaria, D.; Abdelkader, A. Intravenous Ibuprofen Versus Ketorolac for Perioperative Pain Control in Patients with Morbid Obesity Undergoing Bariatric Surgery: A Randomized Controlled Trial. Obes. Surg. 2025, 35, 1350–1356. [Google Scholar] [CrossRef]
- Govindarajan, R.; Ghosh, B.; Sathyamoorthy, M.K.; Kodali, N.S.; Raza, A.; Aronsohn, J.; Rajpal, S.; Ramaswamy, C.; Abadir, A. Efficacy of ketorolac in lieu of narcotics in the operative management of laparoscopic surgery for morbid obesity. Surg. Obes. Relat. Dis. 2005, 1, 530–535. [Google Scholar] [CrossRef]
- Hamed, J.M.E.; Refaat, H.S.; Al-Wadaani, H. Dexmedetomidine compared to remifentanil infusion as adjuvant to sevoflurane anesthesia during laparoscopic sleeve gastrectomy. Anesth. Essays Res. 2019, 13, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Khalil, B.N.; Elderh, M.S.; Khaja, M.A.; El-Shaer, A.N.; Ali, B.E.; Taeimah, M.O. Perioperative use of ketamine infusion versus dexmedetomidine infusion for analgesia in obese patients undergoing bariatric surgery: A double-blinded three-armed randomized controlled trial. BMC Anesthesiol. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halaweh, S.; Obeidat, F.; Absalom, A.R.; AlOweidi, A.; Abu Abeeleh, M.; Qudaisat, I.; Robinson, F.; Mason, K.P. Dexmedetomidine versus morphine infusion following laparoscopic bariatric surgery: Effect on supplemental narcotic requirement during the first 24 h. Surg. Endosc. 2016, 30, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Zeeni, C.; Aouad, M.T.; Daou, D.; Naji, S.; Jabbour-Khoury, S.; Alami, R.S.; Safadi, B.Y.; Siddik-Sayyid, S.M. The Effect of Intraoperative Dexmedetomidine Versus Morphine on Postoperative Morphine Requirements After Laparoscopic Bariatric Surgery. Obes. Surg. 2019, 29, 3800–3808. [Google Scholar] [CrossRef]
- Bakhamees, H.S.; El-Halafawy, Y.M.; El-Kerdawy, H.M.; Gouda, N.M.; Altemyatt, S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East J. Anaesthesiol. 2007, 19, 537–551. [Google Scholar]
- De Oliveira, G.S.; Duncan, K.; Fitzgerald, P.; Nader, A.; Gould, R.W.; McCarthy, R.J. Systemic Lidocaine to Improve Quality of Recovery after Laparoscopic Bariatric Surgery: A Randomized Double-Blinded Placebo-Controlled Trial. Obes. Surg. 2013, 24, 212–218. [Google Scholar] [CrossRef]
- De Oliveira, C.M.B.; Coelho, L.M.G.; Valadão, J.A.; Moura, E.C.R.; da Silva, A.A.M.; de Lima, R.C.; Brunialti, M.K.C.; Salomão, R.; Leal, P.d.C.; Sakata, R.K. Assessment of the Effect of Perioperative Venous Lidocaine on the Intensity of Pain and IL-6 Concentration After Laparoscopic Gastroplasty. Obes. Surg. 2020, 30, 3912–3918. [Google Scholar] [CrossRef]
- Sherif, A.A.; Elsersy, H.E. The impact of dexmedetomidine or xylocaine continuous infusion on opioid consumption and recovery after laparoscopic sleeve gastrectomy. Minerva Anestesiol. 2017, 83, 1274–1282. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Wang, J.; Gao, X.; Wang, G. Effect of Intravenous Infusion of Lidocaine Compared with Ultrasound-Guided Transverse Abdominal Plane Block on the Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery. Drug Des. Dev. Ther. 2022, 16, 739–748. [Google Scholar] [CrossRef]
- Yurttas, T.; Djurdjevic, M.; Schnider, T.W.; Filipovic, M. Analgesic efficacy of systemic lidocaine using lean body mass based dosing regime versus placebo in bariatric surgery: A prospective, randomised, double-blind, placebo-controlled, single-centre study. Br. J. Anaesth. 2023, 131, 122–129. [Google Scholar] [CrossRef]
- Plass, F.; Nicolle, C.; Zamparini, M.; Al Issa, G.; Fiant, A.L.; Le Roux, Y.; Gérard, J.L.; Fischer, M.O.; Alvès, A.; Hanouz, J. Effect of intra-operative intravenous lidocaine on opioid consumption after bariatric surgery: A prospective, randomised, blinded, placebo-controlled study. Anaesthesia 2020, 76, 189–198. [Google Scholar] [CrossRef]
- Sakata, R.K.; de Lima, R.C.; Valadão, J.A.; Leal, P.C.; Moura, E.C.; Cruz, V.P.; de Oliveira, C.M. Randomized, Double-Blind Study of the Effect of Intraoperative Intravenous Lidocaine on the Opioid Consumption and Criteria for Hospital Discharge After Bariatric Surgery. Obes. Surg. 2019, 30, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Alimian, M.; Safaeian, R.; Zaman, B.; Entezari, S.; Abedian, A.E. Comparison of the Effect of Intraoperative 1 mg/kg/h and 2 mg/kg/h IV Lidocaine Infusion on Postoperative Pain and Nausea-Vomiting in Laparoscopic Gastric Bypass Surgery. J. Pharm. Res. Int. 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Hasanein, R.; El-Sayed, W.; Nabil, N.; Elsayed, G. The effect of combined remifentanil and low dose ketamine infusion in patients undergoing laparoscopic gastric bypass. Egypt. J. Anaesth. 2011, 27, 255–260. [Google Scholar] [CrossRef]
- Yang, T.; Mudabbar, M.S.; Liu, B.; Xu, M.; Fu, Q. Intraoperative Esketamine Is Effective at Reducing Acute Postoperative Pain in Bariatric Surgery Patients: A Randomized Control Trial. Obes. Surg. 2023, 33, 2368–2374. [Google Scholar] [CrossRef]
- Das Adhikary, S.; Thiruvenkatarajan, V.; McFadden, A.; Liu, W.M.; Mets, B.; Rogers, A. Analgesic efficacy of ketamine and magnesium after laparoscopic sleeve gastrectomy: A randomized, double-blind, placebo-controlled trial. J. Clin. Anesth. 2021, 68, 110097. [Google Scholar] [CrossRef]
- Mehta, S.D.; Smyth, D.; Vasilopoulos, T.; Friedman, J.; Sappenfield, J.W.; Alex, G. Ketamine infusion reduces narcotic requirements following gastric bypass surgery: A randomized controlled trial. Surg. Obes. Relat. Dis. 2021, 17, 737–743. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Dang, J.; Zheng, H.; Ren, B.; Liu, C.; Zuo, R.; Wang, R.; Liu, T.; Wang, Z. Effect of Intraoperative Infusion of Esketamine on Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery: A Randomized Controlled Trial. Pain Ther. 2023, 12, 979–992. [Google Scholar] [CrossRef]
- Schulmeyer, M.C.C.; de la Maza, J.; Ovalle, C.; Farias, C.; Vives, I. Analgesic Effects of a Single Preoperative Dose of Pregabalin after Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2009, 20, 1678–1681. [Google Scholar] [CrossRef]
- Alimian, M.; Faiz, S.H.-R.; Imani, F.; Navadegi, S.F.; Pournajafian, A.; Safari, S. Effect of Oral Pregabalin Premedication on Post-Operative Pain in Laparoscopic Gastric Bypass Surgery. Anesthesiol. Pain Med. 2012, 2, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.K.; Abdallah, N.M. Multimodal analgesia with pregabalin and dexmedetomidine in morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A prospective randomized double blind placebo controlled study. Egypt. J. Anaesth. 2016, 32, 293–298. [Google Scholar] [CrossRef]
- Hassani, V.; Pazouki, A.; Nikoubakht, N.; Chaichian, S.; Sayarifard, A.; Khankandi, A.S. The Effect of Gabapentin on Reducing Pain After Laparoscopic Gastric Bypass Surgery in Patients with Morbid Obesity; A Randomized Clinical Trial. Anesthesiol. Pain Med. 2015, 5, e22372. [Google Scholar] [CrossRef] [PubMed]
- Mulier, J.P.; Wouters, R.; Dillemans, B.; Dekock, M. A Randomized Controlled, Double-Blind Trial Evaluating the Effect of Opi-oid-Free Versus Opioid General Anaesthesia on Postoperative Pain and Discomfort Measured by the QoR-40. J. Clin. Anesth. Pain Med. 2018, 6, 2. [Google Scholar]
- Mieszczański, P.; Górniewski, G.; Ziemiański, P.; Cylke, R.; Lisik, W.; Trzebicki, J. Comparison between multimodal and intraoperative opioid free anesthesia for laparoscopic sleeve gastrectomy: A prospective, randomized study. Sci. Rep. 2023, 13, 12677. [Google Scholar] [CrossRef]
- Clanet, M.; Touihri, K.; El Haddad, C.; Goldsztejn, N.; Himpens, J.; Fils, J.F.; Gricourt, Y.; Van der Linden, P.; Coeckelenbergh, S.; Joosten, A.; et al. Effect of opioid-free versus opioid-based strategies during multimodal anaesthesia on postoperative morphine consumption after bariatric surgery: A randomised double-blind clinical trial. BJA Open 2024, 9, 100263. [Google Scholar] [CrossRef]
- Dagher, C.; Mattar, R.; Aoun, M.; Tohme, J.; Naccache, N.; Jabbour, H. Opioid-free anesthesia in bariatric surgery: A prospective randomized controlled trial. Eur. J. Med. Res. 2025, 30, 320. [Google Scholar] [CrossRef]
- Menck, J.T.; Tenório, S.B.; de Oliveira, R.M.; Strobel, R.; dos Santos, B.B.; Junior, A.F.F.; de Cesaro, M.P. Opioid-free Anesthesia for Laparoscopic Gastroplasty. A Prospective and Randomized Trial. Open Anesth. J. 2022, 16, e258964582208110. [Google Scholar] [CrossRef]
- Campos-Pérez, W.; Ramírez-Plascencia, L.; Pérez-Robles, M.; Rivera-Valdés, J.J.; Sánchez-Muñoz, P.; Pérez-Vargas, L.; González-Landeros, D.; Cuevas, J.H.M.; Martínez-López, E. A comparison of opioid-containing anesthesia versus opioid-free anesthesia using the Cortínez-Sepúlveda model on differential cytokine responses in obese patients undergoing gastric bypass surgery: A randomized controlled trial. BMC Anesthesiol. 2022, 22, 294. [Google Scholar] [CrossRef]
- Soudi, A.M.; Hammad, R.A.; ElShafie, M.A.; Ahmed, I.M.A.S.; Alhadidy, M.A. Comparing opioid free general anesthesia to traditional balanced general anesthesia regarding achievement of enhanced recovery in laparoscopic bariatric surgeries. Ain-Shams J. Anesthesiol. 2022, 14, 24. [Google Scholar] [CrossRef]
- Macfater, H.; Xia, W.; Srinivasa, S.; Hill, A.G.; Van De Velde, M.; Joshi, G.P.; Beloeil, H.; Bonnet, F.; Hill, A.; Joshi, G.P.; et al. Evidence-Based Management of Postoperative Pain in Adults Undergoing Laparoscopic Sleeve Gastrectomy. World J. Surg. 2019, 43, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Tamburini, E.; Linassi, F.; Pettenuzzo, T.; Boscolo, A.; Navalesi, P. Non-Opioid Analgesics and Adjuvants after Surgery in Adults with Obesity: Systematic Review with Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, R.; Alnajjar, D.; Bin Salamah, R.; Mandoorah, J.; Alghamdi, A.; Aloteibi, R.E.; Almusharaf, L.; Albabtain, B. Dexmedetomidine in Bariatric Surgery: A Systematic Review and Meta-Analysis of Its Effects on Postoperative Pain and Postoperative Nausea and Vomiting. J. Clin. Med. 2025, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.C.; Ortegal, G.H.P.C.; Aguirre, J.M.; Costa, P.R.R.; Ferreira, L.N.; Moreira, L.F.; Silva, G.C.; Filho, P.P.M.F.; Ferreira, D.M. Effects of Intravenous Lidocaine on Quality of Recovery After Laparoscopic Bariatric Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Surg. 2024, 34, 2663–2669. [Google Scholar] [CrossRef]
- Chaouch, M.A.M.A.; Daghmouri, M.A.; Boutron, M.-C.; Ferraz, J.-M.; Usai, S.; Soubrane, O.; Beaussier, M.; Pourcher, G.; Oweira, H. Ketamine as a component of multimodal analgesia for pain management in bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. Surg. 2022, 78, 103783. [Google Scholar] [CrossRef]
- Mao, J.; Price, D.D.; Mayer, D.J. Mechanisms of hyperalgesian and morphine tolerance: A current view of their possible interactions. Pain 1995, 62, 259–274. [Google Scholar] [CrossRef]
- Thorp, A.W.; Brown, L.; Green, S.M. Ketamine-Associated Vomiting. Pediatr. Emerg. Care 2009, 25, 15–18. [Google Scholar] [CrossRef]
- Hung, K.-C.; Chang, L.-C.; Ho, C.-N.; Hsu, C.-W.; Wu, J.-Y.; Lin, Y.-T.; Chen, I.-W. Influence of Intravenous Magnesium Sulfate Infusion on the Subjective Postoperative Quality of Recovery: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 2375. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, S.-C.; Chiang, M.-H.; Hsu, C.-W.; Chen, J.-Y.; Huang, P.-W.; Sun, C.-K. Analgesic Efficacy of Gabapentin and Pregabalin in Patients Undergoing Laparoscopic Bariatric Surgeries: A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 2734–2743. [Google Scholar] [CrossRef]
- Mieszczanski, P.; Gorniewski, G.; Janiak, M.; Trzebicki, J. The effect of pre-emptive oral pregabalin on opioid consumption in patients undergoing laparoscopic sleeve gastrectomy with an analysis of intraoperative hemodynamic stability and quality of recovery: Study protocol for a randomized, prospective, double-blind study. Trials 2024, 25, 367. [Google Scholar] [CrossRef]
- Sultana, A.; Torres, D.; Schumann, R. Special indications for Opioid Free Anaesthesia and Analgesia, patient and procedure related: Including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 547–560. [Google Scholar] [CrossRef]
- Ao, Y.; Ma, J.; Zheng, X.; Zeng, J.; Wei, K. Opioid-Sparing Anesthesia Versus Opioid-Free Anesthesia for the Prevention of Postoperative Nausea and Vomiting after Laparoscopic Bariatric Surgery: A Systematic Review and Network Meta-Analysis. Anesth. Analg. 2024, 140, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Beloeil, H.; Garot, M.; Lebuffe, G.; Gerbaud, A.; Bila, J.; Cuvillon, P.; Dubout, E.; Oger, S.; Nadaud, J.; Becret, A.; et al. Balanced Opioid-free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology 2021, 134, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Didehvar, S.; Viola-Blitz, J.; Haile, M.; Franco, L.; Kline, R.; Kurian, M.; Fielding, G.; Ren, C.; Bekker, A. A Randomized, Double Blind Study to Evaluate the Efficacy of Palonosetron with Dexamethasone Versus Palonosetron Alone for Prevention of Post-Operative Nausea and Vomiting in Subjects Undergoing Bariatric Surgeries with High Emetogenic Risk. Open Anesthesiol. J. 2013, 7, 30–36. [Google Scholar] [CrossRef]
- Benevides, M.L.; Oliveira, S.S.; de Aguilar-Nascimento, J.E. The Combination of Haloperidol, Dexamethasone, and Ondansetron for Prevention of Postoperative Nausea and Vomiting in Laparoscopic Sleeve Gastrectomy: A Randomized Double-Blind Trial. Obes. Surg. 2013, 23, 1389–1396. [Google Scholar] [CrossRef]
- Burcu, B.; Hacım, N.A.; Caliskan, O.; Demirgan, S.; Aktokmakyan, T.V.; Meric, S.; Duymaz, T.; Karabay, O.; Solmaz, A. Impact of body weight-based dosing of palonosetron and ondansetron on postoperative nausea and vomiting following laparoscopic sleeve gastrectomy: A randomized, double-blind study. Acta Chir. Belg. 2024, 124, 41–49. [Google Scholar] [CrossRef]
- Kaloria, N.; Sen, I.M.; Saini, V.; Gupta, R.; Walia, R. Palonosetron-dexamethasone versus ondansetron-dexamethasone to prevent postoperative nausea and vomiting undergoing laparoscopic sleeve gastrectomy: A preliminary, randomized, double blinded study. Indian J. Clin. Anaesth. 2017, 4, 523–529. [Google Scholar]
- Ortiz, E.; González, A.I.; Jaime, V.; Guzmán, J.A.; Esparza, I.; Orozco, J.O.; Guerrero, M.A.; Ramos, A.; Zerrweck, C. The impact of Aprepitant on Nausea and Vomiting following Laparoscopic Sleeve Gastrectomy: A Blinded Randomized Controlled Trial. Obes. Surg. 2024, 34, 1316–1323. [Google Scholar] [CrossRef]
- Sinha, A.C.; Singh, P.M.; Williams, N.W.; Ochroch, E.A.; Goudra, B.G. Aprepitant’s Prophylactic Efficacy in Decreasing Postoperative Nausea and Vomiting in Morbidly Obese Patients Undergoing Bariatric Surgery. Obes. Surg. 2013, 24, 225–231. [Google Scholar] [CrossRef]
- Ashoor, T.M.; Kassim, D.Y.; Esmat, I.M. A Randomized Controlled Trial for Prevention of Postoperative Nausea and Vomiting after Laparoscopic Sleeve Gastrectomy: Aprepitant/Dexamethasone vs. Mirtazapine/Dexamethasone. Anesthesiol. Res. Pract. 2022, 2022, 3541073. [Google Scholar] [CrossRef]
- Shahinpour, S.; Momeni, N.; Yaqubnejad, M.; Khajavi, M.; Pourfakhr, P. Evaluation and Comparison of Two Different Combined Regimens for Prophylaxis of Nausea and Vomiting After Laparoscopic Bariatric Surgery: A Double-Blinded Randomized Clinical Trial. Acta Medica Iran. 2023, 61, 175–180. [Google Scholar] [CrossRef]
- Talebpour, M.; Omrani, N.G.; Imani, F.; Moharari, R.S.; Pourfakhr, P.; Khajavi, M.R. Comparison Effect of Promethazine/Dexamethasone and Metoclopramide/Dexamethasone on Postoperative Nausea and Vomiting after Laparascopic Gastric Placation: A Randomized Clinical Trial. Anesthesiol. Pain Med. 2017, 7, e57810. [Google Scholar] [CrossRef]
- Moussa, A.A.; Oregan, P.J. Prevention of postoperative nausea and vomiting in patients undergoing laparoscopic bariatric surgery—Granisetron alone vs granisetron combined with dexamethasone/droperidol. Middle East J. Anaesthesiol. 2007, 19, 357–367. [Google Scholar]
- Ebrahimian, M.; Mirhashemi, S.-H.; Oshidari, B.; Zamani, A.; Shadidi-Asil, R.; Kialashaki, M.; Ghayebi, N. Effects of ondansetron, metoclopramide and granisetron on perioperative nausea and vomiting in patients undergone bariatric surgery: A randomized clinical trial. Surg. Endosc. 2023, 37, 4495–4504. [Google Scholar] [CrossRef] [PubMed]
- Pourfakhr, P.; Aghabagheri, M.; Mahmoudabadi, H.Z.; Najjari, K.; Talebpour, M.; Khajavi, M.R. Prophylactic Administration of Diphenhydramine/Acetaminophen and Ondansetron Reduced Postoperative Nausea and Vomiting and Pain Following Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Trial. Obes. Surg. 2021, 31, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; El-Halafawy, M.; Abdelhamid, B.; Barghout, F.; Sayed, I. Efficacy of Combining Cyclizine, Metoclopramide, or On-dansetron with Dexamethasone in Prevention of Postoperative Nausea and Vomiting after Laparoscopic Sleeve Gastrectomy; A Randomized Controlled Trial. Middle East J. Anesthesiol. 2025, 32, 42–55. [Google Scholar]
- Atif, Q.A.A.; Al Obaid, O.; Malik, A.M. Effect of intravenous scopolamine before stapling, on postoperative nausea and vomiting in sleeve gastrectomy patients: A randomized controlled trial. Surg. Endosc. 2022, 36, 7717–7721. [Google Scholar] [CrossRef]
- Schumann, R.; Ziemann-Gimmel, P.; Sultana, A.; Eldawlatly, A.A.; Kothari, S.N.; Shah, S.; Wadhwa, A. Postoperative nausea and vomiting in bariatric surgery: A position statement endorsed by the ASMBS and the ISPCOP. Surg. Obes. Relat. Dis. 2021, 17, 1829–1833. [Google Scholar] [CrossRef]
- Polderman, J.A.; Farhang-Razi, V.; Van Dieren, S.; Kranke, P.; DeVries, J.H.; Hollmann, M.W.; Preckel, B.; Hermanides, J. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 2018, 8, CD011940. [Google Scholar] [CrossRef]
- Tricco, A.C.; Soobiah, C.; Blondal, E.; Veroniki, A.A.; Khan, P.A.; Vafaei, A.; Ivory, J.; Strifler, L.; Ashoor, H.; MacDonald, H.; et al. Comparative efficacy of serotonin (5-HT3) receptor antagonists in patients undergoing surgery: A systematic review and network meta-analysis. BMC Med. 2015, 13, 136. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, T.; Wu, Q.; Da, M. The Efficacy of Aprepitant in Preventing Post-bariatric Surgery Nausea and Vomiting: Evidence from Clinical Trials. Obes. Surg. 2024, 34, 2617–2626. [Google Scholar] [CrossRef]
- Singh, P.M.; Borle, A.; Rewari, V.; Makkar, J.K.; Trikha, A.; Sinha, A.C.; Goudra, B. Aprepitant for postoperative nausea and vomiting: A systematic review and meta-analysis. Postgrad. Med. J. 2015, 92, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, F.; Liang, C.; Huang, Y.; Zhao, Y.; Liu, C.; Lin, C.; Zhang, L.; Zhou, S.; Wang, Q.; et al. Fosaprepitant for postoperative nausea and vomiting in patients undergoing laparoscopic gastrointestinal surgery: A randomised trial. Br. J. Anaesth. 2023, 131, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Deitrick, C.L.; Mick, D.J.; Lauffer, V.; Prostka, E.; Nowak, D.; Ingersoll, G. A Comparison of Two Differing Doses of Promethazine for the Treatment of Postoperative Nausea and Vomiting. J. Perianesth. Nurs. 2015, 30, 5–13. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.; Castro-Alves, L.; Chang, R.; Yaghmour, E.; McCarthy, R. Systemic metoclopramide to prevent postoperative nausea and vomiting: A meta-analysis without Fujii’s studies. Br. J. Anaesth. 2012, 109, 688–697. [Google Scholar] [CrossRef]
- Kranke, P.; Morin, A.M.; Roewer, N.; Eberhart, L.H.J. Dimenhydrinate for prophylaxis of postoperative nausea and vomiting: A meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2002, 46, 238–244. [Google Scholar] [CrossRef]
- Apfel, C.C.; Zhang, K.; George, E.; Shi, S.; Jalota, L.; Hornuss, C.; Fero, K.E.; Heidrich, F.; Pergolizzi, J.V.; Cakmakkaya, O.S.; et al. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: A systematic review and meta-analysis. Clin. Ther. 2010, 32, 1987–2002. [Google Scholar] [CrossRef]
- White, P.F.; Tang, J.; Song, D.; Coleman, J.E.; Wender, R.H.; Ogunnaike, B.; Sloninsky, A.; Kapu, R.; Shah, M.; Webb, T. Transdermal Scopolamine: An Alternative to Ondansetron and Droperidol for the Prevention of Postoperative and Postdischarge Emetic Symptoms. Anesth. Analg. 2007, 104, 92–96. [Google Scholar] [CrossRef]
- De Cassai, A.; Tulgar, S.; Carron, M.; Navalesi, P. Regional anesthesia in bariatric surgery. Curr. Opin. Anaesthesiol. 2025, 38, 611–617. [Google Scholar] [CrossRef]
- Shariat, A.; Kadakia, R.; Lin, H.-M.; Egorova, N.; Jin, S.; Latmore, M.; Epstein, J.; Pai BH, P.; Park, K.; Kini, S.; et al. Comparison of Posterior Quadratus Lumborum Block vs Surgical Wound Infiltration in Patients Undergoing Bariatric Sleeve Gastrectomy Surgery. Obes. Surg. 2025, 35, 2673–2679. [Google Scholar] [CrossRef]
- Ma, P.; Lloyd, A.; McGrath, M.; Cung, A.S.; Akusoba, I.; Jackson, A.; Swartz, D.; Boone, K.; Higa, K. Efficacy of liposomal bupivacaine versus bupivacaine in port site injections on postoperative pain within enhanced recovery after bariatric surgery program: A randomized clinical trial. Surg. Obes. Relat. Dis. 2019, 15, 1554–1562. [Google Scholar] [CrossRef]
- Neishaboury, M.; Shokri, S.; Kianpour, P.; Farhadi, K.; Najjari, K.; Sharifnia, H.; MohammadYousef, R.; Khajavi, M. The Effects of Intraperitoneal Dexmedetomidine in Comparison with Ropivacaine in Postoperative Pain After Laparoscopic Sleeve Gastrectomy: A Double-Blind, Randomized, Placebo-Controlled, Clinical Trial. Obes. Surg. 2025, 35, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.M.; Bakhtiyari, M.; Gholizadeh, B.; Shariati, C. Analgesic Effect of Intraperitoneal Bupivacaine Hydrochloride After Laparoscopic Sleeve Gastrectomy: A Randomized Clinical Trial. J. Gastrointest. Surg. 2018, 22, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Omar, I.; Abualsel, A. Efficacy of Intraperitoneal Instillation of Bupivacaine after Bariatric Surgery: Randomized Controlled Trial. Obes. Surg. 2019, 29, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Seal, A.; Lemech, I.; Fisher, O.M.; Williams, N. Intraperitoneal Instillation of Local Anesthetic (IPILA) in Bariatric Surgery and the Effect on Post-operative Pain Scores: A Randomized Control Trial. Obes. Surg. 2022, 32, 2349–2356. [Google Scholar] [CrossRef]
- Sherwinter, D.A.; Ghaznavi, A.M.; Spinner, D.; Savel, R.H.; Macura, J.M.; Adler, H. Continuous Infusion of Intraperitoneal Bupivacaine after Laparoscopic Surgery: A Randomized Controlled Trial. Obes. Surg. 2008, 18, 1581–1586. [Google Scholar] [CrossRef]
- Mittal, T.; Dey, A.; Siddhartha, R.; Nali, A.; Sharma, B.; Malik, V. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postoperative analgesia in laparoscopic gastric sleeve resection: A randomized single blinded case control study. Surg. Endosc. 2018, 32, 4985–4989. [Google Scholar] [CrossRef]
- Emile, S.H.; Abdel-Razik, M.A.; Elbahrawy, K.; Elshobaky, A.; Shalaby, M.; Elbaz, S.A.; Gado, W.A.; Elbanna, H.G. Impact of Ultrasound-Guided Transversus Abdominis Plane Block on Postoperative Pain and Early Outcome After Laparoscopic Bariatric Surgery: A Randomized Double-Blinded Controlled Trial. Obes. Surg. 2019, 29, 1534–1541. [Google Scholar] [CrossRef]
- Ibrahim, M.; El Shamaa, H. Efficacy of ultrasound-guided oblique subcostal transversus abdominis plane block after laparoscopic sleeve gastrectomy: A double blind, randomized, placebo controlled study. Egypt. J. Anaesth. 2014, 30, 285–292. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Wang, X.; Niu, Z.; Wang, P.; Yuan, L.; Wang, P. The Effect of Opioid-Free Anesthesia with Transversus Abdominis Plane Block on Patients Undergoing Laparoscopic Sleeve Gastrectomy: Randomized Controlled Study. J. Pain Res. 2024, 17, 2881–2890. [Google Scholar] [CrossRef]
- Albrecht, E.; Kirkham, K.R.; Endersby, R.V.W.; Chan, V.W.S.; Jackson, T.; Okrainec, A.; Penner, T.; Jin, R.; Brull, R. Ultrasound-Guided Transversus Abdominis Plane (TAP) Block for Laparoscopic Gastric-Bypass Surgery:a Prospective Randomized Controlled Double-Blinded Trial. Obes. Surg. 2013, 23, 1309–1314. [Google Scholar] [CrossRef]
- Saber, A.A.; Lee, Y.C.; Chandrasekaran, A.; Olivia, N.; Asarian, A.; Al-Ayoubi, S.; DiGregorio, R. Efficacy of transversus abdominis plane (TAP) block in pain management after laparoscopic sleeve gastrectomy (LSG): A double-blind randomized controlled trial. Am. J. Surg. 2019, 217, 126–132. [Google Scholar] [CrossRef]
- Karaveli, A.; Kaplan, S.; Kavakli, A.S.; Kosar, M.N.; Mayir, B. The Effect of Ultrasound-Guided Erector Spinae Plane Block on Postoperative Opioid Consumption and Respiratory Recovery in Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Study. Obes. Surg. 2025, 35, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.F.; Abdelghany, M.S.; Abu Elyazed, M.M. Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Laparoscopic Bariatric Surgery: A Prospective Randomized Controlled Trial. Pain Pract. 2021, 21, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.S.; KamalELDin, D.M.; Nofal, W.H. Pre-emptive quadratus lumborum block for laparoscopic bariatric surgery: A prospective randomized controlled study. Ain-Shams J. Anesthesiol. 2021, 13, 21. [Google Scholar] [CrossRef]
- Xue, Q.; Chu, Z.; Zhu, J.; Zhang, X.; Chen, H.; Liu, W.; Jia, B.; Zhang, Y.; Wang, Y.; Huang, C.; et al. Analgesic Efficacy of Transverse Abdominis Plane Block and Quadratus Lumborum Block in Laparoscopic Sleeve Gastrectomy: A Randomized Double-Blinded Clinical Trial. Pain Ther. 2022, 11, 613–626. [Google Scholar] [CrossRef]
- Ozel, E.S.; Kaya, C.; Turunc, E.; Ustun, Y.B.; Cebeci, H.; Dost, B. Analgesic efficacy of the external oblique intercostal fascial plane block on postoperative acute pain in laparoscopic sleeve gastrectomy: A randomized controlled trial. Korean J. Anesthesiol. 2025, 78, 159–170. [Google Scholar] [CrossRef]
- Turunc, E.; Dost, B.; Ozel, E.S.; Kaya, C.; Ustun, Y.B.; Bilgin, S.; Ozbalci, G.S.; Koksal, E. Bilateral Ultrasound-Guided External Oblique Intercostal Block Vs. Modified Thoracoabdominal Nerve Block Through Perichondrial Approach for Postoperative Analgesia in Patients Undergoing Laparoscopic Sleeve Gastrectomy Surgery: A Randomized Controlled Study. Obes. Surg. 2024, 34, 3726–3734. [Google Scholar] [CrossRef]
- McDonnell, J.G.; O’Donnell, B.; Curley, G.; Heffernan, A.; Power, C.; Laffey, J.G. The Analgesic Efficacy of Transversus Abdominis Plane Block After Abdominal Surgery: A Prospective Randomized Controlled Trial. Anesth. Analg. 2007, 104, 193–197. [Google Scholar] [CrossRef]
- Filardi, K.; Filardi, R.; Wegner, B.; Arias, J.; da Silva, G.; Felippe, V. Ultrasound-Guided Transversus Abdominis Plane Block as an Effective Path to Reduce Opioid Consumption After Laparoscopic Bariatric Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2024, 34, 4244–4254. [Google Scholar] [CrossRef]
- Yang, G.; Wang, P.; Yin, Y.; Qu, H.; Zhao, X.; Jin, X.; Chu, Q. Erector spinae plane block versus paravertebral block on postoperative quality of recovery in obese patients undergoing laparoscopic sleeve gastrectomy: A randomized controlled trial. PeerJ 2024, 12, e17431. [Google Scholar] [CrossRef]
- Jinaworn, P.; Pannangpetch, P.; Bunanantanasan, K.; Manomaisantiphap, S.; Udomsawaengsup, S.; Thepsoparn, M.; Saeyup, P. Efficacy of Erector Spinae Plane Block on Postoperative Analgesia for Patients Undergoing Metabolic Bariatric Surgery: A Randomized Controlled Trial. Obes. Surg. 2024, 34, 4211–4219. [Google Scholar] [CrossRef]
- Hung, K.-C.; Liu, W.-C.; Hsu, C.-W.; Wu, J.-Y.; Liao, S.-W.; Chen, I.-W. Efficacy of Erector Spinae Plane Block on Analgesic Outcomes in Patients Undergoing Metabolic Surgery: A Meta-Analysis of Randomized Controlled Trials. Obes. Surg. 2025, 35, 1135–1145. [Google Scholar] [CrossRef]
- Eipe, N.; Budiansky, A.S. Perioperative Pain Management in Bariatric Anesthesia. Saudi J. Anaesth. 2022, 16, 339–346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieszczański, P.; Jurczak, M.; Cylke, R.; Ziemiański, P.; Trzebicki, J. Evidence-Based Perioperative Prevention of Postoperative Nausea and Vomiting (PONV) in Patients Undergoing Laparoscopic Bariatric Surgery: A Scoping Review. J. Clin. Med. 2025, 14, 6901. https://doi.org/10.3390/jcm14196901
Mieszczański P, Jurczak M, Cylke R, Ziemiański P, Trzebicki J. Evidence-Based Perioperative Prevention of Postoperative Nausea and Vomiting (PONV) in Patients Undergoing Laparoscopic Bariatric Surgery: A Scoping Review. Journal of Clinical Medicine. 2025; 14(19):6901. https://doi.org/10.3390/jcm14196901
Chicago/Turabian StyleMieszczański, Piotr, Marcin Jurczak, Radosław Cylke, Paweł Ziemiański, and Janusz Trzebicki. 2025. "Evidence-Based Perioperative Prevention of Postoperative Nausea and Vomiting (PONV) in Patients Undergoing Laparoscopic Bariatric Surgery: A Scoping Review" Journal of Clinical Medicine 14, no. 19: 6901. https://doi.org/10.3390/jcm14196901
APA StyleMieszczański, P., Jurczak, M., Cylke, R., Ziemiański, P., & Trzebicki, J. (2025). Evidence-Based Perioperative Prevention of Postoperative Nausea and Vomiting (PONV) in Patients Undergoing Laparoscopic Bariatric Surgery: A Scoping Review. Journal of Clinical Medicine, 14(19), 6901. https://doi.org/10.3390/jcm14196901






