Abstract
Background and Objective: Postoperative nausea and vomiting (PONV) ranks among the most common postoperative complications, affecting up to 80% of patients undergoing laparoscopic bariatric surgery. This condition negatively impacts patient comfort and well-being while also potentially delaying ambulation and increasing the risk of anastomotic and wound dehiscence. Although various interventions can mitigate the risk of PONV, none are entirely effective; therefore, combined prophylactic strategies are the standard approach. In recent years, numerous techniques and interventions have emerged; consequently, this scoping review aims to assess the current evidence regarding PONV prevention in patients undergoing laparoscopic bariatric procedures. Methods: This review was conducted in accordance with PRISMA guidelines and registered with OSF. A search was performed across the MEDLINE (PubMed), Scopus, Embase, and Web of Science databases. Inclusion criteria encompassed randomized controlled trials (RCTs) published up to May 2025, focusing on adult patients undergoing laparoscopic bariatric surgeries with PONV as a primary or secondary outcome. Results: A total of 81 studies were included in this review, encompassing a broad range of perioperative techniques, including opioid-sparing adjuvants, regional anesthesia, and pharmacological interventions. Conclusions: While there is general consensus and guidance advocating for a multimodal approach to PONV prevention, debates persist regarding the optimal techniques and antiemetic drug regimens to implement. Emerging evidence, particularly concerning regional anesthesia strategies and combined pharmacological prophylaxis, including novel agents, highlights the potential advantages of innovative approaches. Highlights: Effective management of postoperative nausea and vomiting in patients undergoing laparoscopic bariatric surgery is essential, given its impact on patient comfort, recovery, and the potential to prevent wound or anastomotic dehiscence. Although multimodal antiemetic strategies are regarded as standard, disagreements remain regarding specific measures to be adopted. New techniques and strategies, including advanced regional anesthesia techniques, pharmacological, and non-pharmacological methods, offer promising avenues for improved prophylaxis.
1. Introduction
Postoperative nausea and vomiting (PONV) represents a common complication following surgical procedures, affecting approximately 60–80% of patients undergoing laparoscopic bariatric surgery (LBS) [1,2]. This incidence is significantly higher compared to that observed in the general surgical patient population [3]. Numerous factors may contribute to this increased risk, including reduced gastric volume following laparoscopic sleeve gastrectomy (LSG), impaired splanchnic perfusion during pneumoperitoneum, or endocrinological factors [1,3,4,5]; however, the precise etiology of this phenomenon remains unclear.
From the patient’s point of view, PONV, along with its associated complications and effects, holds paramount clinical significance, influencing both patient comfort and safety. This spectrum of adverse factors encompasses dehydration, electrolyte disturbances, tension on sutures with the consequent risk of herniation and other wound-related complications, venous hypertension, bleeding, aspiration, and prolonged hospitalization with elevated healthcare costs [6]. Typically, PONV persists for up to 24 to 48 h following surgery [7]; however, in some cases, it may persist longer or even indicate surgical complications [8]. When nausea and vomiting occur after hospital discharge, they substantially contribute to unplanned, early readmissions, predominantly due to dehydration and electrolyte imbalances [9], thereby increasing healthcare utilization and additional costs [10]. In addition to being a potential hazard, there is compelling evidence that PONV constitutes a leading cause of patient suffering after bariatric surgery [11].
Considering the aforementioned factors, PONV prevention and treatment are one of the main goals of the guidelines for perioperative care in bariatric surgery issued by the Enhanced Recovery After Surgery (ERAS) Society. These guidelines aim to enhance patient comfort and satisfaction, which correlates with higher scores on relevant scales [12,13,14,15]. To mitigate PONV, the guidelines advocate a multimodal approach as a standard practice, including the use of dexamethasone as a supportive pharmacological intervention, shortening of perioperative fasting time, abstaining from routine nasogastric decompression, and implementing opioid-sparing strategies.
Taking into account that a vast majority of patients undergoing LBS are non-smoking women aged < 50 [16] undergoing gastrointestinal surgery [15], effective PONV prophylaxis is challenging. Moreover, although many interventions may reduce PONV incidence, none of them are entirely surefire; therefore, combined multimodal interventions are recommended [15]. The modifiable factors that can be applied to PONV prophylaxis can be divided into anesthetic techniques (including regional anesthesia), as well as both pharmacological and non-pharmacological prophylaxis and rescue therapy. Recently, numerous emerging interventions have been assessed in randomized controlled trials (RCTs). Our scoping review aims to evaluate and map the current evidence on PONV prophylaxis in patients undergoing LBS.
2. Materials and Methods
The scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [17] (Supplementary Material File S1) and was registered with the Open Science Framework (OSF).
Two authors independently searched the MEDLINE (PubMed), Scopus, Embase, and Web of Science databases utilizing the keywords “bariatric surgery,” “metabolic surgery,” “laparoscopic sleeve gastrectomy,” “laparoscopic gastric bypass,” and “postoperative nausea” or “postoperative vomiting.” Furthermore, a manual review of the reference lists of the included publications was conducted to identify additional studies for possible inclusion.
The researchers employed the following sequence of research procedures: Initially, titles were screened; subsequently, abstracts and full papers were reviewed. A paper was deemed potentially relevant, and its full text was subsequently examined to determine whether its relevance could not be excluded based on its title and abstract. The full texts of all papers were subsequently assessed. In instances of disagreement, the consensus among the research supervisors was considered final.
With regard to the eligibility criteria, our review includes RCTs on PONV in laparoscopic bariatric surgery published in English. We focused on high-quality RCTs, which are essential for assessing the efficacy of interventions and providing the most dependable medical evidence, vital for practitioners. Given that the majority of RCTs are published in English, we elected to include only literature in this language due to practical and resource limitations. Studies assessing the impact of surgical techniques, their modifications, and alternative or non-conventional medical interventions on PONV were excluded from our review.
3. Results
The initial data search yielded 1293 articles from PubMed, 741 from Web of Science, 2341 from SCOPUS, and 2442 from Embase. A total of 6817 articles were initially retrieved. After the removal of duplicates utilizing Rayyan (Johnson & Phillips, 2018, Newport, UK), 2426 articles remained for screening.
Through a title and abstract review, 199 studies were further examined, of which 81 relevant articles, after a full-text assessment, were retrieved and included in this scoping review. The selection process is depicted in Figure 1. Data extraction was performed by two independent researchers; any disagreements were resolved by the research supervisors.
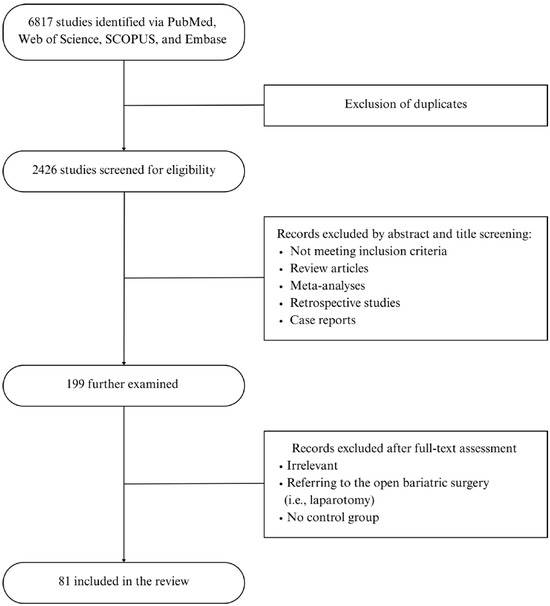
Figure 1.
Flow Chart.
Using the Risk of Bias (ROB2) tool and the Cochrane Intervention Systematic Evaluation Manual (Cochrane Handbook for Systematic Reviews of Interventions), we conducted a methodological quality assessment of the articles that met the inclusion criteria [18]. The qualitative methodological evaluation is illustrated in Figure 2.
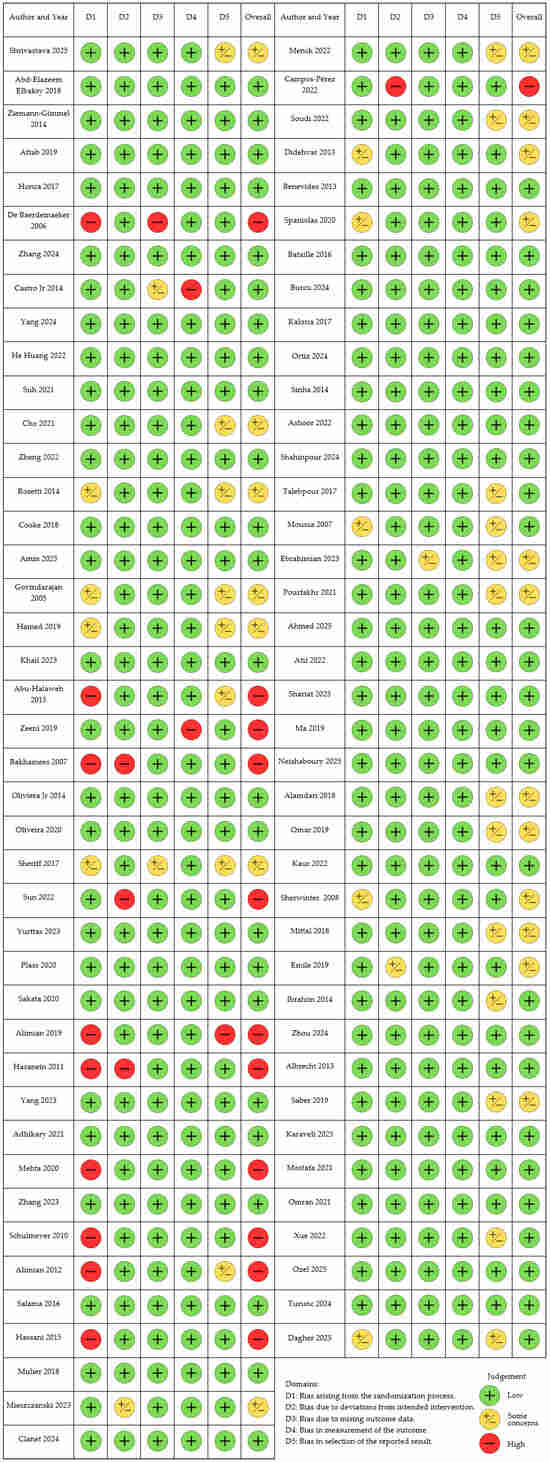
Figure 2.
Traffic light plot for Risk of Bias Assessment.
The relevant 81 RCTs were grouped into four groups: (1) anesthetic factors (n = 15), (2) opioid-sparing agents (n = 33), (3) pharmacological prophylaxis (n = 16), and (4) regional anesthesia techniques (n = 19). Two studies assessed both anesthetic factors and opioid-sparing agents. The relevant studies are depicted in Table 1, Table 2, Table 3 and Table 4.
We wish to emphasize that certain RCTs were designed to assess multiple interventions simultaneously. Furthermore, in the majority of these trials, antiemetic strategies were combined, potentially leading to confounding risks attributable to cointerventions. To mitigate this concern, the tables delineate both the interventions evaluated within the study groups and the antiemetics employed across both groups.
3.1. Anesthetic Factors in PONV Prophylaxis
15 RCTs evaluate anesthetic factors influencing PONV incidence and severity, encompassing a broad spectrum of interventions, including the selection and dosing of anesthetics, muscle relaxants, and fluid therapy. (Table 1).

Table 1.
Anesthetic factors in PONV prophylaxis in bariatric surgery.
Table 1.
Anesthetic factors in PONV prophylaxis in bariatric surgery.
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|
| Shrivastava [19] | 2025 | 168 | LSG | Propofol TIVA, GDFT vs. control | Dexamethasone, azasetron, metoclopramide | 4-point NRS within 24 h postoperatively | Propofol TIVA with GDFT decreased the incidence of PONV at 3–24 h postoperatively by 27.51%. |
| Elbakry [20] | 2018 | 100 | LSG | Propofol TIVA with dexmedetomidine vs. desflurane | Ondansetron | PONV score within 24 h postoperatively | Lower PONV incidence in the study group, 10% vs. 30% compared to desflurane |
| Ziemann-Gimmel [21] | 2014 | 119 | LYGB, LSG, LGB, revisional surgeries | Propofol TIVA, OFA vs. volatile anesthesia with opioids | Dexamethasone, droperidol, or promethazine | 4-point Verbal Rating Scale in the morning after surgery | PONV Risk reduction of 46.4% in the TIVA group |
| Spaniolas [22] | 2020 | 83 | LSG | Propofol TIVA, aprepitant, and transdermal scopolamine vs. volatile anesthesia | Dexamethasone, ondansetron, metoclopramide, compazine | 10-point Verbal Rating Scale at 1, 4, 12, 24 h postoperatively | PONV scores lower at all the time points in the study group |
| Aftab [23] | 2019 | 183 | LSG, LYGB | Propofol TIVA vs. desflurane | Ondansetron, metoclopramide | Visual Analog Scale at PACU, surgical ward, and 24–48 h postoperatively | No difference in PONV incidence compared with desflurane |
| Honca [24] | 2017 | 61 | LSG | Propofol TIVA vs. desflurane | Unspecified | PONV incidence at admission to PACU and 5, 10, 20 min postoperatively | No difference in PONV incidence compared with desflurane |
| De Baerdemaeker [25] | 2006 | 50 | LGB | Desflurane vs. sevoflurane | Ondansetron | Number of PONV episodes at admission to PACU and 30, 60, 120 min postoperatively | No difference in PONV incidence between desflurane and sevoflurane |
| Zhang [26] | 2024 | 90 | LSG | Light (BIS 50) vs. deep (BIS 35) anesthesia | Dexamethasone, palonosetron | 4-point PONV grade 24 h postoperatively | No difference in PONV incidence |
| Castro Jr [27] | 2014 | 88 | LBS | Sugammadex vs. neostigmine | Dexamethasone, ondansetron | PONV incidence before discharge from PACU | Less PONV in the sugammadex group |
| Yang [28] | 2024 | 80 | LSG | Deep (PTC 1–3) neuromuscular block vs. moderate | Dexamethasone, ondansetron | PONV incidence 40 min, 24 h, 48 h postoperatively | No difference between the groups |
| He Huang [29] | 2022 | 150 | LSG | Deep (PTC 1–2) neuromuscular block vs. moderate | Unspecified | PONV incidence at an unspecified period | No difference between the groups |
| Suh [30] | 2021 | 134 | LSG, LYGB | Preoperative carbohydrate loading vs. control | Unspecified | PONV duration | Shorter duration of nausea in the study group |
| Cho [31] | 2021 | 75 | LSG | GDFT, SVV-guided vs. control | Prochlorperazine | 11-point NRS at 0 min, 30 min, 1 h, 24 h, 48 h postoperatively | No difference between the groups |
| Zheng [32] | 2022 | 137 | LSG | GDFT, SVV-guided vs. control | Dexamethasone, tropisetron | 4-point PONV scale at 24 h and 48 h postoperatively | GDFT effective in decreasing the incidence of PONV, 47.1% vs. 71.6% in the control group |
| Rossetti [8] | 2014 | 145 | LSG | Nasogastric decompression | Unspecified | PONV incidence at an unspecified period | No difference between the groups |
3.1.1. Volatile Anesthesia vs. Total Intravenous Anesthesia (TIVA)
In the population of patients with obesity undergoing LBS, the utilization of volatile anesthetics correlates with increased PONV incidence, whereas TIVA has a prophylactic effect [33]. Four studies regarding bariatric surgery support this conclusion [19,20,21,22]. In the RCT by Shirvastava, propofol TIVA, when compared to sevoflurane anesthesia in patients undergoing LSG, decreased the incidence of PONV by 27.51% from 3 to 24 h postoperatively, with no impact observed immediately after surgery [19]. Consistent with these findings, Elbakry et al. reported that patients in the TIVA group experienced PONV approximately 20% less frequently than those receiving desflurane anesthesia [20]. Nonetheless, both studies were influenced by confounding factors, as the concurrent use of fluid goal-directed therapy [19] or dexmedetomidine [20], which may have also impacted the outcomes. Conversely, studies by Aftab [23] and Honca [24] indicate no significant difference between TIVA and volatile anesthesia in the prevention of PONV. This absence of difference might be partly attributable to shorter durations of surgery compared to the aforementioned studies.
In a study by De Baerdemaeker et al., a comparison of the effect of volatile anesthetics in bariatric surgery, including sevoflurane and desflurane, revealed a higher incidence of PONV in the desflurane group at one time point, 120 min postoperatively [25]. The clinical significance of this single finding remains unclear and requires further studies.
Moreover, a single study assessing bispectral index (BIS)-guided monitoring of the depth of anesthesia using TIVA found no beneficial effects on PONV incidence [26], which contradicts studies in the general population [34].
3.1.2. Neuromuscular Block
We identified three studies evaluating the impact of the depth of neuromuscular block and the reversal agent used on the incidence of PONV in bariatric patients. These two questions are connected, as the safe reversal of deep neuromuscular block implies a preference for sugammadex over neostigmine, which has a proven proemetic effect [35]. Such a relation is supported by a study by Castro et al., in which the sugammadex group had a lower incidence of PONV and an opioid-sparing effect compared to the neostigmine group [27].
With regard to deep neuromuscular blockade, consistent findings from two RCTs indicate that no reduction in PONV was observed [28,29]. Nonetheless, some advantageous effects on recovery, pain scores [28], or the restoration of intestinal function were noted [29]. In conclusion, the omission of neostigmine appears to be a crucial factor in mitigating the risk of PONV, and further research involving larger cohorts is necessary to evaluate the impact of neuromuscular blockade depth comprehensively.
3.1.3. Perioperative Fluid Management
Proper hydration can reduce the incidence of PONV [36]. This can be accomplished by minimizing fasting duration and preoperative fluid restrictions, or by administering adequate fluid intra- and postoperatively, possibly using different methods to guide. In studies dedicated to bariatric surgery, an RCT by Suh et al. [30] demonstrated that providing patients scheduled for bariatric surgery with a carbohydrate drink up to three hours before surgery resulted in a reduced duration of PONV postoperatively, without adverse effects such as aspiration or disturbances in glycemic control.
Another approach to preventing both dehydration and excessive fluid administration involves the utilization of hemodynamic-guided goal-directed fluid therapy (GDFT), which has been evaluated in two studies [31,32]. Zheng et al., in their RCT, showed that GDFT based on stroke volume variation (SVV) was an effective method for maintaining the fluid balance, resulting in a lower incidence of PONV observed in the goal-directed group, 47.1% compared to 71.6% within the first 48 h [32]. Furthermore, the severity of symptoms and the requirement for rescue antiemetics were significantly reduced. Conversely, these promising findings are not supported by another study conducted by Cho et al. [31], which showed no significant benefit of SVV-guided fluid therapy concerning PONV in bariatric patients receiving crystalloids. Due to the limited research, more RCTs are warranted to assess the relationship and implications of various fluid administration strategies on the incidence of PONV.
3.1.4. Nasogastric Tube Placement
The placement of a nasogastric tube is currently not recommended, not only in bariatric surgery [15], but also in other surgical procedures involving the upper gastrointestinal tract [37,38,39], as it fails to prevent complications, including PONV. Consistently, a single identified RCT dedicated to bariatric surgery demonstrated that nasogastric decompression with tube placement and maintenance in the postoperative period did not reduce the incidence of PONV or leakage in patients undergoing LSG [8]. To the contrary, nasogastric tube placement may hinder early oral intake, which is undesirable according to ERAS guidelines [15].
3.2. Opioid-Sparing Agents
The reduction in opioid dosage is a modifiable factor to decrease PONV incidence; therefore, opioid-sparing multimodal analgesia is a standard according to the ERAS guidelines [15]. However, the evidence supporting specific perioperative interventions varies, and in some instances, it may be limited. We identified 33 RCTs assessing the impact of opioid-sparing agents such as non-opioid analgesics, dexmedetomidine, lidocaine, N-methyl-D-aspartate (NMDA) receptor antagonists, ketamine and magnesium sulfate, gabapentinoids, and ultimately opioid-free anesthesia (OFA), which are detailed in Table 2. Dexamethasone is further included in pharmacological prophylaxis, although it also possesses co-analgesic properties.

Table 2.
Opioid-sparing agents in bariatric surgery.
Table 2.
Opioid-sparing agents in bariatric surgery.
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Dosing | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|---|
| Cooke [40] | 2018 | 127 | LSG | Paracetamol vs. placebo | Paracetamol 1000 mg i.v. | Aprepitant or droperidol, dexamethasone, ondansetron | 11-point PONV scale until discharge | No difference between the groups |
| Amin [41] | 2025 | 106 | LSG, LYGB | Ketorolac vs. ibuprofen | Ketorolac 30 mg i.v. ibuprofen 800 mg i.v. | Dexamethasone, ondansetron | PONV incidence at an unspecified period | Ketorolac superior to ibuprofen |
| Govindarajan [42] | 2005 | 50 | LYGB | Ketorolac vs. remifentanil | Ketorolac infusion 6–9 mg/h i.v., postoperatively, infusion 4.5–9 mg/h i.v. | Ondansetron | PONV incidence at an unspecified period | PONV reduction 4% vs. 28% in the placebo group |
| Hamed [43] | 2019 | 132 | LSG | Dexmedetomidine vs. remifentanil | Dexmedetomidine 0.2–0.5 mcg/kg/h i.v. intraoperatively | Dexamethasone, droperidol | PONV incidence at an unspecified period | PONV reduction 3% vs. 10% in the placebo group |
| Ziemann-Gimmel [21] | 2014 | 124 | LSG, LYGB, LGB, revisional procedures | Propofol TIVA, OFA vs. volatile anesthesia with opioids | Dexmedetomidine loading dose 0.5 mcg/kg and infusion 0.1–0.3 mcg/kg/h i.v., ketamine 0.5 mg/kg i.v. | Dexamethasone, ondansetron, droperidol, promethazine | 4-point Verbal Rating Scale in the morning postoperatively | PONV absolute risk reduction of 17.3% in the OFA group |
| Khail [44] | 2023 | 90 | LSG | Ketamine vs. Dexmedetomidine vs. placebo | Ketamine loading dose 0.3 mg/kg IBW, infusion 0.3/kg/h i.v., Dexmedetomidine loading dose 0.5 mcg/kg IBW, infusion 0.5 mcg/kg/h i.v. | Ondansetron | 4-point PONV scale at 0, 6, 12, 24 h postoperatively | Less PONV in the dexmedetomidine group compared to ketamine and placebo |
| Abu-Halaweh [45] | 2015 | 60 | LBS | Dexmedetomidine vs. morphine | Dexmedetomidine 0.3 mcg/kg i.v. | Unspecified | PONV incidence within 24 h postoperatively | Less PONV in the dexmedetomidine group, 26.7% vs. 63.3% |
| Zeeni [46] | 2019 | 60 | LSG | Dexmedetomidine vs. morphine | Dexmedetomidine loading dose 1 mcg/kg, infusion 0.5 mcg/kg/h i.v. | Dexamethasone, ondansetron | NRS PONV score at 30 min intervals at PACU and 24 h postoperatively | No difference between the groups |
| Bakhamees [47] | 2007 | 80 | LYGB | Dexmedetomidine vs. placebo | Dexmedetomidine loading dose 0.8 mcg/kg, infusion 0.4 mcg/kg/h i.v. | Unspecified | PONV incidence within 24 h postoperatively | No difference between the groups |
| De Oliveira [48] | 2014 | 50 | LSG | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/h i.v. | Ondansetron | PONV incidence at PACU | No difference between the groups |
| De Oliveira [49] | 2020 | 60 | LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/h i.v. | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the lidocaine group |
| Sheriff [50] | 2017 | 150 | LSG | Lidocaine vs. dexmedetomidine vs. placebo | Lidocaine loading dose 2 mg/kg, infusion 1.5 mg/kg/h i.v., dexmedetomidine loading dose 1 mcg/kg, infusion 0.4 mcg/kg/h i.v. | Unspecified | PONV incidence within 6 h postoperatively | No difference between the groups |
| Sun [51] | 2022 | 99 | LSG, LYGB | Lidocaine vs. TAP Block vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/kg/h i.v. | Dexamethasone | PONV incidence within 24 h postoperatively | No difference between lidocaine and TAP Block |
| Yurttas [52] | 2023 | 137 | LSG, LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 1.5 mg/kg/h i.v. | Unspecified | 3-point PONV scale within 48 h postoperatively | No difference between the groups |
| Plass [53] | 2020 | 178 | LSG, LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/kg/h i.v. | Dexamethasone, droperidol | PONV incidence until discharge | No difference between the groups |
| Sakata [54] | 2020 | 58 | LYGB | Lidocaine vs. placebo | Lidocaine loading dose 1.5 mg/kg, infusion 2 mg/kg/h i.v. | Ondansetron | PONV incidence until discharge from | No difference between the groups |
| Alimian [55] | 2019 | 42 | LYGB | Lidocaine 1 mg/kg/h vs. lidocaine 2 mg/kg/h | Lidocaine infusion 1 mg/kg/h vs. 2 mg/kg/h i.v. | Unspecified | PONV incidence at 0, 30 min, 1 h, 6 h, 12 h, 24 h postoperatively | No difference between the groups |
| Hasanein [56] | 2011 | 60 | LYGB | Ketamine and remifentanil vs. placebo | Ketamine 1 mcg/kg/min i.v. | Dexamethasone, metoclopramide | PONV incidence at an unspecified period | No difference between the groups |
| Yang [57] | 2023 | 68 | LSG | Esketamine | Esketamine loading dose 0.2 mg/kg, infusion 0.2 mg/kg/h i.v. | Dexamethasone | PONV score within 48 h postoperatively | No difference between the groups |
| Adhikary [58] | 2021 | 126 | LSG | Ketamine vs. Ketamine + Magnesium sulfate vs. placebo | Ketamine 0.5 mg/kg i.v., Magnesium sulfate 2 g i.v. | Dexamethasone, ondansetron | 4-point PONV scale every 4 h until 24 h postoperatively | No difference between the groups |
| Mehta [59] | 2020 | 54 | LYGB | Ketamine vs. control | Ketamine loading dose 20 mg i.v., infusion 5 mcg/kg/min i.v. | Dexamethasone | Need for antiemetics within 24 h postoperatively | No difference between the groups |
| Zhang [60] | 2023 | 74 | LBS | Esketamine vs. placebo | Esketamine infusion 0.5 mcg/kg/h i.v. | Dexamethasone, Palonosetron | PONV incidence during the 1-day postoperatively | No difference between the groups |
| Schulmeyer [61] | 2010 | 80 | LSG | Pregabalin vs. placebo | Pregabalin 150 mg p.o. | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the pregabalin group, 25.6% vs. 46.3% placebo |
| Alimian [62] | 2012 | 60 | LYGB | Pregabalin vs. placebo | Pregabalin 300 mg p.o. | Unspecified | PONV incidence within 24 h postoperatively | Absolute risk reductions of 36.7% for nausea and 30% for vomiting in the pregabalin group vs. placebo |
| Salama [63] | 2016 | 60 | LSG | Pregabalin and dexmedetomidine vs. placebo | Pregabalin 75 mg p.o., dexmedetomidine infusion 0.4 mcg/kg/h i.v. | Dexamethasone, ondansetron, metoclopramide | 11-point Verbal Rating Scale every 30 min at PACU, every 2 h on the first post-surgery day | Significantly less PONV within the first 18 h postoperatively |
| Hassani [64] | 2015 | 76 | LYGB | Gabapentin vs. placebo | Gabapentin 100 mg p.o. | Unspecified | Incidence of PONV within 6 h postoperatively | Less PONV in the gabapentin group 10% vs. 33% placebo |
| Mulier [65] | 2018 | 50 | LSG, LYGB, revisional procedures | OFA vs. sufentanil | Dexmedetomidine loading dose 0.5 mcg/kg, infusion 0.25–1 mcg/kg/h i.v., ketamine loading dose 0.25 mg/kg i.v., lidocaine loading dose 1.5 mg/kg, infusion 1.5–3 mg/kg/h i.v. | Ondansetron | Incidence of PONV at PACU | PONV reduction 13% vs. 63% in the sufentanil group |
| Mieszczanski [66] | 2023 | 59 | LSG | OFA vs. remifentanil | Dexmedetomidine loading dose 1 mcg/kg, infusion max 1 mcg/kg/h i.v., ketamine loading dose 0.5 mg/kg i.v., lidocaine loading dose 1.5 mg/kg, infusion max 3 mg/kg/h i.v., magnesium sulfate 40–50 mg/kg i.v. | Dexamethasone, ondansetron | PONV Impact scale within 24 h postoperatively | Less PONV in the OFA group, limited to the first postoperative hour |
| Clanet [67] | 2024 | 172 | LYGB | OFA vs. remifentanil | Dexmedetomidine loading dose 0.5 mcg/kg, infusion 0.4–0.8 mcg/kg/h | Dexamethasone, ondansetron | Incidence of PONV at PACU and 4 h, 24 h postoperatively | Less PONV in the OFA group in the first 4 h after the surgery |
| Dagher [68] | 2025 | 58 | LSG, LYGB | OFA vs. opioid-based anesthesia | Dexmedetomidine infusion 0.2–0.5 mcg/kg/h i.v., Dexamethasone 8 mg i.v., Ketamine loading dose 0.2 mg/kg, infusion 0.15 mg/kg/h i.v., lidocaine loading dose 1.5 mg/kg, infusion 1.5 mg/kg/h i.v., magnesium sulfate loading dose 50 mg/kg, infusion 8 mg/kg/h i.v. | Ondansetron | Incidence of PONV 4 h, 24 h postoperatively | No difference between the groups |
| Menck [69] | 2022 | 60 | LYGB | OFA vs. opioid-based anesthesia | Dexmedetomidine loading dose 0.5 mcg/kg i.v., Ketamine 25 mg i.v., Lidocaine loading dose 1.5–2 mg/kg, infusion 1 mg/kg/h i.v., Magnesium sulfate loading dose 40 mg/kg i.v., infusion 5 mg/kg/h i.v., clonidine infusion 0.15 mcg/kg/h i.v. | Dexamethasone, droperidol, ondansetron | PONV incidence at PACU and on the first post-surgery day | No difference between the groups |
| Campos-Pérez [70] | 2022 | 40 | LYGB | OFA vs. opioid-based anesthesia | Dexmedetomidine loading dose 1–1.5 mcg/kg, infusion 0.3–0.7 mcg/kg/min i.v., ketamine loading dose 0.12 mg/kg, infusion 0.15 mcg/kg/min i.v., lidocaine 1 mg/kg, infusion 1 mg/kg i.v., magnesium sulfate loading dose 30–50 mg/kg, infusion 10 mg/kg/min i.v. | Unspecified | PONV incidence within 24 h postoperatively | No difference between the groups |
| Soudi [71] | 2022 | 60 | LBS | OFA vs. opioid-based anesthesia | Dexmedetomidine loading 1 mcg/kg, infusion 0.5 mcg/kg/h i.v., ketamine loading 0.5 mg/kg, infusion 0.25 mg/kg/h i.v. | Unspecified | Number of PONV episodes within 24 h postoperatively | No difference between the groups |
3.2.1. Non-Opioid Analgesics
The utilization of nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol is advocated by both the ERAS [15] and Procedure Specific Postoperative Pain Management (PROSPECT) guidelines for LSG [72], based on data extrapolated from the broader obese population [73], as specific evidence regarding PONV incidence is scarce. Yet, 3 RCTs assessing various bariatric procedures consistently endorse the use of NSAIDs and paracetamol owing to their opioid-sparing effects, which are associated with a reduced incidence of PONV. In a study conducted by Cooke et al., patients undergoing LSG and receiving paracetamol experienced shorter hospital stays and a lower incidence of PONV; however, these differences did not achieve statistical significance, likely due to the limited sample size [40]. A recent study by Amin et al. demonstrated that ketorolac was highly effective, as its administration was correlated with a decrease in postoperative nausea from 87% to 42% and vomiting from 64% to 13%, compared to the ibuprofen group, alongside reductions in both intraoperative and postoperative opioid consumption [41]. These findings align with a study by Govindarajan et al., which demonstrated a reduction in PONV incidence from 28% to 4% in the ketorolac group compared to the placebo group [42], along with improved pain and recovery scores.
3.2.2. Dexmedetomidine
Dexmedetomidine, an alpha-2 adrenergic agonist, has demonstrated a beneficial effect on the incidence of PONV in patients undergoing bariatric surgery [74] by decreasing the need for opioids and anesthetics, with most RCTs endorsing its protective role [21,43,44,45]. Hamed et al. compared dexmedetomidine infusion with remifentanil and reported a significantly lower incidence of PONV: 10% vs. 3% in the dexmedetomidine group, with better pain scores [43]. In this study, dexmedetomidine supplanted opioids intraoperatively without hemodynamic instability. In line with these results, Ziemann-Gimmel et al. demonstrated that fewer patients experienced PONV in the TIVA with propofol, dexmedetomidine, and ketamine group (20%) compared to 37.3% in the conventional, opioid-based anesthesia utilizing volatile anesthetics [21]. However, in their study, multiple interventions overlapped, making it difficult to estimate the isolated impact of dexmedetomidine. Furthermore, Khail et al. [44] in a three-arm study showed superiority in PONV reduction using dexmedetomidine over both the control group and the ketamine group and also improved recovery. Finally, Abu-Halaweh et al., in a single study comparing 24 h postoperative dexmedetomidine and morphine infusion, demonstrated a decreased incidence of nausea in the dexmedetomidine group (26.7% vs. 63.3%) [45]. Still, the morphine dosing regimen as a comparator was relatively high (3 mg/h) for LBS and more than standard in most of the centers.
In contrast to these RCTs, Zeeni et al. [46] found no difference between patients administered a loading dose (1 mcg/kg) of dexmedetomidine with a 30 min infusion and those receiving a placebo. Nevertheless, in this study, dexmedetomidine infusion was limited to a brief period during the surgical procedure, which may have attenuated its potential beneficial effects. Similarly, in a study conducted by Backhamees [47], no reduction in PONV was identified; however, their research demonstrated that dexmedetomidine enhanced pain scores, improved the recovery profile, and decreased opioid requirements relative to placebo.
3.2.3. Lidocaine
The prevailing view recognizes intravenous administration of lidocaine, a sodium channel blocker, as a co-analgesic that improves recovery, decreases opioid consumption, and reduces the PONV incidence in patients undergoing LBS [75]. However, not only is the evidence not unequivocal and conflicting, but also concerns arise from the potential risk of bradycardia or hypotension and the risk of toxicity, especially if combined with different forms of regional analgesia.
In studies comparing lidocaine infusion with placebo, lidocaine superiority with regard to PONV reduction was shown in four studies [48,49,50,51]. In a study by Oliveira Jr., investigators observed improved recovery outcomes and a decreased incidence of PONV in Postoperative Care Unit (PACU) among patients who received a 1.5 mg/kg bolus and a 2 mg/kg/h infusion intraoperatively; however, due to a limited sample size of 25 patients per group, the difference did not reach statistical significance [48]. Notably, the same dose used in a study by Oliveira et al. was effective in preventing PONV for up to 24 h post-surgery [49]. In this latter study, the lidocaine group had both lower opioid consumption and lower pain scores during the immediate postoperative period. A study by Sherif et al., which examined various dosing regimens of lidocaine, including a bolus of 2 mg/kg and an infusion of 1.5 mg/kg/h, found that patients experienced less vomiting; however, the differences observed were not statistically significant [50]. Consistent with previous RCTs, Sun et al. demonstrated the beneficial effects of a lidocaine bolus of 1.5 mg IV combined with a 2 mg/kg/h infusion compared to placebo, by decreasing nausea from 63.63% in the control group to 42.42% in the lidocaine group, and reducing vomiting from 42.42% to 15.15%, respectively [51]. Interestingly, their study also indicated that lidocaine infusion was equally effective in reducing PONV and enhancing recovery as a transversus abdominis plane (TAP) block in obese patients undergoing LSG or LYGB.
Conversely, multiple investigations have cast doubts on the justification for administering lidocaine as an adjunct analgesic in bariatric surgery [52,53,54]. An RCT conducted by Yurttas et al., involving 137 patients, revealed no significant advantages of lidocaine administration concerning the incidence of PONV or opioid consumption within the first four hours postoperatively [52]. Unlike other studies, Yurttas et al. utilized lean body weight (LBW) for dosing adjustments, which may have resulted in a lower dosage compared to studies that employed adjusted body weight (ABW). Consistent with their findings, Plass et al. also failed to demonstrate the superiority of lidocaine infusion, finding no significant differences in PONV incidence, pain scores, or recovery metrics [53]. Furthermore, this study is among the few that examined potential hemodynamic instability in bariatric patients receiving perioperative lidocaine infusion; notably, refractory hypotension was observed in 6% of patients, whereas none were recorded in the control group. As a result, patients in the treatment group required increased vasopressor support, raising concerns regarding the safety profile of such a strategy. Lastly, Sakata et al. [54] reported no significant improvement in PONV incidence following lidocaine use; however, patients in the study group had significantly lower morphine consumption. It is essential to note that their study had a relatively small sample size (n = 58), which may have limited its statistical power to detect potential differences.
An important practical issue concerning the lidocaine dosing protocol in patients undergoing LBS is investigated by Alimian et al., who conducted a comparative study between a 1 mg/kg/h infusion and a 2 mg/kg/h infusion utilizing ideal body weight (IBW) [55]. The investigators found no significant differences in any of the assessed parameters, thereby concluding that lower dosages may be equally efficacious as higher dosages in managing postoperative pain and PONV within this patient population.
3.2.4. NMDA Receptor Antagonists
Ketamine
Ketamine and its isomer S-ketamine are antagonists of NMDA receptors. Due to their analgesic and opioid-sparing properties, they are used as co-analgesics in bariatric surgery, exerting a beneficial influence on pain scores and opioid requirements [76]. Nevertheless, their impact on PONV prevalence remains controversial, as six identified RCTs have consistently shown no improvement in this aspect [44,56,57,58,59,60]. In a study conducted by Khail et al., patients receiving ketamine bolus and infusion demonstrated reductions in both pain scores and postoperative morphine consumption compared to placebo and dexmedetomidine groups [44]. Hasanein et al. [56] compared 60 patients assigned to either a remifentanil and ketamine infusion group or a placebo group. While this comparison revealed a substantial reduction in postoperative opioid consumption, which was potentially attributable, in part, to the attenuating effect of NMDA receptor antagonists on remifentanil-induced hyperalgesia [77], lower morphine consumption was not associated with a decreased incidence of PONV [56]. A similar relationship was observed in studies by Yang et al., Zhang et al., and Mehta et al. [57,59,60]. To some extent, this discrepancy may be explained by specific emetic properties of ketamine [78], which could balance the positive effects of opioid reduction.
Contrary to the aforementioned studies, Adhikary et al. [58], in their comparatively larger study involving 126 patients, did not demonstrate an opioid-sparing effect or enhanced pain scores, and consistently observed no difference in PONV. However, in contrast to other studies, patients received only a single bolus of ketamine rather than an infusion, which may have been a significant factor.
Magnesium Sulfate
Magnesium sulfate, also acting as an NMDA receptor antagonist, not only has opioid-sparing properties but also reduces PONV in the general population [79]. It is frequently incorporated as part of a multimodal drug regimen, including in bariatric surgery. Although the studies outlined in the OFA section utilized magnesium sulfate, only a single RCT evaluated its effect independently as a subgroup [58], and this study demonstrated no significant beneficial outcomes. Nevertheless, in that study, the dosage of magnesium sulfate was relatively low (2 g) and administered regardless of patient weight, potentially leading to underdosing in obese patients and, consequently, reduced efficacy. Given the limited data available, further RCTs are required to comprehensively evaluate the impact of magnesium sulfate on PONV in bariatric surgical procedures.
3.2.5. Gabapentinoids: Gabapentin and Pregabalin
Gabapentin and pregabalin are derivatives of the neurotransmitter gamma-aminobutyric acid (GABA), which bind to the α2δ subunit of the voltage-gated calcium channel, thereby inhibiting excitatory synaptic transmission. In addition to their use as anticonvulsants or for the treatment of neuropathic pain, these medications may be administered as co-analgesics during various surgical procedures, including bariatric surgery, to enhance pain control and reduce PONV [80]. Nevertheless, gabapentinoids represent the least studied co-analgesics used in LBS, with only four studies evaluating their impact on PONV and one study in progress [61,62,63,64,81].
Schulmeyer et al. demonstrated a beneficial impact of a pregabalin dose of 150 mg compared to placebo regarding PONV incidence and postoperative pain [61] in their study of 80 enrolled patients. The pregabalin group had a lower incidence of PONV at 25.6% (10/39) compared to 46.3% in the placebo group (19/41), respectively, resulting in an absolute risk reduction of approximately 20%, which is clinically significant. Additionally, morphine use and pain scores were superior to those of the placebo. Moreover, the pregabalin group experienced no apparent side effects, such as excessive sedation, which would impair recovery and diminish the mentioned benefits [61]. However, a critical limitation of this study is the assumption that multimodal analgesia or simple analgesics are not used, restricting pregabalin’s administration to cases where these alternatives are not feasible, which is mainly hypothetical [72]. Moreover, Alimian et al. found that 300 mg of pregabalin reduced the risk of PONV compared to placebo [62], achieving absolute risk reductions of 36.7% for nausea and 30% for vomiting. The study’s shortcoming is the lack of reporting on the incidence of oversedation and delayed recovery, as pregabalin, especially at higher doses, can have a sedative effect that should be avoided in the ERAS protocol [15]. In line with these results, Salama et al. reported a significant reduction in PONV in patients receiving 75 mg of pregabalin with dexmedetomidine infusion [63] up to 18 h after the LSG. However, in their study, the potentially beneficial impact of pregabalin overlaps with that of dexmedetomidine, therefore making it difficult to determine the effect of pregabalin as a sole co-analgesic. Finally, PONV risk reduction is also supported by Hassani et al. [64], who reported a 10% vs. 33.3% PONV incidence in a group that received gabapentin 100 mg. The demonstrated efficiency of even such a small dose of gabapentinoids in PONV prophylaxis is promising, and it should entail larger, multi-center dose-finding studies to fully assess their potential in improving post-operative outcomes in LBS.
3.2.6. Opioid-Free Anesthesia (OFA)
OFA is characterized as a heterogeneous group of techniques wherein opioids are not administered during general anesthesia [82]. OFA encompasses comprehensive multimodal analgesia, advancing from opioid-sparing strategies to the complete elimination of intraoperative opioids. Due to variations in dosing regimens and the potential application of regional anesthesia techniques across studies, RCTs evaluating this method are heterogeneous, and comparing them yields conflicting results. Yet, concerning PONV prophylaxis, the recent network meta-analyses demonstrate OFA superiority compared to opioid-based and opioid-sparing anesthesia, with an overall relative risk reduction of 40% [83]. Conversely, although the prevailing consensus regards OFA as a protective measure against PONV, the number of RCTs is limited. There are significant discrepancies among these studies, and debates persist concerning the specific time points at which the incidence and severity of PONV are evaluated. Furthermore, OFA raises concerns regarding potential hemodynamic instability and patient safety, which warrant careful consideration [84].
In their pivotal study, Mulier et al. demonstrated a statistically significant PONV reduction from 63% in the opioid-based group using sufentanil to 13% in the OFA group, measured during the immediate postoperative period in PACU [65]. The benefits of OFA also encompassed reduced morphine consumption and improved recovery outcomes. However, the authors did not assess PONV in the following hours, and it could not be concluded whether the antiemetic protective effect was prolonged. Aligning with these findings, a study by Mieszczanski et al. reported that the superiority of OFA concerning PONV incidence was observed only during the first hour post-surgery, with no significant difference from the sixth hour onwards. The authors attributed this pattern to the short half-lives of the co-analgesics administered exclusively during surgery [66]. Supporting these observations, Clanet et al. noted a reduction in PONV within the first four hours postoperatively in the OFA group [67]. Nevertheless, in a previous study by Ziemann-Gimmel et al. [21], this beneficial effect persisted up to 24 h in patients receiving OFA with propofol TIVA, rendering it more clinically significant. Conversely, several studies have failed to demonstrate any improvement in PONV associated with the use of OFA [68,69,70,71]. These discrepancies, together with the absence of definitive evidence, contribute to the ongoing debate regarding the role of OFA in bariatric surgical procedures.
3.3. Pharmacological Prophylaxis
Given that PONV prophylaxis using a single drug in bariatric surgery may prove insufficient, current ERAS guidelines advocate for a multimodal approach [15]. This strategy involves the administration of one antiemetic agent from at least three of the following six classes of drugs: long-acting corticosteroids, such as dexamethasone; 5-hydroxytryptamine receptor antagonists (5-HT); butyrophenones; neurokinin-1 (NK-1) receptor antagonists; antihistamines; and anticholinergics [15]. We identified 16 RCTs evaluating such antiemetics listed in Table 3.

Table 3.
Pharmacological PONV prophylaxis.
Table 3.
Pharmacological PONV prophylaxis.
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Dosing Regimen | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|---|
| Didehvar [85] | 2013 | 76 | LBS | Dexamethasone vs. placebo | Dexamethasone 8 mg i.v. | Palonosetron | 4-point PONV scale at 2, 6, 12, 24, 72 h postoperatively | No difference between the groups |
| Benevides [86] | 2013 | 90 | LSG | Ondansetron vs. dexamethasone + ondansetron + haloperidol | Dexamethasone 8 mg i.v., Ondansetron 8 mg i.v., Haloperidol 2 mg i.v. | Unspecified | 11-point Verbal Rating Scale at 0–2, 2–12, 12–24, 24–36 h postoperatively | Less nausea in the dexamethasone, haloperidol, and dexamethasone group compared to the ondansetron-only group |
| Spaniolas [22] | 2020 | 83 | LSG | Propofol TIVA, aprepitant, and transdermal scopolamine vs. volatile anesthesia | Aprepitant 80 mg p.o., Dexamethasone 8 mg i.v., ondansetron 4 mg iv., scopolamine transdermal patch | Metoclopramide, compazine | 10-point Verbal Rating Scale at 1, 4, 12, 24 h postoperatively | PONV scores lower at all the time points in the study group |
| Bataille [4] | 2016 | 117 | LSG | Dexamethasone and ondansetron vs. placebo | Dexamethasone 4 mg i.v., ondansetron 4 mg i.v. | TIVA | PONV incidence within 24 h postoperatively and 11-point Verbal Rating Scale | Less PONV in the study group 45% vs. 54% in the control group |
| Burcu [87] | 2024 | 100 | LSG | Ondansetron vs. Palonosetron | Ondansetron 8 mg i.v., Palonosetron 75 mcg i.v. | Unspecified | PONV incidence during hospitalization | Less PONV in the palonosetron group |
| Kaloria [88] | 2017 | 22 | LSG | Ondansetron vs. palonosetron | Ondansetron max 8 mg i.v., palonosetron max 75 mcg | Dexamethasone | PONV incidence at 0, 0–6, 6–12, 12–24, 24–48, 48–72 h postoperatively | No difference between the groups |
| Ortiz [89] | 2024 | 400 | LSG | Aprepitant vs. placebo | Aprepitant 80 mg p.o. | Dexamethasone, ondansetron, metoclopramide | Rhodes index at 0, 6, 12, 24 h postoperatively | Less PONV in the aprepitant group in the first 24 h postoperatively |
| Sinha [90] | 2014 | 125 | LYGB, LGB | Aprepitant vs. placebo | Aprepitant 80 mg p.o. | Ondansetron | PONV incidence at 30 min, 1, 2, 6, 24, 48, 72 h postoperatively | Less vomiting in the aprepitant group, 3% vs. 15% in the placebo group |
| Ashoor [91] | 2022 | 90 | LSG | Aprepitant vs. mirtazapine vs. placebo | Aprepitant 80 mg p.o., mirtazapine 30 mg p.o. | Dexamethasone | 4-point Verbal Descriptive Scale every 4 h within 24 h postoperatively | Less PONV in the aprepitant group 34.5% vs. mirtazapine, 35.7% and placebo, 93.1% |
| Shahinpour [92] | 2024 | 83 | LSG, LYGB | Haloperidol vs. promethazine | Haloperidol 2 mg i.m., promethazine 25 mg i.m. | Dexamethasone, ondansetron | Numeric Verbal Rating Scale at 6 and 24 h postoperatively | Less PONV in the haloperidol group, 20% vs. promethazine 40% |
| Talebpour [93] | 2017 | 80 | Laparoscopic gastric plication | Metoclopramide vs. promethazine | Metoclopramide 10 mg i.v., promethazine 50 mg i.m. | Dexamethasone, ondansetron | 4-point PONV scale within 48 h postoperatively | Less PONV in the promethazine group 41% vs. 97.5% in the metoclopramide group |
| Moussa [94] | 2007 | 120 | LSG, LYGB, LGB | Granisetron vs. granisetron + droperidol vs. granisetron + dexamethasone vs. placebo | Dexamethasone 8 mg i.v., droperidol 1.25 mg i.v., granisetron 1 mg i.v. | Unspecified | PONV incidence within 24 h postoperatively | PONV incidence 30% in granisetron, 30% granisetron + droperidol, 20% granisetron + dexamethasone vs. 67% in the placebo group |
| Ebrahimian [95] | 2023 | 130 | LSG | Ondansetron vs. metoclopramide vs. granisetron vs. metoclopramide + ondansetron | Ondansetron max 8 mg i.v., metoclopramide max 10 mg i.v., granisetron 2 mg i.v. | Dexamethasone | PONV impact scale within 48 h postoperatively | No difference between the groups |
| Pourfakhr [96] | 2021 | 82 | LSG | Diphenhydramine vs. control | Diphenhydramine 0.4 mg/kg i.v. | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the diphendydramine group 30% vs. 56% in the control group |
| Ahmed [97] | 2025 | 210 | LSG | Cyclizine vs. metoclopramide vs. ondansetron | Cyclizine 50 mg i.v., metoclopramide 10 mg i.v., ondansetron 8 mg i.v. | Dexamethasone | 11-point Likert scale within 24 h postoperatively | No difference between the groups |
| Atif [98] | 2022 | 100 | LSG | Scopolamine vs. control | Scopolamine 10 mg i.v. | Metoclopramide | PONV intensity scale at 2 and 24 h postoperatively | No difference between the groups |
3.3.1. Dexamethasone
Dexamethasone is an established antiemetic agent used to decrease PONV incidence [36] and is endorsed for routine application in bariatric surgery by the American Society for Metabolic and Bariatric Surgery (ASMBS) [99]. It is regarded as particularly advantageous in laparoscopic surgeries, as it may enhance pain scores and decrease opioid consumption [15]. Consequently, it is incorporated into most multi-drug prophylactic regimens against PONV in laparoscopic bariatric surgery (LBS), given that its single-dose benefits outweigh potential risks, such as transient hyperglycemia [100]. Nonetheless, the number of RCTs dedicated explicitly to assessing its isolated efficacy in preventing PONV within this context remains limited. Didehvar et al. reported in their study that the addition of dexamethasone to the 5-HT3 antagonist palonosetron was ineffective in reducing PONV risk among high-risk bariatric surgery patients [85]. Consistent with these findings, Benevides et al. [86] observed no statistically significant difference in PONV incidence between the groups receiving ondansetron alone, ondansetron combined with dexamethasone, and ondansetron with haloperidol, respectively. However, their study noted that groups administered dexamethasone experienced lower pain scores and reduced postoperative opioid consumption.
Dexamethasone is more frequently evaluated as part of a multimodal therapeutic approach. In such a study, Spaniolas et al. demonstrated that dexamethasone, combined with aprepitant, transdermal scopolamine, and ondansetron, significantly improved verbal scores related to nausea and vomiting at all measured time points compared to the control group [22]. Of clinical importance is the elimination of discharge delays associated with PONV, contrasting with the 9.5% incidence observed in the control group. In contrast to these results, and highlighting the difficulty of preventing PONV among high-risk bariatric patients, an RCT conducted by Bataille et al. revealed that the combination of dexamethasone and ondansetron did not effectively prevent PONV in patients undergoing laparoscopic sleeve gastrectomy (LSG) [4]. It is noteworthy that the dose of dexamethasone used in this study was relatively low (4 mg), which may have contributed to the lack of efficacy of this prophylactic strategy.
3.3.2. 5-HT3 Antagonists
5-HT3 antagonists, such as ondansetron, palonosetron, or granisetron, are recommended for both prophylaxis and rescue treatment of PONV and are regarded as the gold standard for these applications [36,101]. Consequently, they are frequently incorporated into combination therapy with antiemetics of different classes. Although they are considered first-line treatments, there exists limited evidence supporting the superiority of one particular agent over others. Burcu et al. demonstrated the superiority of palonosetron, administered at a dose of 1 mcg/kg of total body weight, in comparison to ondansetron, given at a fixed standard dose of 8 mg intravenously [87]. In their RCT, palonosetron was associated with a lower incidence of PONV during the first 24 h. Furthermore, only 2% of patients in the study group required rescue antiemetics, compared to 68% of patients who received ondansetron, marking a significant difference. The authors attribute this discrepancy to the longer duration of action of palonosetron, its weight-adjusted dosing regimen, and enhanced efficacy in preventing PONV [87]. Conversely, a study conducted by Kaloria et al. [88] found that the difference in PONV incidence between the 8 mg ondansetron group and the 75 mcg palonosetron group, both with dexamethasone, was too minor to attain statistical significance. However, the study’s limited sample size, as it was designed as a pilot study, indicates the need for larger RCTs.
3.3.3. NK-1 Receptor Antagonists
RCTs conducted in patients undergoing bariatric surgery have demonstrated that aprepitant, an NK-1 receptor antagonist, is a promising and potent agent for preventing PONV [102], particularly since it has no sedative effects. In a recent meta-analysis, patients receiving aprepitant had a lower incidence of PONV, at 17.7% compared to 30.7% in the control group [102]. Furthermore, the protective effect was sustained for up to 12 h postoperatively following a single dose of aprepitant. An extensive study involving 400 participants, conducted by Ortiz et al., revealed that administering 80 mg of aprepitant one hour preoperatively, in addition to a multimodal prophylaxis regimen comprising dexamethasone, ondansetron, and metoclopramide, significantly reduced nausea, vomiting, and the need for rescue medication up to 24 h after the LSG [89]. Similarly, Sinha et al. reported a reduction in the incidence of postoperative vomiting, along with a delay in the onset of the first vomiting episode [90], following the administration of 80 mg of aprepitant one hour before the surgery. However, their study found no statistically significant differences in the mean nausea verbal score. Aligning with the aforementioned RCTs, Ashoor et al. demonstrated a superior antiemetic effect of 80 mg of aprepitant combined with 8 mg of dexamethasone compared to dexamethasone alone or mirtazapine [91], revealing a PONV incidence of 34.5% in the aprepitant group versus 93.1% in the dexamethasone-alone group. Additionally, the authors reported that patients in the aprepitant group achieved the highest satisfaction scores, which constitutes a clinically significant finding.
Considering the increasing popularity of aprepitant, a significant knowledge gap remains: the optimal dosage has yet to be identified. This matter is of particular importance, as the standard dose recommended by the European Society of Anesthesiology and Intensive Care (ESAIC) guidelines is 40 mg [36]; 80 mg is the most extensively studied in the LBS, and 125 mg is also evaluated in some studies [103]. Moreover, to our knowledge, no RCTs dedicated to bariatric surgery have evaluated other NK-1 antagonists such as fosaprepitant and their efficacy in preventing PONV, which would be of considerable interest given their proven effectiveness in gastrointestinal surgery [104].
3.3.4. Dopamine Receptor Antagonists
This drug class encompasses dopamine receptor antagonists, including antipsychotics such as butyrophenones, derivatives of phenothiazine, and the prokinetic agent metoclopramide. Butyrophenones, exemplified by droperidol and haloperidol, possess established antiemetic properties and have been used as first-line PONV prophylaxis. Yet, their usage has diminished due to concerns regarding their safety profile and sedative effects, which are considered undesirable within the ERAS protocol [15]. These disadvantages must be carefully considered, rendering this class of drugs more suitable as a rescue therapy rather than a first-line treatment [36].
In a study conducted by Shahinpour et al., a multi-drug prophylactic regimen comprising dexamethasone, ondansetron, and haloperidol demonstrated greater efficacy in preventing PONV compared to promethazine [92]. The study reported a twofold reduction in PONV incidence within the haloperidol group, with 20% of patients experiencing PONV versus 40% in the control group during recovery among those undergoing bariatric surgery. Furthermore, both the incidence and severity of PONV were diminished following haloperidol administration. Nevertheless, the authors did not evaluate the sedation levels or the time to ambulation in the patients, which raises concerns regarding whether the benefits of the observed effect surpass potential risks [92].
Promethazine, alternatively, possesses both antipsychotic and antihistaminic properties, with a dose-dependent sedative effect [36,105]. Talebpour et al. observed that promethazine was more effective than metoclopramide in preventing PONV, reducing the incidence of PONV by 41% in comparison to 95.5% [93]. Nonetheless, oversedation occurred more frequently in the promethazine group, with a statistically significant difference observed in the mean duration of ambulation during the post-operative period [93], which constitutes a profound adverse effect given that early ambulation is a priority for this patient population.
On the other hand, Moussa et al. [94] demonstrated that the combination of haloperidol with granisetron did not produce a statistically or clinically significant effect on the incidence of PONV among patients undergoing LBS, thereby questioning its role within a multi-drug antiemetic regimen.
Although metoclopramide seems to have low antiemetic efficacy with a number needed to treat (NNT) of 8–10 [106], an RCT by Ebrahimian et al. demonstrated a synergistic effect of the combination of ondansetron and metoclopramide, exceeding the efficacy of monotherapy with metoclopramide, ondansetron, or granisetron [95]. However, the sample size was relatively small, requiring larger RCTs to confirm this effect.
3.3.5. Histamine Receptor Antagonists
Antihistamines such as dimenhydrinate have demonstrated efficacy in preventing PONV [107]. However, there is a limited number of studies on the bariatric population, and concerns remain regarding their sedative effects, which restrict their use [36]. Addressing these issues, Pourfakhr et al. reported that patients undergoing LSG who received diphenhydramine, in comparison to those receiving a placebo, experienced a significantly lower incidence of PONV (30% versus 56% in PACU, and 40% versus 66% within 24 h), as well as improved pain scores [96]. While increased sedation scores indicated a sedative effect of diphenhydramine, this was not associated with prolonged PACU stays or delayed recovery, which holds clinical significance [96].
Conversely, Ahmed et al. observed no significant difference in the incidence of PONV between the groups administered dexamethasone and those receiving histamine receptor antagonists such as cyclizine, metoclopramide, or ondansetron [97], considering these interventions to be equally efficacious in preventing PONV without any adverse effects attributable to the medications.
3.3.6. Muscarinic Receptor Antagonists
Scopolamine is an anticholinergic medication that, when used as a long-acting transdermal patch, possesses the potential to reduce the incidence of PONV during the first 24 h following surgery and potentially beyond [108]. The primary adverse effects associated with this strategy include xerostomia, blurred vision, and sedative effects. In one RCT dedicated to bariatric surgery, Atif et al. observed no advantage of intravenous scopolamine administration before stapling the gastric sleeve regarding PONV incidence [98] in patients undergoing LSG. Similarly, in one study involving major laparoscopic surgeries, including bariatric procedures, limited benefits from the transdermal application of scopolamine were demonstrated [109]. On the other hand, Spaniolas et al. [22] successfully integrated the transdermal scopolamine patch into multimodal prophylaxis for PONV; however, due to the study design, the efficacy of this technique cannot be evaluated independently.
In conclusion, the role of scopolamine in preventing PONV in bariatric surgery remains unclear; however, the extended duration of action associated with the transdermal patch may be advantageous and warrants further investigation.
3.4. Regional Anesthesia
Current evidence consistently recommends utilizing regional anesthesia techniques in obese patients undergoing bariatric surgery, as they reduce opioid use and, therefore, decrease the risk of PONV [15,110]. Such methods include local wound infiltration, intraperitoneal administration of local anesthetics, TAP Block, Erectus Spinae Plane Block (ESPB), Quadratus Lumborum Block (QLB), and External Oblique Intercostal Fascial Plane Block (EOIB). We identified 19 such studies, which are listed in Table 4.

Table 4.
Regional Anesthesia in Bariatric Surgery.
Table 4.
Regional Anesthesia in Bariatric Surgery.
| Author | Year | Number of Participants | Type of Surgery | Intervention Assessed | Control group | Antiemetics Used | PONV Outcome Measure | Main Results Regarding PONV |
|---|---|---|---|---|---|---|---|---|
| Shariat [111] | 2025 | 40 | LSG | QLB | Wound infiltration with local anesthetics | Ondansetron | PONV incidence within 24 h postoperatively | No difference between the groups |
| Ma [112] | 2019 | 179 | LYGB | Wound infiltration with liposomal bupivacaine | Wound infiltration with bupivacaine alone | Dexamethasone, transdermal scopolamine | PONV score every 4 h until discharge | No difference between the groups |
| Neishaboury [113] | 2025 | 72 | LSG | Intraperitoneal ropivacaine alone vs. intraperitoneal ropivacaine with dexmedetomidine | Intraperitoneal placebo | Ondansetron | PONV incidence within 24 h postoperatively | Less PONV in the intraperitoneal ropivacaine with dexmedetomidine 8.3% vs. ropivacaine alone 29.2% vs. 50% placebo |
| Alamdari [114] | 2018 | 120 | LSG | Intraperitoneal bupivacaine | Control group | Unspecified | PONV incidence at unspecified time | Less PONV in the bupivacaine group 11.7% vs. 41.7% control |
| Omar [115] | 2019 | 100 | LBS | Intraperitoneal bupivacaine | Intraperitoneal placebo | Dexamethasone, ondansetron | PONV incidence within 24 h postoperatively | No difference between the groups |
| Kaur [116] | 2022 | 104 | LBS | Intraperitoneal ropivacaine | Intraperitoneal placebo | Dexamethasone, droperidol, ondansetron | Antiemetic use at 1, 2, 4, 6, 24, 48 h postoperatively | No difference between the groups |
| Sherwinter [117] | 2008 | 30 | Laparoscopic adjustable banding | Intraperitoneal bupivacaine | Intraperitoneal placebo | Dexamethasone, metoclopramide | PONV incidence at 30 min, 6, 12, 24, 48 h postoperatively | No difference between the groups |
| Mittal [118] | 2018 | 60 | LSG | TAP Block | No intervention | Unspecified | PONV incidence at 30 min, 3, 6, 12, 24, 48 h postoperatively | Less PONV in the TAP Block group |
| Emile [119] | 2019 | 92 | LBS | TAP Block | No intervention | Ondansetron | 4-point PONV scale within 24 h postoperatively | Less PONV in the TAP Block group |
| Ibrahim [120] | 2014 | 63 | LSG | Subcostal TAP Block | Wound infiltration with local anesthetics | Dexamethasone, ondansetron | PONV incidence at PACU and at 24 h postoperatively | No difference between the groups |
| Zhou [121] | 2024 | 71 | LSG | TAP Block | No intervention | Dexamethasone, ondansetron, droperidol | PONV score at 2, 6, 12, 24 h postoperatively | Less PONV in the TAP Block group |
| Albrecht [122] | 2013 | 70 | LYGB | Subcostal TAP Block + wound infiltration with local anesthetic | Wound infiltration with local anesthetic | Dexamethasone, ondansetron, granisetron | PONV incidence during phase I recovery, 0–24, 24–48 h postoperatively | No difference between the groups |
| Saber [123] | 2019 | 90 | LSG | TAP Block with bupivacaine, TAP Block with bupivacaine + adrenaline | No intervention | Dexamethasone, metoclopramide | 5-point PONV score every 6 h for 48 h | No difference between the groups |
| Karaveli [124] | 2025 | 40 | LSG | ESP Block | No intervention | Ondansetron | 4-point Likert scale within 24 h postoperatively | No difference between the groups |
| Mostafa [125] | 2021 | 60 | LBS | ESP Block | Sham block | Dexamethasone | PONV incidence within 24 h postoperatively | No difference between the groups |
| Omran [126] | 2021 | 30 | LSG, LYGB | QLB | Sham block | Unspecified | PONV incidence within 24 h postoperatively | Less PONV in the QLB group 13.3% vs. 46.7% |
| Xue [127] | 2022 | 225 | LSG | TAP Block, QLB | No intervention | Granisetron | PONV incidence | No difference between the groups |
| Ozel [128] | 2025 | 60 | LSG | EOIB | No intervention | Dexamethasone, ondansetron | 5-point Verbal Descriptive Scale within 24 h postoperatively | Fewer patients requiring antiemetics in the EOIB group, 16.7% vs. 40% |
| Turunc [129] | 2024 | 58 | LSG | EOIB | M-TAPA Block | Ondansetron | 5-point Verbal Descriptive Scale at 0, 3, 6, 12, 18, 24 h postoperatively | No difference between the groups |
3.4.1. Local Wound Infiltration
Local wound and trocar insertion site infiltration is regarded as a practical alternative to regional blocks owing to its simplicity [110]. However, the research concerning the incidence is minimal. In an RCT by Shariat et al., the authors found no difference regarding PONV incidence comparing local anesthetic wound infiltration to posterior QLB in patients undergoing LSG [111], thereby indicating the efficacy of the former method. Additionally, the type of local anesthetic employed does not appear to have a definitive impact [112].
3.4.2. Intraperitoneal Local Anesthetic Installation
Although the intraperitoneal administration of local anesthetics is currently not recommended in bariatric surgery due to ongoing controversies [72], emerging evidence suggests potential benefits, including an improvement in the incidence. Neishaboury et al. observed that intraperitoneal administration of 0.5% ropivacaine with 1 mcg/kg dexmedetomidine resulted in a significantly reduced incidence of PONV within 24 h, compared to both placebo (8.3% versus 50%) and ropivacaine alone (29.2%) [113]. The precise mechanism, whether attributable to local or systemic effects of dexmedetomidine, remains a topic of debate. Consistent with these findings, Alamdari et al. reported a lower incidence of PONV in patients receiving intraperitoneal bupivacaine (11.7% versus 41.7%) [114]. On the other hand, other studies have not demonstrated any improvement in PONV incidence [115,116,117], thereby casting doubt on the overall efficacy of this approach. Although further large-scale RCTs are necessary to comprehensively evaluate this technique, alternative methods supported by more unequivocal evidence may be preferred.
3.4.3. TAP Block
The TAP block is a regional anesthetic technique that provides analgesia to the anterior and lateral abdominal wall [130]. Two RCTs by Emile and Mittal demonstrated the superiority of the TAP block in comparison to control groups [118,119]. In these studies, it was consistently observed that not only was the incidence and severity decreased in the TAP group, but also the time to ambulation, pain scores, and overall patient satisfaction [118,119]. Similarly, a study evaluating the subcostal TAP block compared to local anesthetic infiltration by Ibrahim et al. noted a marginal reduction in nausea and vomiting among patients receiving TAP blocks [120]. Zhou reported a decrease in PONV in patients who received TAP blocks with dexmedetomidine and esketamine, compared to those under opioid-based anesthesia. However, the benefit of regional anesthesia in this study is difficult to ascertain due to numerous confounding variables between groups [121]. In contrast, two randomized trials conducted by Albrecht and Saber found no significant differences in the rates of nausea or vomiting between the experimental and control groups [122,123]. Considering that only some of the RCTs evaluating TAP block in bariatric surgery assess PONV, and given their small sample sizes, the overall impact of TAP block, specifically on PONV incidence, is subject to debate and necessitates further randomized controlled trials, particularly comparisons with other regional anesthesia techniques. Nonetheless, the use of the TAP block as part of multimodal analgesia presents undeniable benefits, including a significant reduction in opioid consumption [131].
3.4.4. ESPB
ESPB is a fascial plane block wherein local anesthetic is administered between the erector spinae muscle and the thoracic transverse process [132]. The main objective is to inhibit the dorsal and ventral branches of the thoracic and abdominal nerves. In an RCT by Karaveli et al., it was demonstrated that bilateral ESPB significantly diminishes analgesic consumption; however, the sample size was too small to determine its effect on PONV [124]. Similar findings and limitations were reported in a study by Mostafa et al. [125]. In contrast, a randomized trial by Jinaworn et al. revealed no significant difference in perioperative opioid consumption in patients undergoing laparoscopic bariatric surgery (LBS). The authors suggested that this may have been attributable to routine infiltration of local anesthetic by surgeons in both the control and experimental groups, as well as the routine administration of other analgesics [133]. This study, however, did not report on PONV incidence. In conclusion, ESPB provides superior pain control compared to placebo in patients undergoing metabolic surgery and appears to decrease the risk of PONV, likely due to reduced intraoperative and postoperative opioid consumption [134].
3.4.5. QLB
One of the other regional anesthesia techniques is the QLB, wherein the needle tip is positioned between the quadratus lumborum muscle and the psoas major muscle for the administration of local anesthetic. Omran et al. demonstrated that bilateral posterior QLB significantly reduced the incidence from 46.7% in the control group to 13.3% in the QLB group, with improved pain scores and decreased opioid consumption [126]. On the other hand, Xue et al. demonstrated that despite providing better analgesia in TAPB and QLB groups, no corresponding improvement in PONV was achieved [127].
3.4.6. EOIB
EOIB is a novel technique that may reduce pain by blocking both the lateral and anterior cutaneous branches of the intercostal nerves. It may offer benefits, as it is easier to perform due to the superficial location of the fascial plane. We identified only 2 RCTs evaluating this technique in bariatric surgery. Ozel et al. demonstrated that it provides adequate analgesia in patients undergoing LSG and reduces PONV scores compared to the control group, with fewer patients requiring antiemetics 16.7% vs. 40% [128]. Another option is performing a Modified Thoracoabdominal Nerve Block Through Perichondrial Approach (M-TAPA), which has effects similar to EOIB, as demonstrated by Turunc et al. [129], and shows no significant differences in PONV incidence or pain scores. However, the number of studies investigating these novel blocks remains limited.
4. Discussion
This scoping review provides a synthesis of current evidence based on RCTs concerning perioperative methods aimed at reducing the risk and incidence of PONV in patients undergoing bariatric surgery. Although the included studies demonstrate a broad spectrum of potential preventative strategies, PONV prophylaxis remains a significant challenge and a prevalent issue among a considerable proportion of patients [1]. A key conclusion derived from this review is that PONV prophylaxis, particularly in this vulnerable group, must be multimodal, integrating diverse mechanisms of action, as no single intervention is sufficient to ensure patient comfort in this regard.
The factors influencing PONV discussed in this review include anesthesia techniques, opioid-sparing strategies such as the use of co-analgesics, pharmacological prophylaxis, and regional anesthesia.
In patients undergoing bariatric surgery, despite the practical utility and potential benefits of volatile anesthetics, propofol TIVA is deemed superior for the prevention of PONV [19,20,22,34,35]. Regarding muscle relaxation, although the effectiveness of deep neuromuscular blockade in preventing PONV remains uncertain, sugammadex should be preferred over neostigmine to mitigate the associated risks [27]. Furthermore, avoiding dehydration through the oral administration of carbohydrate fluids and ensuring adequate intravenous fluid therapy is recommended [30,31]. However, the application of hemodynamic-guided fluid therapy produces conflicting outcomes and necessitates further research [31,32], as it is not yet regarded as a standard treatment.
Given that opioid utilization is among the primary modifiable factors contributing to PONV, the implementation of opioid-sparing strategies within multimodal analgesia is unequivocally endorsed [15]. Besides the consensus that NSAIDs and paracetamol possess opioid-sparing properties, thereby reducing the likelihood of PONV [135], the selection of co-analgesics remains less definitive. Although accumulating evidence supports the administration of dexmedetomidine [21,43,44,45,74] to decrease the incidence of PONV, enhance pain management and recovery outcomes, some studies question its efficacy in preventing PONV and highlight potential adverse effects [46,47,84]. Similarly, various studies have demonstrated that lidocaine, as a co-analgesic, can decrease PONV [48,49,50,51]; however, controversies persist concerning optimal dosing regimens, safety profiles, and the combination with regional anesthesia modalities, each involving local anesthetics and presenting a potential risk of toxicity. Furthermore, conflicting results have emerged regarding lidocaine’s effectiveness and the duration of its PONV-reducing effects [52,53,54]. On the other hand, ketamine, known for enhancing pain scores and reducing opioid requirements [76], does not appear to significantly lower PONV risk [44,47,56,57,58,59], possibly due to its emetogenic properties [78]. Magnesium sulfate, although promising, currently lacks sufficient evidence to support its use for PONV mitigation [58]. Lastly, gabapentinoids are considered potentially advantageous in reducing PONV risk [61,62,63,64]; however, the optimal dosing regimen remains unresolved.
The elimination of opioids through the utilization of OFA may represent a significant, evidence-based strategy to mitigate the risk of PONV, which is supported by the increasing amount of literature [21,65,66,83]. However, as OFA is a highly heterogeneous group of techniques, differing in dosing regimens and the selection of co-analgesics, no specific model can be recommended. Moreover, as there are concerns about its safety, progressing from multimodal opioid-sparing anesthesia to OFA is not currently regarded as standard practice.
Pharmacological multi-drug prophylaxis for PONV remains a standard approach [15,36]. Given its concomitant opioid-sparing and co-analgesic properties, dexamethasone should be regarded as a first-line component of such a strategy [36], with negligible side effects associated with a single dose [36,100]. Another primary drug class, widely used and effective, includes 5-HT3 antagonists, applicable for both prophylaxis and rescue therapy [36,101]. Furthermore, emerging and promising evidence suggests that NK-1 receptor antagonists, such as aprepitant, may constitute a potent class of drugs for mitigating PONV [102], with comparative advantages over other antiemetics [91]. These agents are particularly advantageous due to their lack of sedative effects, in contrast to dopamine, histamine, or muscarinic receptor antagonists, which provide a significant benefit in fast-track bariatric surgery within the ERAS protocol [36,93,96,105].
Considering the substantial advancements in recent research regarding various techniques of regional anesthesia, it can be concluded that at least one form of this method, even if limited to local wound infiltration, is recommended for every patient undergoing LBS [15]. Regional anesthesia has been shown to enhance pain management, reduce the requirement for opioids, and consequently diminish opioid-related side effects such as PONV [110]. Nonetheless, discrepancies exist among RCTs evaluating the efficacy of diverse methods, including local wound infiltration, intraperitoneal local anesthetic installation, ESPB, TAP Block, EOI, and QLB, in the prevention of PONV. Often, these studies are designed to compare each technique to a placebo, with limited direct comparisons between specific methods. This represents a significant knowledge gap that necessitates large-scale RCTs or network meta-analyses to facilitate comprehensive comparisons.
In our department, based on our experience, guidelines, and RCTs, we adopted a multimodal approach to optimize PONV prophylaxis and treatment. We implemented a three-drug pharmacological prophylaxis regimen comprising aprepitant 80 mg administered orally one hour prior to surgery, dexamethasone 8 mg given intravenously, and ondansetron 4 mg administered intravenously at the conclusion of the operation, as a rescue therapy. Patients are permitted to consume water up to two hours before the procedure to prevent dehydration. During the intraoperative period, anesthesia is induced and maintained using BIS-guided propofol TIVA and the lowest feasible remifentanil infusion using the target-controlled infusion (TCI) Minto model to maintain hemodynamic stability. We decided against adopting OFA as a standard, considering the limited benefits demonstrated in RCTs conducted within our department [66]. We use TOF-guided rocuronium dosing to achieve neuromuscular blockade with sugammadex reversal as a standard protocol. At the commencement of surgery, surgeons perform a routine local infiltration of the trocar insertion site with 40 mL of 0.25% bupivacaine. Regarding postoperative analgesia, we administer paracetamol and metamizole, and each patient is administered oxycodone 0.1 mg/kg of lean body weight at the end of the surgery. In the PACU, patients may receive additional doses based on the NRS score. We also use the PCA oxycodone administration technique in selected patients.
4.1. Limitations of the Study
This scoping review has several limitations. Firstly, there exists significant heterogeneity among the included RCTs, including variation in study design. Overall, no standardized outcome measure has been employed to assess the presence and severity of PONV. Multiple scales are utilized, such as the PONV impact scale or the Likert scale. Furthermore, some studies differentiate between nausea assessment and vomiting, which complicates comparison and hampers definitive conclusions. Additionally, in a minority of RCTs, the Apfel scale is used to predict risk and guide prophylaxis, whereas others do not utilize it. Moreover, our review excluded operational techniques that may influence PONV as well as alternative medicine approaches, which could be components of a multimodal strategy. Aside from these factors, our analysis included exclusively RCTs to mitigate bias; consequently, the scope of the reviewed research, particularly concerning specific techniques, does not encompass all existing published literature. Lastly, only articles published in English were considered, potentially introducing language bias. These methodological limitations warrant cautious interpretation of the findings, which should not be regarded as definitive.
4.2. Future Directions
There is limited evidence regarding the optimal drug dosing for co-analgesics such as gabapentinoids or lidocaine, as well as antiemetics, particularly promising NK-1 antagonists. Future research should include large-scale dose-finding RCTs for these agents. Additionally, a substantial knowledge gap exists concerning the efficacy and practical applicability of novel regional blocks such as EOI or QLB in LBS. Finally, given that a broad spectrum of regional anesthesia techniques often cannot be combined due to limitations in the maximum permissible doses of local anesthetics and logistical considerations, a network meta-analysis would be necessary to compare the efficacy of various methods against each other and against a placebo.
5. Conclusions
The prevention and management of PONV continue to pose substantial clinical challenges. Although a multimodal strategy is regarded as standard practice for mitigating PONV, debates persist concerning the selection of specific techniques and antiemetic drug regimens. Recent evidence, especially in relation to regional anesthesia techniques and combined pharmacological prophylaxis, including new agents, underlines the potential advantages of innovative approaches.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14196901/s1, File S1: PRISMA-ScR checklist.
Author Contributions
Conceptualization, P.M. and JT; methodology, P.M. and M.J.; data analysis, P.Z., M.J. and R.C.; writing and original draft preparation, P.M. and M.J.; supervision, J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing does not apply to this article, as no new datasets were generated or analyzed during the current study. All data analyzed in this review were derived from previously published studies, which are cited within the manuscript. Further information regarding data sources can be found in the references section.
Conflicts of Interest
All the authors have seen the contents of this manuscript and agree they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PONV | Postoperative Nausea and Vomiting |
| RCTs | Randomized controlled trial |
| LBS | Laparoscopic bariatric surgery |
| LSG | Laparoscopic sleeve gastrectomy |
| ERAS | Enhanced Recovery After Surgery |
| TIVA | Total intravenous anesthesia |
| BIS | Bispectral index |
| GDFT | Goal-directed fluid therapy |
| SVV | Stroke Volume Variation |
| NMDA | N-methyl-d-aspartate |
| OFA | Opioid-free anesthesia |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PACU | Postoperative care unit |
| TAP | Transversus Abdominis Plane |
| LYGB | Laparoscopic Roux-en-Y gastric bypass |
| LGB | Laparoscopic gastric banding |
| PTC | Post-tetanic count |
| IBW | Ideal body weight |
| GABA | Gamma-aminobutyric acid |
| 5-HT3 | 5-hydroxytryptamine |
| NK-1 | Neurokinin-1 |
| ESPB TCI | Erectus Spinae Plane Block Target-Controlled Infusion |
| QLB | Quadratus Lumborum Block |
| EOIB | External Oblique Intercostal Fascial Plane Block |
| ABW | Adjusted body weight |
References
- Kushner, B.S.; Freeman, D.; Sparkman, J.; Salles, A.; Eagon, J.C.; Eckhouse, S.R. Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg. Obes. Relat. Dis. 2020, 16, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Groene, P.; Eisenlohr, J.; Zeuzem, C.; Dudok, S.; Karcz, K.; Hofmann-Kiefer, K. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Videosurg. Other Miniinvasive Tech. 2019, 14, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Halliday, T.A.; Sundqvist, J.; Hultin, M.; Walldén, J. Post-operative nausea and vomiting in bariatric surgery patients: An observational study. Acta Anaesthesiol. Scand. 2017, 61, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Bataille, A.; Letourneulx, J.-F.; Charmeau, A.; Lemedioni, P.; Léger, P.; Chazot, T.; Le Guen, M.; Diemunsch, P.; Fischler, M.; Liu, N. Impact of a prophylactic combination of dexamethasone–ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol–remifentanil anaesthesia. Eur. J. Anaesthesiol. 2016, 33, 898–905. [Google Scholar] [CrossRef]
- Liao, B.; Liao, W.; Wu, X.; Liu, S.; Li, Y.; Qin, R.; Yin, S. Analysis of influencing factors and construction of prediction model for postoperative nausea and vomiting in patients undergoing laparoscopic sleeve gastrectomy: A single-center retrospective cohort study. BMC Anesthesiol. 2024, 24, 131. [Google Scholar] [CrossRef]
- Scuderi, P.E.; Conlay, L.A. Postoperative Nausea and Vomiting and Outcome. Int. Anesthesiol. Clin. 2003, 41, 165–174. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Rossetti, G.; Fei, L.; Docimo, L.; Del Genio, G.; Micanti, F.; Belfiore, A.; Brusciano, L.; Moccia, F.; Cimmino, M.; Marra, T. Is Nasogastric Decompression Useful in Prevention of Leaks After Laparoscopic Sleeve Gastrectomy? A Randomized Trial. J. Investig. Surg. 2014, 27, 234–239. [Google Scholar] [CrossRef]
- Rashdan, M.; Al-Sabe, L.; Salameh, M.; Halaseh, S.; Al-Mikhi, B.; Sha’bIn, S.; Alqirem, L.; Alsaadi, T.; Ahmad, J.; Sabbagh, A.; et al. Predictive factors for readmission after bariatric surgery: Experience of an obesity center. Medicine 2024, 103, e39242. [Google Scholar] [CrossRef]
- Gress, K.; Urits, I.; Viswanath, O.; Urman, R.D. Clinical and economic burden of postoperative nausea and vomiting: Analysis of existing cost data. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 681–686. [Google Scholar] [CrossRef]
- Sizemore, D.C. Postoperative Nausea. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500029/ (accessed on 14 September 2025).
- Ruiz-Tovar, J.; Garcia, A.; Ferrigni, C.; Gonzalez, J.; Castellon, C.; Duran, M. Impact of implementation of an enhanced recovery after surgery (ERAS) program in laparoscopic Roux-en-Y gastric bypass: A prospective randomized clinical trial. Surg. Obes. Relat. Dis. 2019, 15, 228–235. [Google Scholar] [CrossRef]
- Elvir-Lazo, O.L.; White, P.F.; Yumul, R.; Eng, H.C. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: An updated review. F1000Research 2020, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wu, X.; Chen, Y.; Yuan, H. Effect of multimodal intervention on postoperative nausea and vomiting in patients undergoing gynecological laparoscopy. J. Int. Med. Res. 2019, 47, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; dos Reis Falcão, L.F.; O’KAne, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.M.; Greim, C.-A.; Roewer, N. A Simplified Risk Score for Predicting Postoperative Nausea and Vomiting. Anesthesiology 1999, 91, 693. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Pierre, S.; Whelan, R. Nausea and vomiting after surgery. Contin. Educ. Anaesth. Crit. Care Pain 2013, 13, 28–32. [Google Scholar] [CrossRef]
- Shrivastava, M.; Ugile, A.B.; Nam, P.B.; Bhalerao, J. A study of different anaesthetic methods on postoperative nausea and vomiting in laparoscopic sleeve gastrectomy in a tertiary hospital in Central India. Eur. J. Cardiovasc. Med. 2025, 15, 109–114. [Google Scholar] [CrossRef]
- Elbakry, A.-E.; Sultan, W.-E.; Ibrahim, E. A comparison between inhalational (Desflurane) and total intravenous anaesthesia (Propofol and dexmedetomidine) in improving postoperative recovery for morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A double-blinded randomised controlled trial. J. Clin. Anesth. 2018, 45, 6–11. [Google Scholar] [CrossRef]
- Ziemann-Gimmel, P.; Goldfarb, A.A.; Koppman, J.; Marema, R.T. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br. J. Anaesth. 2014, 112, 906–911. [Google Scholar] [CrossRef]
- Spaniolas, K.; Nie, L.; Moller, D.; Tatarian, T.; Hesketh, A.; Yang, J.; Docimo, S.; Bates, A.; Gan, T.J.; Pryor, A. A Comprehensive Approach for the Prevention of Nausea and Vomiting Following Sleeve Gastrectomy: A Randomized Controlled Trial. Obes. Surg. 2020, 30, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Aftab, H.; Fagerland, M.W.; Gondal, G.; Ghanima, W.; Olsen, M.K.; Nordby, T. Pain and nausea after bariatric surgery with total intravenous anesthesia versus desflurane anesthesia: A double blind, randomized, controlled trial. Surg. Obes. Relat. Dis. 2019, 15, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Honca, M.; Honca, T. Comparison of Propofol with Desflurane for Laparoscopic Sleeve Gastrectomy in Morbidly Obese patients: A Prospective Randomized Trial. Bariatr. Surg. Pract. Patient Care 2017, 12, 49–54. [Google Scholar] [CrossRef]
- De Baerdemaeker, L.; Jacobs, S.; Blauwen, N.M.M.D.; Pattyn, P.; Herregods, L.L.G.; Mortier, E.P.; Struys, M.M.R.F. Postoperative Results after Desflurane or Sevoflurane Combined with Remifentanil in Morbidly Obese Patients. Obes. Surg. 2006, 16, 728–733. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.-Y.; Gao, R.-J.; Huang, Y.; Mao, S.-M.; Feng, J.-Y. The Effect of Depth of Anesthesia on Postoperative Pain in Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Trial. Obes. Surg. 2024, 34, 1793–1800. [Google Scholar] [CrossRef]
- Castro, D.S.; Leão, P.; Borges, S.; Gomes, L.; Pacheco, M.; Figueiredo, P. Sugammadex Reduces Postoperative Pain After Laparoscopic Bariatric Surgery. Surg. Laparosc. Endosc. Percutaneous Tech. 2014, 24, 420–423. [Google Scholar] [CrossRef]
- Yang, W.-L.; Wen, Y.-L.; Xu, W.-M.; Xu, C.-L.; Yin, W.-Q.; Lin, J.-Y. Effect of deep neuromuscular block on the quality of early recovery after sleeve gastrectomy in obese patients: A randomized controlled trial. BMC Anesthesiol. 2024, 24, 101. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, L.; Yu, Y.; Liu, S.; Xu, H.; Xu, Z.; Yang, C.; Liu, C. Comparison of Deep and Moderate Neuromuscular Blockade on Intestinal Mucosal Barrier in Laparoscopic Gastrectomy: A Prospective, Randomized, Double-Blind Clinical Trial. Front. Med. 2022, 8, 789597. [Google Scholar] [CrossRef]
- Suh, S.; Hetzel, E.; Alter-Troilo, K.; Lak, K.; Gould, J.C.; Kindel, T.L.; Higgins, R.M. The influence of preoperative carbohydrate loading on postoperative outcomes in bariatric surgery patients: A randomized, controlled trial. Surg. Obes. Relat. Dis. 2021, 17, 1480–1488. [Google Scholar] [CrossRef]
- Cho, H.-J.; Huang, Y.-H.; Poon, K.-S.; Chen, K.-B.; Liao, K.H. Perioperative hemodynamic optimization in laparoscopic sleeve gastrectomy using stroke volume variation to reduce postoperative nausea and vomiting. Surg. Obes. Relat. Dis. 2021, 17, 1549–1557. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, K.; Liu, L.; Ma, J.; Liu, D.; Zhang, J. The Impact of Goal-Directed Fluid Therapy on Postoperative Nausea and Vomiting in High-Risk Patients Undergoing Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2022, 32, 3533–3540. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Tian, C.; Lu, J.; Lee, Y. Total Intravenous Anesthesia Versus Inhalation Anesthesia on Postoperative Analgesia and Nausea and Vomiting After Bariatric Surgery: A Systematic Review and Meta-Analysis. Asian J. Anesthesiol. 2021, 59, 135–151. [Google Scholar] [CrossRef]
- Oliveira, C.R.D.; Bernardo, W.M.; Nunes, V.M. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz. J. Anesthesiol. 2017, 67, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Tramèr, M.R.; Fuchs-Buder, T. Omitting antagonism of neuromuscular block: Effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. Br. J. Anaesth. 1999, 82, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Palomba, G.; Basile, R.; Capuano, M.; Pesce, M.; Rurgo, S.; Sarnelli, G.; De Palma, G.D.; Aprea, G. Nasogastric tube after laparoscopic Heller-Dor surgery: Do you really need it? Curr. Probl. Surg. 2024, 61, 101457. [Google Scholar] [CrossRef]
- Weijs, T.J.; Kumagai, K.; Berkelmans, G.H.K.; Nieuwenhuijzen, G.A.P.; Nilsson, M.; Luyer, M.D.P. Nasogastric decompression following esophagectomy: A systematic literature review and meta-analysis. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.; Varghese, C.; Thejasvin, K.; Prabakaran, V.; Robinson, S.; Pathak, S.; Dasari, B.V.M.; Pandanaboyana, S. Impact of routine nasogastric decompression versus no nasogastric decompression after pancreaticoduodenectomy on perioperative outcomes: Meta-analysis. BJS Open 2021, 5, zrab111. [Google Scholar] [CrossRef]
- Cooke, F.E.; Samuels, J.D.; Pomp, A.; Gadalla, F.; Wu, X.; Afaneh, C.; Dakin, G.F.; Goldstein, P.A. A Randomized, Double-Blind, Placebo-Controlled Trial of Intravenous Acetaminophen on Hospital Length of Stay in Obese Individuals Undergoing Sleeve Gastrectomy. Obes. Surg. 2018, 28, 2998–3006. [Google Scholar] [CrossRef]
- Amin, S.; Hasanin, A.; Soliman, S.; Mostafa, M.; Abdallah, A.S.; Zakaria, D.; Abdelkader, A. Intravenous Ibuprofen Versus Ketorolac for Perioperative Pain Control in Patients with Morbid Obesity Undergoing Bariatric Surgery: A Randomized Controlled Trial. Obes. Surg. 2025, 35, 1350–1356. [Google Scholar] [CrossRef]
- Govindarajan, R.; Ghosh, B.; Sathyamoorthy, M.K.; Kodali, N.S.; Raza, A.; Aronsohn, J.; Rajpal, S.; Ramaswamy, C.; Abadir, A. Efficacy of ketorolac in lieu of narcotics in the operative management of laparoscopic surgery for morbid obesity. Surg. Obes. Relat. Dis. 2005, 1, 530–535. [Google Scholar] [CrossRef]
- Hamed, J.M.E.; Refaat, H.S.; Al-Wadaani, H. Dexmedetomidine compared to remifentanil infusion as adjuvant to sevoflurane anesthesia during laparoscopic sleeve gastrectomy. Anesth. Essays Res. 2019, 13, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Khalil, B.N.; Elderh, M.S.; Khaja, M.A.; El-Shaer, A.N.; Ali, B.E.; Taeimah, M.O. Perioperative use of ketamine infusion versus dexmedetomidine infusion for analgesia in obese patients undergoing bariatric surgery: A double-blinded three-armed randomized controlled trial. BMC Anesthesiol. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halaweh, S.; Obeidat, F.; Absalom, A.R.; AlOweidi, A.; Abu Abeeleh, M.; Qudaisat, I.; Robinson, F.; Mason, K.P. Dexmedetomidine versus morphine infusion following laparoscopic bariatric surgery: Effect on supplemental narcotic requirement during the first 24 h. Surg. Endosc. 2016, 30, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Zeeni, C.; Aouad, M.T.; Daou, D.; Naji, S.; Jabbour-Khoury, S.; Alami, R.S.; Safadi, B.Y.; Siddik-Sayyid, S.M. The Effect of Intraoperative Dexmedetomidine Versus Morphine on Postoperative Morphine Requirements After Laparoscopic Bariatric Surgery. Obes. Surg. 2019, 29, 3800–3808. [Google Scholar] [CrossRef]
- Bakhamees, H.S.; El-Halafawy, Y.M.; El-Kerdawy, H.M.; Gouda, N.M.; Altemyatt, S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East J. Anaesthesiol. 2007, 19, 537–551. [Google Scholar]
- De Oliveira, G.S.; Duncan, K.; Fitzgerald, P.; Nader, A.; Gould, R.W.; McCarthy, R.J. Systemic Lidocaine to Improve Quality of Recovery after Laparoscopic Bariatric Surgery: A Randomized Double-Blinded Placebo-Controlled Trial. Obes. Surg. 2013, 24, 212–218. [Google Scholar] [CrossRef]
- De Oliveira, C.M.B.; Coelho, L.M.G.; Valadão, J.A.; Moura, E.C.R.; da Silva, A.A.M.; de Lima, R.C.; Brunialti, M.K.C.; Salomão, R.; Leal, P.d.C.; Sakata, R.K. Assessment of the Effect of Perioperative Venous Lidocaine on the Intensity of Pain and IL-6 Concentration After Laparoscopic Gastroplasty. Obes. Surg. 2020, 30, 3912–3918. [Google Scholar] [CrossRef]
- Sherif, A.A.; Elsersy, H.E. The impact of dexmedetomidine or xylocaine continuous infusion on opioid consumption and recovery after laparoscopic sleeve gastrectomy. Minerva Anestesiol. 2017, 83, 1274–1282. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Wang, J.; Gao, X.; Wang, G. Effect of Intravenous Infusion of Lidocaine Compared with Ultrasound-Guided Transverse Abdominal Plane Block on the Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery. Drug Des. Dev. Ther. 2022, 16, 739–748. [Google Scholar] [CrossRef]
- Yurttas, T.; Djurdjevic, M.; Schnider, T.W.; Filipovic, M. Analgesic efficacy of systemic lidocaine using lean body mass based dosing regime versus placebo in bariatric surgery: A prospective, randomised, double-blind, placebo-controlled, single-centre study. Br. J. Anaesth. 2023, 131, 122–129. [Google Scholar] [CrossRef]
- Plass, F.; Nicolle, C.; Zamparini, M.; Al Issa, G.; Fiant, A.L.; Le Roux, Y.; Gérard, J.L.; Fischer, M.O.; Alvès, A.; Hanouz, J. Effect of intra-operative intravenous lidocaine on opioid consumption after bariatric surgery: A prospective, randomised, blinded, placebo-controlled study. Anaesthesia 2020, 76, 189–198. [Google Scholar] [CrossRef]
- Sakata, R.K.; de Lima, R.C.; Valadão, J.A.; Leal, P.C.; Moura, E.C.; Cruz, V.P.; de Oliveira, C.M. Randomized, Double-Blind Study of the Effect of Intraoperative Intravenous Lidocaine on the Opioid Consumption and Criteria for Hospital Discharge After Bariatric Surgery. Obes. Surg. 2019, 30, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Alimian, M.; Safaeian, R.; Zaman, B.; Entezari, S.; Abedian, A.E. Comparison of the Effect of Intraoperative 1 mg/kg/h and 2 mg/kg/h IV Lidocaine Infusion on Postoperative Pain and Nausea-Vomiting in Laparoscopic Gastric Bypass Surgery. J. Pharm. Res. Int. 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Hasanein, R.; El-Sayed, W.; Nabil, N.; Elsayed, G. The effect of combined remifentanil and low dose ketamine infusion in patients undergoing laparoscopic gastric bypass. Egypt. J. Anaesth. 2011, 27, 255–260. [Google Scholar] [CrossRef]
- Yang, T.; Mudabbar, M.S.; Liu, B.; Xu, M.; Fu, Q. Intraoperative Esketamine Is Effective at Reducing Acute Postoperative Pain in Bariatric Surgery Patients: A Randomized Control Trial. Obes. Surg. 2023, 33, 2368–2374. [Google Scholar] [CrossRef]
- Das Adhikary, S.; Thiruvenkatarajan, V.; McFadden, A.; Liu, W.M.; Mets, B.; Rogers, A. Analgesic efficacy of ketamine and magnesium after laparoscopic sleeve gastrectomy: A randomized, double-blind, placebo-controlled trial. J. Clin. Anesth. 2021, 68, 110097. [Google Scholar] [CrossRef]
- Mehta, S.D.; Smyth, D.; Vasilopoulos, T.; Friedman, J.; Sappenfield, J.W.; Alex, G. Ketamine infusion reduces narcotic requirements following gastric bypass surgery: A randomized controlled trial. Surg. Obes. Relat. Dis. 2021, 17, 737–743. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Dang, J.; Zheng, H.; Ren, B.; Liu, C.; Zuo, R.; Wang, R.; Liu, T.; Wang, Z. Effect of Intraoperative Infusion of Esketamine on Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery: A Randomized Controlled Trial. Pain Ther. 2023, 12, 979–992. [Google Scholar] [CrossRef]
- Schulmeyer, M.C.C.; de la Maza, J.; Ovalle, C.; Farias, C.; Vives, I. Analgesic Effects of a Single Preoperative Dose of Pregabalin after Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2009, 20, 1678–1681. [Google Scholar] [CrossRef]
- Alimian, M.; Faiz, S.H.-R.; Imani, F.; Navadegi, S.F.; Pournajafian, A.; Safari, S. Effect of Oral Pregabalin Premedication on Post-Operative Pain in Laparoscopic Gastric Bypass Surgery. Anesthesiol. Pain Med. 2012, 2, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.K.; Abdallah, N.M. Multimodal analgesia with pregabalin and dexmedetomidine in morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A prospective randomized double blind placebo controlled study. Egypt. J. Anaesth. 2016, 32, 293–298. [Google Scholar] [CrossRef]
- Hassani, V.; Pazouki, A.; Nikoubakht, N.; Chaichian, S.; Sayarifard, A.; Khankandi, A.S. The Effect of Gabapentin on Reducing Pain After Laparoscopic Gastric Bypass Surgery in Patients with Morbid Obesity; A Randomized Clinical Trial. Anesthesiol. Pain Med. 2015, 5, e22372. [Google Scholar] [CrossRef] [PubMed]
- Mulier, J.P.; Wouters, R.; Dillemans, B.; Dekock, M. A Randomized Controlled, Double-Blind Trial Evaluating the Effect of Opi-oid-Free Versus Opioid General Anaesthesia on Postoperative Pain and Discomfort Measured by the QoR-40. J. Clin. Anesth. Pain Med. 2018, 6, 2. [Google Scholar]
- Mieszczański, P.; Górniewski, G.; Ziemiański, P.; Cylke, R.; Lisik, W.; Trzebicki, J. Comparison between multimodal and intraoperative opioid free anesthesia for laparoscopic sleeve gastrectomy: A prospective, randomized study. Sci. Rep. 2023, 13, 12677. [Google Scholar] [CrossRef]
- Clanet, M.; Touihri, K.; El Haddad, C.; Goldsztejn, N.; Himpens, J.; Fils, J.F.; Gricourt, Y.; Van der Linden, P.; Coeckelenbergh, S.; Joosten, A.; et al. Effect of opioid-free versus opioid-based strategies during multimodal anaesthesia on postoperative morphine consumption after bariatric surgery: A randomised double-blind clinical trial. BJA Open 2024, 9, 100263. [Google Scholar] [CrossRef]
- Dagher, C.; Mattar, R.; Aoun, M.; Tohme, J.; Naccache, N.; Jabbour, H. Opioid-free anesthesia in bariatric surgery: A prospective randomized controlled trial. Eur. J. Med. Res. 2025, 30, 320. [Google Scholar] [CrossRef]
- Menck, J.T.; Tenório, S.B.; de Oliveira, R.M.; Strobel, R.; dos Santos, B.B.; Junior, A.F.F.; de Cesaro, M.P. Opioid-free Anesthesia for Laparoscopic Gastroplasty. A Prospective and Randomized Trial. Open Anesth. J. 2022, 16, e258964582208110. [Google Scholar] [CrossRef]
- Campos-Pérez, W.; Ramírez-Plascencia, L.; Pérez-Robles, M.; Rivera-Valdés, J.J.; Sánchez-Muñoz, P.; Pérez-Vargas, L.; González-Landeros, D.; Cuevas, J.H.M.; Martínez-López, E. A comparison of opioid-containing anesthesia versus opioid-free anesthesia using the Cortínez-Sepúlveda model on differential cytokine responses in obese patients undergoing gastric bypass surgery: A randomized controlled trial. BMC Anesthesiol. 2022, 22, 294. [Google Scholar] [CrossRef]
- Soudi, A.M.; Hammad, R.A.; ElShafie, M.A.; Ahmed, I.M.A.S.; Alhadidy, M.A. Comparing opioid free general anesthesia to traditional balanced general anesthesia regarding achievement of enhanced recovery in laparoscopic bariatric surgeries. Ain-Shams J. Anesthesiol. 2022, 14, 24. [Google Scholar] [CrossRef]
- Macfater, H.; Xia, W.; Srinivasa, S.; Hill, A.G.; Van De Velde, M.; Joshi, G.P.; Beloeil, H.; Bonnet, F.; Hill, A.; Joshi, G.P.; et al. Evidence-Based Management of Postoperative Pain in Adults Undergoing Laparoscopic Sleeve Gastrectomy. World J. Surg. 2019, 43, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Tamburini, E.; Linassi, F.; Pettenuzzo, T.; Boscolo, A.; Navalesi, P. Non-Opioid Analgesics and Adjuvants after Surgery in Adults with Obesity: Systematic Review with Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, R.; Alnajjar, D.; Bin Salamah, R.; Mandoorah, J.; Alghamdi, A.; Aloteibi, R.E.; Almusharaf, L.; Albabtain, B. Dexmedetomidine in Bariatric Surgery: A Systematic Review and Meta-Analysis of Its Effects on Postoperative Pain and Postoperative Nausea and Vomiting. J. Clin. Med. 2025, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.C.; Ortegal, G.H.P.C.; Aguirre, J.M.; Costa, P.R.R.; Ferreira, L.N.; Moreira, L.F.; Silva, G.C.; Filho, P.P.M.F.; Ferreira, D.M. Effects of Intravenous Lidocaine on Quality of Recovery After Laparoscopic Bariatric Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Surg. 2024, 34, 2663–2669. [Google Scholar] [CrossRef]
- Chaouch, M.A.M.A.; Daghmouri, M.A.; Boutron, M.-C.; Ferraz, J.-M.; Usai, S.; Soubrane, O.; Beaussier, M.; Pourcher, G.; Oweira, H. Ketamine as a component of multimodal analgesia for pain management in bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. Surg. 2022, 78, 103783. [Google Scholar] [CrossRef]
- Mao, J.; Price, D.D.; Mayer, D.J. Mechanisms of hyperalgesian and morphine tolerance: A current view of their possible interactions. Pain 1995, 62, 259–274. [Google Scholar] [CrossRef]
- Thorp, A.W.; Brown, L.; Green, S.M. Ketamine-Associated Vomiting. Pediatr. Emerg. Care 2009, 25, 15–18. [Google Scholar] [CrossRef]
- Hung, K.-C.; Chang, L.-C.; Ho, C.-N.; Hsu, C.-W.; Wu, J.-Y.; Lin, Y.-T.; Chen, I.-W. Influence of Intravenous Magnesium Sulfate Infusion on the Subjective Postoperative Quality of Recovery: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 2375. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, S.-C.; Chiang, M.-H.; Hsu, C.-W.; Chen, J.-Y.; Huang, P.-W.; Sun, C.-K. Analgesic Efficacy of Gabapentin and Pregabalin in Patients Undergoing Laparoscopic Bariatric Surgeries: A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 2734–2743. [Google Scholar] [CrossRef]
- Mieszczanski, P.; Gorniewski, G.; Janiak, M.; Trzebicki, J. The effect of pre-emptive oral pregabalin on opioid consumption in patients undergoing laparoscopic sleeve gastrectomy with an analysis of intraoperative hemodynamic stability and quality of recovery: Study protocol for a randomized, prospective, double-blind study. Trials 2024, 25, 367. [Google Scholar] [CrossRef]
- Sultana, A.; Torres, D.; Schumann, R. Special indications for Opioid Free Anaesthesia and Analgesia, patient and procedure related: Including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 547–560. [Google Scholar] [CrossRef]
- Ao, Y.; Ma, J.; Zheng, X.; Zeng, J.; Wei, K. Opioid-Sparing Anesthesia Versus Opioid-Free Anesthesia for the Prevention of Postoperative Nausea and Vomiting after Laparoscopic Bariatric Surgery: A Systematic Review and Network Meta-Analysis. Anesth. Analg. 2024, 140, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Beloeil, H.; Garot, M.; Lebuffe, G.; Gerbaud, A.; Bila, J.; Cuvillon, P.; Dubout, E.; Oger, S.; Nadaud, J.; Becret, A.; et al. Balanced Opioid-free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology 2021, 134, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Didehvar, S.; Viola-Blitz, J.; Haile, M.; Franco, L.; Kline, R.; Kurian, M.; Fielding, G.; Ren, C.; Bekker, A. A Randomized, Double Blind Study to Evaluate the Efficacy of Palonosetron with Dexamethasone Versus Palonosetron Alone for Prevention of Post-Operative Nausea and Vomiting in Subjects Undergoing Bariatric Surgeries with High Emetogenic Risk. Open Anesthesiol. J. 2013, 7, 30–36. [Google Scholar] [CrossRef][Green Version]
- Benevides, M.L.; Oliveira, S.S.; de Aguilar-Nascimento, J.E. The Combination of Haloperidol, Dexamethasone, and Ondansetron for Prevention of Postoperative Nausea and Vomiting in Laparoscopic Sleeve Gastrectomy: A Randomized Double-Blind Trial. Obes. Surg. 2013, 23, 1389–1396. [Google Scholar] [CrossRef]
- Burcu, B.; Hacım, N.A.; Caliskan, O.; Demirgan, S.; Aktokmakyan, T.V.; Meric, S.; Duymaz, T.; Karabay, O.; Solmaz, A. Impact of body weight-based dosing of palonosetron and ondansetron on postoperative nausea and vomiting following laparoscopic sleeve gastrectomy: A randomized, double-blind study. Acta Chir. Belg. 2024, 124, 41–49. [Google Scholar] [CrossRef]
- Kaloria, N.; Sen, I.M.; Saini, V.; Gupta, R.; Walia, R. Palonosetron-dexamethasone versus ondansetron-dexamethasone to prevent postoperative nausea and vomiting undergoing laparoscopic sleeve gastrectomy: A preliminary, randomized, double blinded study. Indian J. Clin. Anaesth. 2017, 4, 523–529. [Google Scholar]
- Ortiz, E.; González, A.I.; Jaime, V.; Guzmán, J.A.; Esparza, I.; Orozco, J.O.; Guerrero, M.A.; Ramos, A.; Zerrweck, C. The impact of Aprepitant on Nausea and Vomiting following Laparoscopic Sleeve Gastrectomy: A Blinded Randomized Controlled Trial. Obes. Surg. 2024, 34, 1316–1323. [Google Scholar] [CrossRef]
- Sinha, A.C.; Singh, P.M.; Williams, N.W.; Ochroch, E.A.; Goudra, B.G. Aprepitant’s Prophylactic Efficacy in Decreasing Postoperative Nausea and Vomiting in Morbidly Obese Patients Undergoing Bariatric Surgery. Obes. Surg. 2013, 24, 225–231. [Google Scholar] [CrossRef]
- Ashoor, T.M.; Kassim, D.Y.; Esmat, I.M. A Randomized Controlled Trial for Prevention of Postoperative Nausea and Vomiting after Laparoscopic Sleeve Gastrectomy: Aprepitant/Dexamethasone vs. Mirtazapine/Dexamethasone. Anesthesiol. Res. Pract. 2022, 2022, 3541073. [Google Scholar] [CrossRef]
- Shahinpour, S.; Momeni, N.; Yaqubnejad, M.; Khajavi, M.; Pourfakhr, P. Evaluation and Comparison of Two Different Combined Regimens for Prophylaxis of Nausea and Vomiting After Laparoscopic Bariatric Surgery: A Double-Blinded Randomized Clinical Trial. Acta Medica Iran. 2023, 61, 175–180. [Google Scholar] [CrossRef]
- Talebpour, M.; Omrani, N.G.; Imani, F.; Moharari, R.S.; Pourfakhr, P.; Khajavi, M.R. Comparison Effect of Promethazine/Dexamethasone and Metoclopramide/Dexamethasone on Postoperative Nausea and Vomiting after Laparascopic Gastric Placation: A Randomized Clinical Trial. Anesthesiol. Pain Med. 2017, 7, e57810. [Google Scholar] [CrossRef]
- Moussa, A.A.; Oregan, P.J. Prevention of postoperative nausea and vomiting in patients undergoing laparoscopic bariatric surgery—Granisetron alone vs granisetron combined with dexamethasone/droperidol. Middle East J. Anaesthesiol. 2007, 19, 357–367. [Google Scholar]
- Ebrahimian, M.; Mirhashemi, S.-H.; Oshidari, B.; Zamani, A.; Shadidi-Asil, R.; Kialashaki, M.; Ghayebi, N. Effects of ondansetron, metoclopramide and granisetron on perioperative nausea and vomiting in patients undergone bariatric surgery: A randomized clinical trial. Surg. Endosc. 2023, 37, 4495–4504. [Google Scholar] [CrossRef] [PubMed]
- Pourfakhr, P.; Aghabagheri, M.; Mahmoudabadi, H.Z.; Najjari, K.; Talebpour, M.; Khajavi, M.R. Prophylactic Administration of Diphenhydramine/Acetaminophen and Ondansetron Reduced Postoperative Nausea and Vomiting and Pain Following Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Trial. Obes. Surg. 2021, 31, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; El-Halafawy, M.; Abdelhamid, B.; Barghout, F.; Sayed, I. Efficacy of Combining Cyclizine, Metoclopramide, or On-dansetron with Dexamethasone in Prevention of Postoperative Nausea and Vomiting after Laparoscopic Sleeve Gastrectomy; A Randomized Controlled Trial. Middle East J. Anesthesiol. 2025, 32, 42–55. [Google Scholar]
- Atif, Q.A.A.; Al Obaid, O.; Malik, A.M. Effect of intravenous scopolamine before stapling, on postoperative nausea and vomiting in sleeve gastrectomy patients: A randomized controlled trial. Surg. Endosc. 2022, 36, 7717–7721. [Google Scholar] [CrossRef]
- Schumann, R.; Ziemann-Gimmel, P.; Sultana, A.; Eldawlatly, A.A.; Kothari, S.N.; Shah, S.; Wadhwa, A. Postoperative nausea and vomiting in bariatric surgery: A position statement endorsed by the ASMBS and the ISPCOP. Surg. Obes. Relat. Dis. 2021, 17, 1829–1833. [Google Scholar] [CrossRef]
- Polderman, J.A.; Farhang-Razi, V.; Van Dieren, S.; Kranke, P.; DeVries, J.H.; Hollmann, M.W.; Preckel, B.; Hermanides, J. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 2018, 8, CD011940. [Google Scholar] [CrossRef]
- Tricco, A.C.; Soobiah, C.; Blondal, E.; Veroniki, A.A.; Khan, P.A.; Vafaei, A.; Ivory, J.; Strifler, L.; Ashoor, H.; MacDonald, H.; et al. Comparative efficacy of serotonin (5-HT3) receptor antagonists in patients undergoing surgery: A systematic review and network meta-analysis. BMC Med. 2015, 13, 136. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, T.; Wu, Q.; Da, M. The Efficacy of Aprepitant in Preventing Post-bariatric Surgery Nausea and Vomiting: Evidence from Clinical Trials. Obes. Surg. 2024, 34, 2617–2626. [Google Scholar] [CrossRef]
- Singh, P.M.; Borle, A.; Rewari, V.; Makkar, J.K.; Trikha, A.; Sinha, A.C.; Goudra, B. Aprepitant for postoperative nausea and vomiting: A systematic review and meta-analysis. Postgrad. Med. J. 2015, 92, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, F.; Liang, C.; Huang, Y.; Zhao, Y.; Liu, C.; Lin, C.; Zhang, L.; Zhou, S.; Wang, Q.; et al. Fosaprepitant for postoperative nausea and vomiting in patients undergoing laparoscopic gastrointestinal surgery: A randomised trial. Br. J. Anaesth. 2023, 131, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Deitrick, C.L.; Mick, D.J.; Lauffer, V.; Prostka, E.; Nowak, D.; Ingersoll, G. A Comparison of Two Differing Doses of Promethazine for the Treatment of Postoperative Nausea and Vomiting. J. Perianesth. Nurs. 2015, 30, 5–13. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.; Castro-Alves, L.; Chang, R.; Yaghmour, E.; McCarthy, R. Systemic metoclopramide to prevent postoperative nausea and vomiting: A meta-analysis without Fujii’s studies. Br. J. Anaesth. 2012, 109, 688–697. [Google Scholar] [CrossRef]
- Kranke, P.; Morin, A.M.; Roewer, N.; Eberhart, L.H.J. Dimenhydrinate for prophylaxis of postoperative nausea and vomiting: A meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2002, 46, 238–244. [Google Scholar] [CrossRef]
- Apfel, C.C.; Zhang, K.; George, E.; Shi, S.; Jalota, L.; Hornuss, C.; Fero, K.E.; Heidrich, F.; Pergolizzi, J.V.; Cakmakkaya, O.S.; et al. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: A systematic review and meta-analysis. Clin. Ther. 2010, 32, 1987–2002. [Google Scholar] [CrossRef]
- White, P.F.; Tang, J.; Song, D.; Coleman, J.E.; Wender, R.H.; Ogunnaike, B.; Sloninsky, A.; Kapu, R.; Shah, M.; Webb, T. Transdermal Scopolamine: An Alternative to Ondansetron and Droperidol for the Prevention of Postoperative and Postdischarge Emetic Symptoms. Anesth. Analg. 2007, 104, 92–96. [Google Scholar] [CrossRef]
- De Cassai, A.; Tulgar, S.; Carron, M.; Navalesi, P. Regional anesthesia in bariatric surgery. Curr. Opin. Anaesthesiol. 2025, 38, 611–617. [Google Scholar] [CrossRef]
- Shariat, A.; Kadakia, R.; Lin, H.-M.; Egorova, N.; Jin, S.; Latmore, M.; Epstein, J.; Pai BH, P.; Park, K.; Kini, S.; et al. Comparison of Posterior Quadratus Lumborum Block vs Surgical Wound Infiltration in Patients Undergoing Bariatric Sleeve Gastrectomy Surgery. Obes. Surg. 2025, 35, 2673–2679. [Google Scholar] [CrossRef]
- Ma, P.; Lloyd, A.; McGrath, M.; Cung, A.S.; Akusoba, I.; Jackson, A.; Swartz, D.; Boone, K.; Higa, K. Efficacy of liposomal bupivacaine versus bupivacaine in port site injections on postoperative pain within enhanced recovery after bariatric surgery program: A randomized clinical trial. Surg. Obes. Relat. Dis. 2019, 15, 1554–1562. [Google Scholar] [CrossRef]
- Neishaboury, M.; Shokri, S.; Kianpour, P.; Farhadi, K.; Najjari, K.; Sharifnia, H.; MohammadYousef, R.; Khajavi, M. The Effects of Intraperitoneal Dexmedetomidine in Comparison with Ropivacaine in Postoperative Pain After Laparoscopic Sleeve Gastrectomy: A Double-Blind, Randomized, Placebo-Controlled, Clinical Trial. Obes. Surg. 2025, 35, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.M.; Bakhtiyari, M.; Gholizadeh, B.; Shariati, C. Analgesic Effect of Intraperitoneal Bupivacaine Hydrochloride After Laparoscopic Sleeve Gastrectomy: A Randomized Clinical Trial. J. Gastrointest. Surg. 2018, 22, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Omar, I.; Abualsel, A. Efficacy of Intraperitoneal Instillation of Bupivacaine after Bariatric Surgery: Randomized Controlled Trial. Obes. Surg. 2019, 29, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Seal, A.; Lemech, I.; Fisher, O.M.; Williams, N. Intraperitoneal Instillation of Local Anesthetic (IPILA) in Bariatric Surgery and the Effect on Post-operative Pain Scores: A Randomized Control Trial. Obes. Surg. 2022, 32, 2349–2356. [Google Scholar] [CrossRef]
- Sherwinter, D.A.; Ghaznavi, A.M.; Spinner, D.; Savel, R.H.; Macura, J.M.; Adler, H. Continuous Infusion of Intraperitoneal Bupivacaine after Laparoscopic Surgery: A Randomized Controlled Trial. Obes. Surg. 2008, 18, 1581–1586. [Google Scholar] [CrossRef]
- Mittal, T.; Dey, A.; Siddhartha, R.; Nali, A.; Sharma, B.; Malik, V. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postoperative analgesia in laparoscopic gastric sleeve resection: A randomized single blinded case control study. Surg. Endosc. 2018, 32, 4985–4989. [Google Scholar] [CrossRef]
- Emile, S.H.; Abdel-Razik, M.A.; Elbahrawy, K.; Elshobaky, A.; Shalaby, M.; Elbaz, S.A.; Gado, W.A.; Elbanna, H.G. Impact of Ultrasound-Guided Transversus Abdominis Plane Block on Postoperative Pain and Early Outcome After Laparoscopic Bariatric Surgery: A Randomized Double-Blinded Controlled Trial. Obes. Surg. 2019, 29, 1534–1541. [Google Scholar] [CrossRef]
- Ibrahim, M.; El Shamaa, H. Efficacy of ultrasound-guided oblique subcostal transversus abdominis plane block after laparoscopic sleeve gastrectomy: A double blind, randomized, placebo controlled study. Egypt. J. Anaesth. 2014, 30, 285–292. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Wang, X.; Niu, Z.; Wang, P.; Yuan, L.; Wang, P. The Effect of Opioid-Free Anesthesia with Transversus Abdominis Plane Block on Patients Undergoing Laparoscopic Sleeve Gastrectomy: Randomized Controlled Study. J. Pain Res. 2024, 17, 2881–2890. [Google Scholar] [CrossRef]
- Albrecht, E.; Kirkham, K.R.; Endersby, R.V.W.; Chan, V.W.S.; Jackson, T.; Okrainec, A.; Penner, T.; Jin, R.; Brull, R. Ultrasound-Guided Transversus Abdominis Plane (TAP) Block for Laparoscopic Gastric-Bypass Surgery:a Prospective Randomized Controlled Double-Blinded Trial. Obes. Surg. 2013, 23, 1309–1314. [Google Scholar] [CrossRef]
- Saber, A.A.; Lee, Y.C.; Chandrasekaran, A.; Olivia, N.; Asarian, A.; Al-Ayoubi, S.; DiGregorio, R. Efficacy of transversus abdominis plane (TAP) block in pain management after laparoscopic sleeve gastrectomy (LSG): A double-blind randomized controlled trial. Am. J. Surg. 2019, 217, 126–132. [Google Scholar] [CrossRef]
- Karaveli, A.; Kaplan, S.; Kavakli, A.S.; Kosar, M.N.; Mayir, B. The Effect of Ultrasound-Guided Erector Spinae Plane Block on Postoperative Opioid Consumption and Respiratory Recovery in Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Study. Obes. Surg. 2025, 35, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.F.; Abdelghany, M.S.; Abu Elyazed, M.M. Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Laparoscopic Bariatric Surgery: A Prospective Randomized Controlled Trial. Pain Pract. 2021, 21, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.S.; KamalELDin, D.M.; Nofal, W.H. Pre-emptive quadratus lumborum block for laparoscopic bariatric surgery: A prospective randomized controlled study. Ain-Shams J. Anesthesiol. 2021, 13, 21. [Google Scholar] [CrossRef]
- Xue, Q.; Chu, Z.; Zhu, J.; Zhang, X.; Chen, H.; Liu, W.; Jia, B.; Zhang, Y.; Wang, Y.; Huang, C.; et al. Analgesic Efficacy of Transverse Abdominis Plane Block and Quadratus Lumborum Block in Laparoscopic Sleeve Gastrectomy: A Randomized Double-Blinded Clinical Trial. Pain Ther. 2022, 11, 613–626. [Google Scholar] [CrossRef]
- Ozel, E.S.; Kaya, C.; Turunc, E.; Ustun, Y.B.; Cebeci, H.; Dost, B. Analgesic efficacy of the external oblique intercostal fascial plane block on postoperative acute pain in laparoscopic sleeve gastrectomy: A randomized controlled trial. Korean J. Anesthesiol. 2025, 78, 159–170. [Google Scholar] [CrossRef]
- Turunc, E.; Dost, B.; Ozel, E.S.; Kaya, C.; Ustun, Y.B.; Bilgin, S.; Ozbalci, G.S.; Koksal, E. Bilateral Ultrasound-Guided External Oblique Intercostal Block Vs. Modified Thoracoabdominal Nerve Block Through Perichondrial Approach for Postoperative Analgesia in Patients Undergoing Laparoscopic Sleeve Gastrectomy Surgery: A Randomized Controlled Study. Obes. Surg. 2024, 34, 3726–3734. [Google Scholar] [CrossRef]
- McDonnell, J.G.; O’Donnell, B.; Curley, G.; Heffernan, A.; Power, C.; Laffey, J.G. The Analgesic Efficacy of Transversus Abdominis Plane Block After Abdominal Surgery: A Prospective Randomized Controlled Trial. Anesth. Analg. 2007, 104, 193–197. [Google Scholar] [CrossRef]
- Filardi, K.; Filardi, R.; Wegner, B.; Arias, J.; da Silva, G.; Felippe, V. Ultrasound-Guided Transversus Abdominis Plane Block as an Effective Path to Reduce Opioid Consumption After Laparoscopic Bariatric Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2024, 34, 4244–4254. [Google Scholar] [CrossRef]
- Yang, G.; Wang, P.; Yin, Y.; Qu, H.; Zhao, X.; Jin, X.; Chu, Q. Erector spinae plane block versus paravertebral block on postoperative quality of recovery in obese patients undergoing laparoscopic sleeve gastrectomy: A randomized controlled trial. PeerJ 2024, 12, e17431. [Google Scholar] [CrossRef]
- Jinaworn, P.; Pannangpetch, P.; Bunanantanasan, K.; Manomaisantiphap, S.; Udomsawaengsup, S.; Thepsoparn, M.; Saeyup, P. Efficacy of Erector Spinae Plane Block on Postoperative Analgesia for Patients Undergoing Metabolic Bariatric Surgery: A Randomized Controlled Trial. Obes. Surg. 2024, 34, 4211–4219. [Google Scholar] [CrossRef]
- Hung, K.-C.; Liu, W.-C.; Hsu, C.-W.; Wu, J.-Y.; Liao, S.-W.; Chen, I.-W. Efficacy of Erector Spinae Plane Block on Analgesic Outcomes in Patients Undergoing Metabolic Surgery: A Meta-Analysis of Randomized Controlled Trials. Obes. Surg. 2025, 35, 1135–1145. [Google Scholar] [CrossRef]
- Eipe, N.; Budiansky, A.S. Perioperative Pain Management in Bariatric Anesthesia. Saudi J. Anaesth. 2022, 16, 339–346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).