Neoadjuvant Treatment Approaches to Oral Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
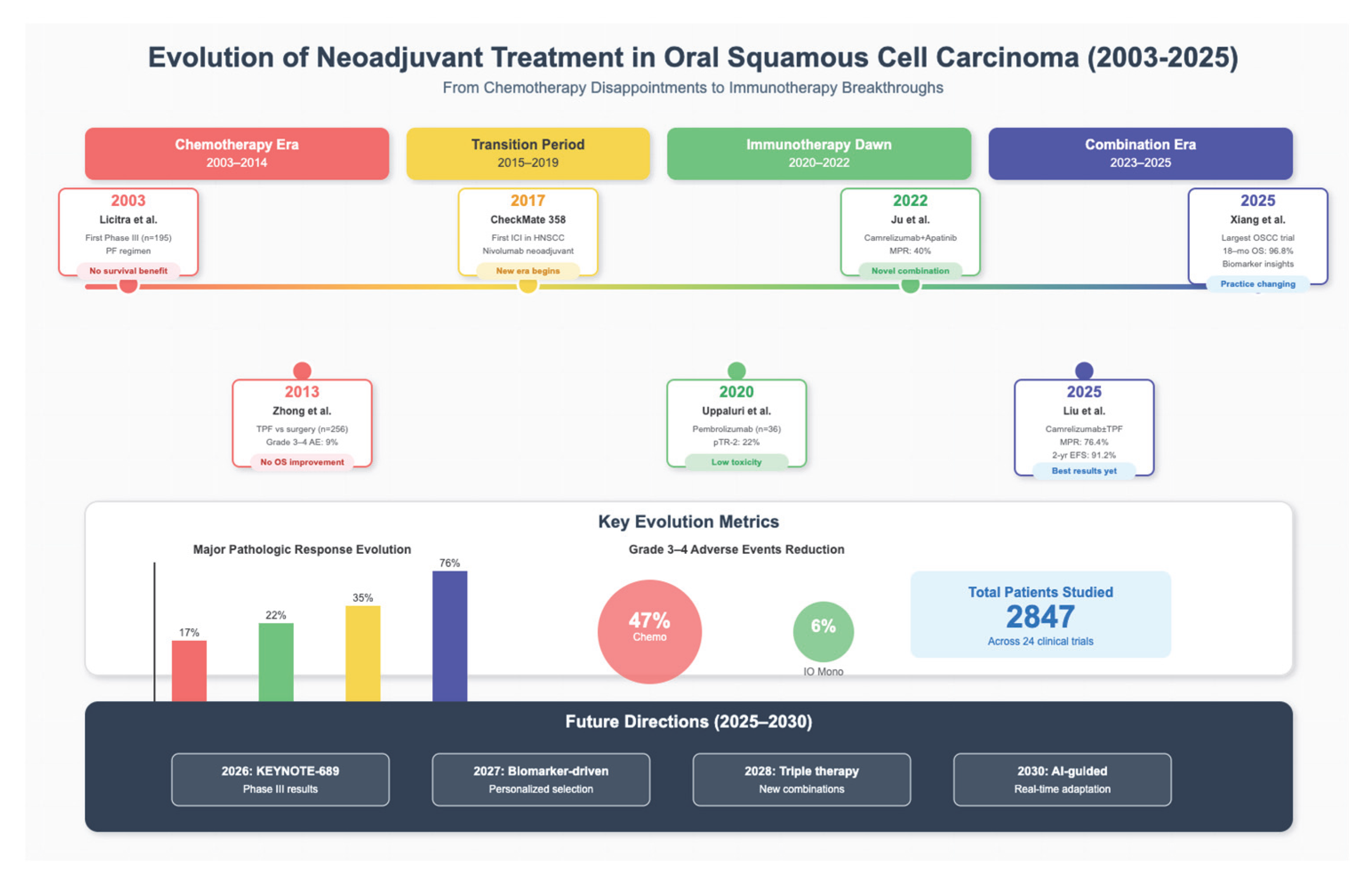
3.1. Study Selection and Characteristics
3.2. Completed Clinical Trials
| Study | Clinical Trial Identifier | Phase | Participants No. | Disease | Treatment Regimen | Primary Endpoint | Response Rate | Survival Outcomes | Key Findings | ≥10 PR, % | cPR, % | Grade 3–4 AEs | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Licitra et al. [2] | NA | III | 195, randomized (98 PF, 97 surgery alone) | T2-4 N0-2M0 OSCC | PF × 3 cycles vs. Surgery alone | Overall survival (OS) | Clinical: 80% vs. NA | 5-yr OS: 55% in both arms (p = 0.767) | No survival benefit with neoadjuvant PF. | 80% | x | 3% | Moderate |
| Zhong et al. [6] | NA | III | 256, randomized (128 TPF, 128 surgery alone) | Locally advanced resectable Stage III–IVA OSCC | TPF vs. Surgery alone | OS | Clinical: 80.6% vs. NA | 3-yr OS: 74.1% vs. 74.3% (p = 0.83) | No survival benefit; trend toward reduced distant. | 80.6% | x | 9% | Short treatment window Moderate |
| Bossi et al. [11] | NA | III | 198; randomized | T2–T4, N0–N2 OSCC | cisplatin 100 mg/m2 and fluorouracil 1000 mg/m2 × 3 cycles, vs. upfront surgery | Occurrence of locoregional or distant tumor relapse, death. | x | 10-yr OS: 46.5%; 10-yr DFS: 48.5% | No difference in the incidence of locoregional relapse between groups, nor in distant mets. No difference in OS. | x | 27 | x | Moderate |
| Ghi et al. [5] | NA | II–III | 421; randomized (206 TPF + chemoradiation (CRT), 208 CRT) | Stage III–IV locally advanced head and neck squamous cell carcinoma (LAHNSCC) | TPF × 3 cycles and CRT vs. CRT alone | OS | overall response rate (ORR) was 76% after induction chemo | Significantly higher OS (57.5% vs. 46.5%; p = 0.031) and 3-yr DFS (47 vs. 38.5% p = 0.013) in TPF + CRT arm | Median OS and the 3- year OS was higher in the IC arm. | x | x | Neutropenia G3–4 was significantly higher in the IC arm (4% versus 1%). No significant differences were observed in other G3–4 toxicities | Moderate to high risk |
| Noronha et al. [10] | NA | III | 495; randomized (248 TP; 247 TPF) | Stage III–IVA OSCC | TPF vs. TP × 2 cycles | Overall survival | pCR: 10.7% vs. 15.5% | 5-year OS was significantly higher in the TPF arm (23.9% vs. 18.5%; HR = 0.778, CI 0.637–0.952, p = 0.015) | x | x | 39.1% TP, 72.5% TPF | Moderate | |
| Chaukar et al. [19] | CTRI/2021/03/032390 | II | 68; randomized (34 upfront surgery and adjuvant treatment, 34 TPF) | cT2-T4N0/N + M0 | TPF (docetaxel 75 mg/m2 day 1, cisplatin 75 mg/m2 day 1, fluorouracil 750 mg/m2 days 1–5) × 2 cycles, surgery, and adjuvant chemoradiotherapy × 6 cycles (treatment arm) vs. surgery and adjuvant treatment × 6 cycles (control arm) | Mandible preservation rate | Complete clinical response: 2.9% (treatment arm) Partial response (defined as >50% reduction: 35.2% (treatment arm) | Mandibular preservation rate: 47% in treatment arm. DFS (p = 0.715, HR 0.911, CI 0.516–1.607) and OS (p = 0.747, HR 0.899, 95% CI 0.510–1.587) were not significantly different between both arms. 5.8% of patients in the treatment arm experienced disease progression. | Chemotherapy-induced toxicity G3–4 observed in 73.6% in the treatment arm | Low |
| Study | Clinical Trial Identifier | Phase | Participants No. | Disease | Treatment Regimen | Primary Endpoint | Key Outcomes | ≥10 PR, % | cPR, % | Grade 3–4 AEs, No. | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoadjuvant Immunotherapy | |||||||||||
| Timár et al. [20] | NA | II | 39; non-randomized (single arm) | T2-3N0M0 OSCC | Local neoadjuvant IL-2 (interleukin-2) injection (800 IU/d) Low-dose cyclophosphamide, indomethacin, zinc and multivitamins (5 doses/week over 3 weeks) | Clinical response Pathologic response | Overall response rate: 42%, pCR: 5%, MPR: 5% (defined as >50%). | x | 5% | None | Multicenter clinical trial Moderate |
| Knochelmann et al. [13] | NCT03021993 | II | 12; non-randomized (single arm) | Resectable stage II-IVA OSCC | Nivolumab (3 mg/kg 3 to 4 biweekly doses) | Objective response rate = complete + partial response rate | Overall response rate: 30%.All patients with stable disease alive and 2 deaths due to progression after median follow-up time of 10 months (immunotherapy response rate). | x | x | None | High risk |
| Schoenfeld et al. [21] | NCT02919683 | II | 29 (14 pts nivolumab, 15 pts nivolumab/ipilimumab) | Untreated oral squamous cell carcinoma (≥T2, or clinically node positive) | Nivolumab alone (3 mg/kg on week 1 and 3) or nivolumab and ipilimumab (1 mg/kg on week 1) | Safety and volumetric response | 4 patients had major/complete pathologic response greater than 90%. 1-year progression-free survival was 85% (N) & overall survival was 89% (N + I). | x | x | grade 3 to 4 events in 2 (N), and 5 (N + I) patients | Moderate |
| Uppaluri et al. [12] | NCT02296684 | II | 36; non-randomized (single arm) | Resectable HPV-ve OSCC | Pembrolizumab (single dose of 200 mg) | pTR-2 (pathologic tumor response ≥ 50% resection bed with tumor necrosis, keratinous debris, and giant cells/histiocytes)1-year relapse rate if high-risk pathology | pTR-2 22%. 1-year relapse rate was 16.7% in high-risk pathology (lower than historical rate of 35%). | x | x | None | Moderate to high risk |
| Yoon et al. [22] | NCT04883645 | Pilot clinical trial | 15; non-randomized (single arm) | T1-2N0M0 resectable OSCC | Topical imiquimod 5% | irMPR (immune-related pathologic response) ≥ 50% reduction in tumor cell count in response to treatment | irMPR 60%. Partial response 40%. % RVT (residual viable tumor) 25–65%. >50% reduction in tumor cell count in 60% of patients. 1-year recurrence free survival 93%. | x | x | 13% | High risk |
| Neoadjuvant Immuno(chemo)therapy | |||||||||||
| Huang et al. [16] | NCT04473716 | I | 20; non-randomized (single intervention) | locally advanced resectable III/IVA OSCC | Toripalimab (PD-1 inhibitor) 240 mg + albumin paclitaxel (260 mg/m2) and cisplatin 75 mg/m2 (TTP) × q3w for 2 cycles | Safety, MPR | pCR 30%, MPR 60%, ORR 60%. | x | 30% | 30% | Moderate |
| Wu et al. [17] | ChiCTR2200056354 | II | 31; non-randomized(18 OSCC, 13 OPSCC; single intervention) | stage III-IV resectable or potentially resectable locally advanced OSCC or OPSCC (oropharyngeal squamous cell carcinoma) | Tislelizumab (200 mg), albumin-bound paclitaxel (260 mg/m2), and cisplatin (60–75 mg/m2) q3w for 2 cycles | MPR | MPR 65.5%, ORR 61.3%, pCR 41.4%. | x | 41.4% | 10% | x |
| Liu et al. [15] | NCT04649476 | II | 68 (34 per arm) | resectable locally advanced III-IVA OSCC | Camrelizumab (200 mg q3w for 3 cycles +/− TPF chemotherapy q3w for 2 cycles (docetaxel 75 mg/m2, cisplatin 75 mg/m2, 5-fluorouracil 750 mg/m2 days 1–5, days 22–26) | MPR | MPR (Cam) 14.7%, MPR (Cam + TPF) 76.4%. 2-year EFS Cam and Cam + TPF 52.9% and 91.2%, respectively.91.2% respectively. | Arm Cam 14.7%, Arm Cam + TPF 76.4% | Arm Cam 0% Arm Cam + TPF 29.4% | Arm Cam 6% Arm Cam + TPF 47% | Low–moderate |
| Xiang et al. [18] | x | II | 31; non-randomized (single arm) | OSCC | Neoadjuvant camrelizumab (200 mg) + nab-paclitaxel (260 mg/m2) + cisplatin (75 mg/m2), adjuvant chemoradiotherapy and camrelizumab q3w for 2 cycles | MPR | pCR 41.4%, MPR 69%, ORR 82.8%. 18-month OS 96.8%. 18-month disease-free survival 85.71%. CD4_Tfh_CXCL13 cells predictive of MPR. | x | 41.4 | 6.5% | Moderate |
| Other Combinations | |||||||||||
| Ju et al. [14] | NCT04393506 | I | 20; non-randomized (single arm) | locally advanced resectable OSCC | Camrelizumab (200 mg) q2w + apatinib (250 mg/daily) | safety & MPR, defined as ≤10% residual viable tumor cells | MPR rate = 40%. 18-monthlocoregional recurrence and survival rates of 10.5% and 95%. All patients with PDL-1 CPS > 10 reached MPR. | x | 5% | none | Moderate to high risk |
3.3. Ongoing Clinical Trials
- -
- NCT05798793: A phase III multicenter randomized trial evaluating camrelizumab combined with docetaxel and cisplatin chemotherapy versus docetaxel and cisplatin chemotherapy alone in resectable locally advanced OSCC;
- -
- NCT06277791: An exploratory single-arm study of adrelimab plus docetaxel and cisplatin in stage IVB OSCC;
- -
- NCT06219980: A phase II single-arm trial combining stereotactic body radiotherapy (SBRT) with sindilizumab, docetaxel, and cisplatin in locally advanced OSCC and oropharyngeal squamous cell carcinoma;
- -
- NCT06353685: A phase II single-arm study examining neoadjuvant immunotherapy plus chemotherapy followed by adjuvant continuous hyperfractionated accelerated radiotherapy (CHART);
- -
- NCT05125055 (Illuminate-2): A phase II/III randomized trial comparing neoadjuvant toripalimab plus chemotherapy (TTP) versus TPF chemotherapy in locally advanced resectable OSCC.
3.4. Study Outcomes and Characteristics
4. Discussion
4.1. Current Treatment Landscape and Rationale for Neoadjuvant Therapy
4.2. Neoadjuvant Chemotherapy in OSCC
4.3. Neoadjuvant Immunotherapy (Checkpoint Inhibitors)
4.4. Emerging Clinical Trial Data and Ongoing Studies
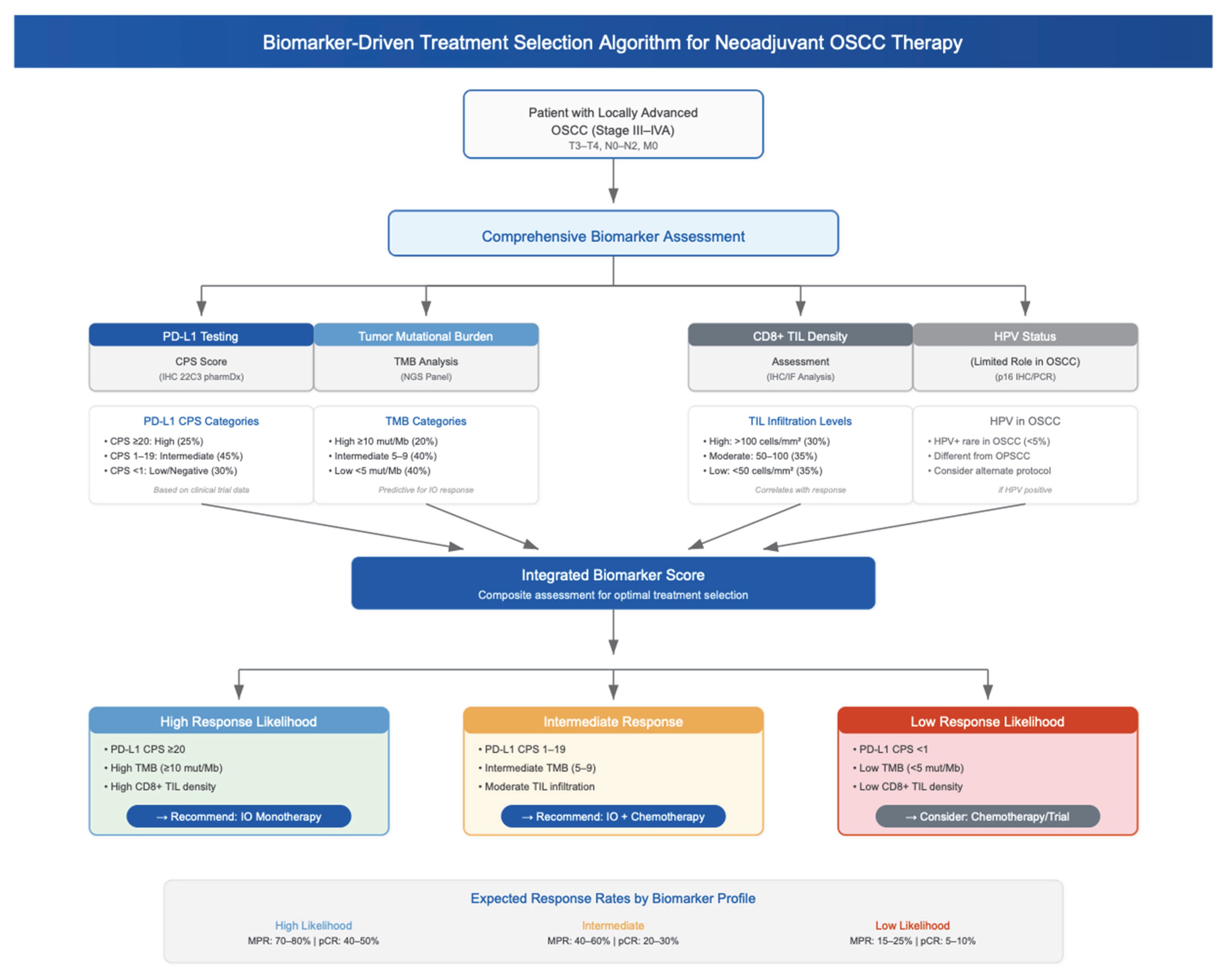
4.5. Biomarker-Based Patient Selection
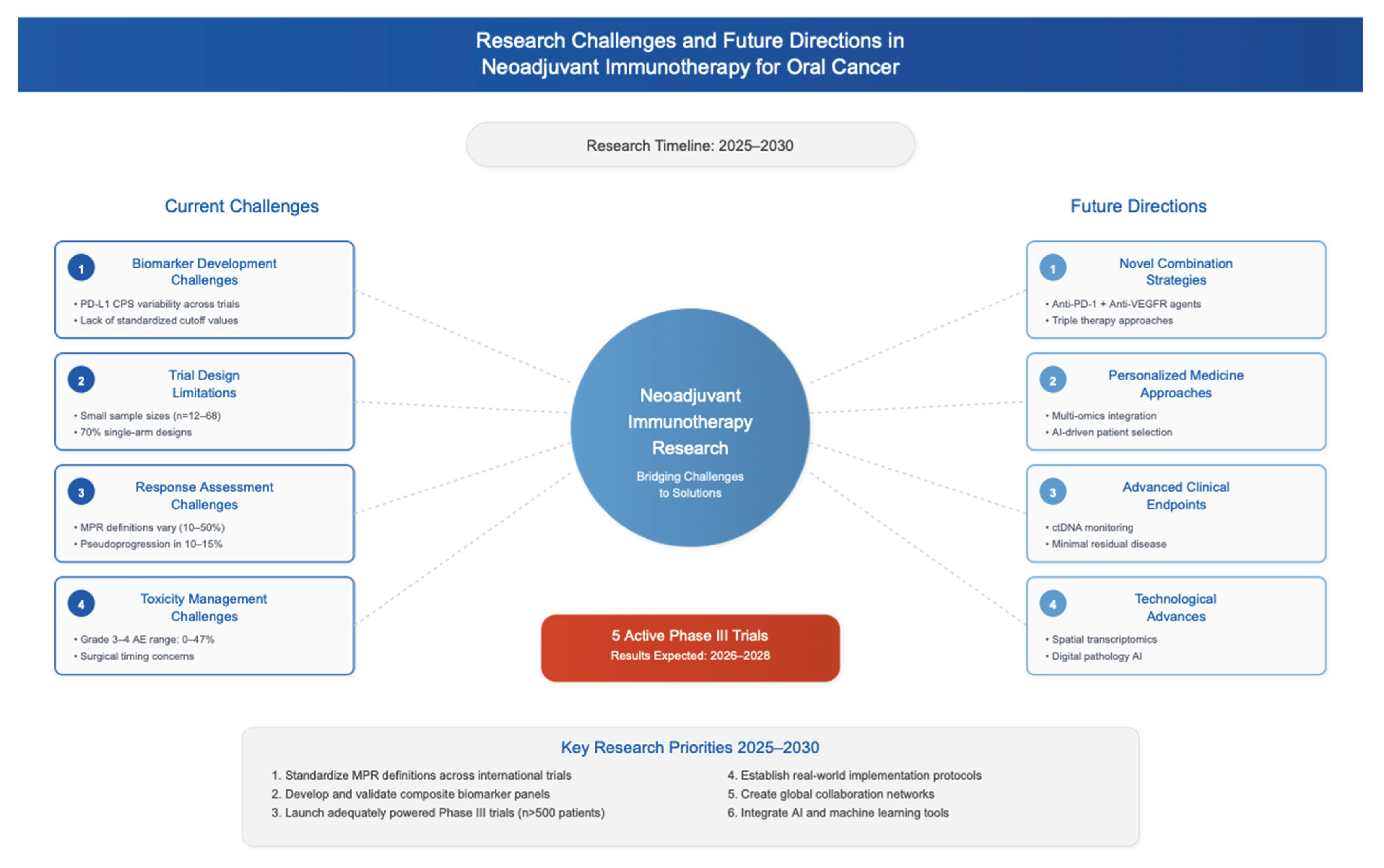
4.6. Global Perspectives and Challenges
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEs | Adverse events |
| CHART | Continuous hyperfractionated accelerated radiotherapy |
| CPS | Combined positive score |
| CRT | Chemoradiotherapy |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DFS | Disease-free survival |
| EFS | Event-free survival |
| ENE | Extranodal extension |
| HNSCC | Head and neck squamous cell carcinoma |
| IC | Induction chemotherapy |
| IL-2 | Interleukin-2 |
| IO | Immuno-oncology |
| irAEs | Immune-related adverse events |
| LAHNSCC | Locally advanced head and neck squamous cell carcinoma |
| MPR | Major Pathologic Response |
| MSI | Microsattelite instability |
| NPR | No pathologic response |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| OS | Overall survival |
| OSCC | Oral squamous cell carcinoma |
| pCR | Pathologic Complete Response |
| PD-L1 | Programmed Death Ligand-1 |
| PD1 | Programmed Cell Death Protein 1 |
| PF | Cisplatin and 5-fluorouracil |
| pPR | Partial pathologic response |
| PR | Pathologic response |
| R1 | Positive surgical margins |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RVT | Residual viable Tumor cell |
| SBRT | Stereotactic Body Radiotherapy |
| SOC | Standard of care (surgery, adjuvant radiotherapy +/− cisplatin) |
| TIL | Tumor-infiltrating lymphocytes |
| TMB | Tumor mutational burden |
| TPF | Docetaxel, Cisplatin, 5-FU |
| TPF | Docetaxel-cisplatin-5-fluorouracil |
References
- Windon, M.J.; Saba, N.F.; Amit, M. Ushering in Neoadjuvant Immune Checkpoint Inhibitors for Mucosal Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol.–Head Neck Surg. 2025, 151, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Grandi, C.; Guzzo, M.; Mariani, L.; Vullo, S.L.; Valvo, F.; Quattrone, P.; Valagussa, P.; Bonadonna, G.; Molinari, R.; et al. Primary Chemotherapy in Resectable Oral Cavity Squamous Cell Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2003, 21, 327–333. [Google Scholar] [CrossRef]
- Amin, N.; Maroun, C.A.; El Asmar, M.; Alkhatib, H.H.; Guller, M.; Herberg, M.E.; Zhu, G.; Seiwert, T.Y.; Pardoll, D.; Eisele, D.W.; et al. Neoadjuvant Immunotherapy Prior to Surgery for Mucosal Head and Neck Squamous Cell Carcinoma: Systematic Review. Head Neck 2022, 44, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Indu, S.; Gautami, D.; VarRuchi, S. Oral Squamous Cell Carcinoma (OSCC) in Humans: Etiological Factors, Diagnostic and Therapeutic Relevance. Res. J. Biotechnol. 2020, 15, 10. [Google Scholar]
- Ghi, M.G.; Paccagnella, A.; Ferrari, D.; Foa, P.; Alterio, D.; Codecà, C.; Nolè, F.; Verri, E.; Orecchia, R.; Morelli, F.; et al. Induction TPF Followed by Concomitant Treatment versus Concomitant Treatment Alone in Locally Advanced Head and Neck Cancer. A Phase II–III Trial. Ann. Oncol. 2017, 28, 2206–2212. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, C.; Ren, G.; Guo, W.; William, W.N.; Sun, J.; Zhu, H.; Tu, W.; Li, J.; Cai, Y.; et al. Randomized Phase III Trial of Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil Followed by Surgery Versus Up-Front Surgery in Locally Advanced Resectable Oral Squamous Cell Carcinoma. J. Clin. Oncol. 2013, 31, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant Checkpoint Blockade for Cancer Immunotherapy. Science 2020, 367, eaax0182. [Google Scholar] [CrossRef]
- Contrera, K.J.; Kansara, S.; Goyal, N.; Mady, L.J.; Puram, S.V.; Young, G.D.; Zandberg, D.P.; Uppaluri, R.; Ferrarotto, R.; Holsinger, F.C.; et al. Neoadjuvant Therapy for Mucosal Head and Neck Squamous Cell Carcinoma: A Review from the American Head and Neck Society. JAMA Otolaryngol. Neck Surg. 2025, 151, 615. [Google Scholar] [CrossRef]
- Uppaluri, R.; Haddad, R.I.; Tao, Y.; Le Tourneau, C.; Lee, N.Y.; Westra, W.; Chernock, R.; Tahara, M.; Harrington, K.J.; Klochikhin, A.L.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2025, 393, 37–50. [Google Scholar] [CrossRef]
- Noronha, V.; Patil, V.; Chaturvedi, P.; Mathrudev, V.; Menon, N.; Bhattacharjee, A.; Singh, A.; Peelay, Z.; Chakraborty, S.; Jadhav, M.; et al. Phase 3 RCT Comparing Docetaxel-Platinum with Docetaxel-Platinum-5FU as Neoadjuvant Chemotherapy in Borderline Resectable Oral Cancer. Eur. J. Cancer 2024, 200, 113560. [Google Scholar] [CrossRef]
- Bossi, P.; Lo Vullo, S.; Guzzo, M.; Mariani, L.; Granata, R.; Orlandi, E.; Locati, L.; Scaramellini, G.; Fallai, C.; Licitra, L. Preoperative Chemotherapy in Advanced Resectable OCSCC: Long-Term Results of a Randomized Phase III Trial. Ann. Oncol. 2014, 25, 462–466. [Google Scholar] [CrossRef]
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase 2 Trial. Clin. Cancer Res. 2020, 26, 5140–5152. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Horton, J.D.; Liu, S.; Armeson, K.; Kaczmar, J.M.; Wyatt, M.M.; Richardson, M.S.; Lomeli, S.H.; Xiong, Y.; Graboyes, E.M.; et al. Neoadjuvant Presurgical PD-1 Inhibition in Oral Cavity Squamous Cell Carcinoma. Cell Rep. Med. 2021, 2, 100426. [Google Scholar] [CrossRef]
- Ju, W.; Xia, R.; Zhu, D.; Dou, S.; Zhu, G.; Dong, M.; Wang, L.; Sun, Q.; Zhao, T.; Zhou, Z.; et al. A Pilot Study of Neoadjuvant Combination of Anti-PD-1 Camrelizumab and VEGFR2 Inhibitor Apatinib for Locally Advanced Resectable Oral Squamous Cell Carcinoma. Nat. Commun. 2022, 13, 5378. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Xiong, X.-P.; Yu, Z.-L.; Shao, Z.; Chen, G.-L.; Liu, Y.-T.; Wang, X.-X.; Fu, Q.-Y.; Cheng, X.-X.; Li, J.; et al. Neoadjuvant Immunotherapy with or without Chemotherapy in Locally Advanced Oral Squamous Cell Carcinoma: Randomized, Two-Arm, Phase 2 Trial. Cell Rep. Med. 2025, 6, 101930. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, J.; Li, J.; Zhu, D.; Dong, M.; Dou, S.; Tang, Y.; Shi, W.; Sun, Q.; Zhao, T.; et al. Neoadjuvant Immunochemotherapy for Locally Advanced Resectable Oral Squamous Cell Carcinoma: A Prospective Single-Arm Trial (Illuminate Trial). Int. J. Surg. 2023, 109, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yao, J.; Zhang, T.; Zhang, J. Neoadjuvant Tislelizumab Combined with Chemotherapy in Locally Advanced Oral or Oropharyngeal Squamous Cell Carcinoma: A Single-Arm, Phase II Trial. J. Clin. Oncol. 2023, 41, e18072. [Google Scholar] [CrossRef]
- Xiang, Z.; Wei, X.; Zhang, Z.; Tang, Y.; Chen, L.; Tan, C.; Zeng, Y.; Wang, J.; Zhao, G.; Dai, Z.; et al. Efficacy, Safety and Single-Cell Analysis of Neoadjuvant Immunochemotherapy in Locally Advanced Oral Squamous Cell Carcinoma: A Phase II Trial. Nat. Commun. 2025, 16, 3968. [Google Scholar] [CrossRef]
- Chaukar, D.; Prabash, K.; Rane, P.; Patil, V.M.; Thiagarajan, S.; Ghosh-Laskar, S.; Sharma, S.; Pai, P.S.; Chaturvedi, P.; Pantvaidya, G.; et al. Prospective Phase II Open-Label Randomized Controlled Trial to Compare Mandibular Preservation in Upfront Surgery with Neoadjuvant Chemotherapy Followed by Surgery in Operable Oral Cavity Cancer. J. Clin. Oncol. 2022, 40, 272–281. [Google Scholar] [CrossRef]
- Tímár, J.; Ladányi, A.; Forster-Horváth, C.; Lukits, J.; Döme, B.; Remenár, É.; Godény, M.; Kásler, M.; Bencsik, B.; Répássy, G.; et al. Neoadjuvant Immunotherapy of Oral Squamous Cell Carcinoma Modulates Intratumoral CD4/CD8 Ratio and Tumor Microenvironment: A Multicenter Phase II Clinical Trial. J. Clin. Oncol. 2005, 23, 3421–3432. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.-H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563. [Google Scholar] [CrossRef]
- Yoon, A.J.; Carvajal, R.D.; Graboyes, E.M.; Kaczmar, J.M.; Albergotti, W.G.; Kejner, A.E.; Troob, S.H.; Philipone, E.; Anoma, J.-S.; Armeson, K.E.; et al. Pilot Clinical Trial of Neoadjuvant Toll-like Receptor 7 Agonist (Imiquimod) Immunotherapy in Early-Stage Oral Squamous Cell Carcinoma. Front. Immunol. 2025, 16, 1530262. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Head and Neck Cancers, Version 4.2025; National Comprehensive Cancer Network (NCCN): Plymouth Meeting, PA, USA, 2025.
- Zinner, R.G.; Obasaju, C.K.; Spigel, D.R.; Weaver, R.W.; Beck, J.T.; Waterhouse, D.M.; Modiano, M.R.; Hrinczenko, B.; Nikolinakos, P.G.; Liu, J.; et al. PRONOUNCE: Randomized, Open-Label, Phase III Study of First-Line Pemetrexed + Carboplatin Followed by Maintenance Pemetrexed versus Paclitaxel + Carboplatin + Bevacizumab Followed by Maintenance Bevacizumab in Patients Ith Advanced Nonsquamous Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 134–142. [Google Scholar] [CrossRef]
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without Tremelimumab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: EAGLE, a Randomized, Open-Label Phase III Study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Liu, S.; Bellile, E.; Nguyen, A.; Zarins, K.; D’Silva, N.; Rozek, L.; Wolf, G.T.; Sartor, M.A.; Moyer, J.; Patel, M.; et al. Characterization of the Immune Response in Patients with Cancer of the Oral Cavity after Neoadjuvant Immunotherapy with the IRX-2 Regimen. Oral Oncol. 2021, 123, 105587. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; Van De Wiel, B.; Kvistborg, P.; Krijgsman, O.; Van Den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus Adjuvant Ipilimumab plus Nivolumab in Macroscopic Stage III Melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, N.Y. JAVELIN Head and Neck 100: A Phase III Trial of Avelumab and Chemoradiation for Locally Advanced Head and Neck Cancer. Future Oncol. 2019, 15, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.J.; Agrawal, N.; Juloori, A.; Cursio, J.; Gooi, Z.; Blair, E.; Chin, J.; Ginat, D.; Pasternak-Wise, O.; Hasina, R.; et al. Neoadjuvant Nivolumab Plus Chemotherapy Followed by Response-Adaptive Therapy for HPV+ Oropharyngeal Cancer: OPTIMA II Phase 2 Open-Label Nonrandomized Clinical Trial. JAMA Oncol. 2024, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.G.; Erstad, D.J.; Wargo, J.A. Neoadjuvant Therapy for Melanoma: Rationale for Neoadjuvant Therapy and Pivotal Clinical Trials. Ther. Adv. Med. Oncol. 2022, 14, 17588359221083052. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Campbell, P.J.; Stratton, M.R. Deciphering Signatures of Mutational Processes Operative in Human Cancer. Cell Rep. 2013, 3, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-camarero, S.; Merino-Menéndez, S.; Cabrera-Martín, M.; Sotelo, M.; Plaza-Hernández, J.; Falahat, F.; Iglesias-Moreno, M.; Pérez-Segura, P. Safety and Preliminary Activity of Pembrolizumab-carboplatin-paclitaxel in Heavily Pretreated and/or Fragile Patients with PDL1-positive Recurrent/Metastatic Head and Neck Cancer. Oncol. Lett. 2022, 25, 37. [Google Scholar] [CrossRef]
- Parekh, J.; Parikh, K.; Reuss, J.E.; Friedlaender, A.; Addeo, A. Current Approaches to Neoadjuvant Immunotherapy in Resectable Non-Small Cell Lung Cancer. Curr. Oncol. Rep. 2023, 25, 913–922. [Google Scholar] [CrossRef]
- Vos, J.L.; Elbers, J.B.W.; Krijgsman, O.; Traets, J.J.H.; Qiao, X.; Van Der Leun, A.M.; Lubeck, Y.; Seignette, I.M.; Smit, L.A.; Willems, S.M.; et al. Neoadjuvant Immunotherapy with Nivolumab and Ipilimumab Induces Major Pathological Responses in Patients with Head and Neck Squamous Cell Carcinoma. Nat. Commun. 2021, 12, 7348. [Google Scholar] [CrossRef]
- Ferris, R.L.; Gonçalves, A.; Baxi, S.S.; Martens, U.M.; Gauthier, H.; Langenberg, M.; Spanos, W.C.; Leidner, R.S.; Kang, H.; Russell, J.; et al. An Open-Label, Multicohort, Phase 1/2 Study in Patients with Virus-Associated Cancers (CheckMate 358): Safety and Efficacy of Neoadjuvant Nivolumab in Squamous Cell Carcinoma of the Head and Neck (SCCHN). Ann. Oncol. 2017, 28, v628–v629. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Bell, D.; Rubin, M.L.; Hutcheson, K.A.; Johnson, J.M.; Goepfert, R.P.; Phan, J.; Elamin, Y.Y.; Torman, D.K.; Warneke, C.L.; et al. Impact of Neoadjuvant Durvalumab with or without Tremelimumab on CD8+ Tumor Lymphocyte Density, Safety, and Efficacy in Patients with Oropharynx Cancer: CIAO Trial Results. Clin. Cancer Res. 2020, 26, 3211–3219. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Li, G.; Yi, M.; Zhang, Z.; Zhong, G.; Xu, L.; Jiang, R.; Zheng, Y.; Huang, L.; et al. Neoadjuvant with Low-Dose Radiotherapy, Tislelizumab, Albumin-Bound Paclitaxel, and Cisplatin for Resectable Locally Advanced Head and Neck Squamous Cell Carcinoma: Phase II Single-Arm Trial. Nat. Commun. 2025, 16, 4608. [Google Scholar] [CrossRef]
- Bourhis, J.; Auperin, A.; Borel, C.; Lefebvre, G.; Racadot, S.; Geoffrois, L.; Sun, X.S.; Saada, E.; Cirauqui, B.; Rutkowski, T.; et al. NIVOPOSTOP (GORTEC 2018-01): A Phase III Randomized Trial of Adjuvant Nivolumab Added to Radio-Chemotherapy in Patients with Resected Head and Neck Squamous Cell Carcinoma at High Risk of Relapse. J. Clin. Oncol. 2025, 43, LBA2. [Google Scholar] [CrossRef]
- Liu, J.; O’Donnell, J.S.; Yan, J.; Madore, J.; Allen, S.; Smyth, M.J.; Teng, M.W.L. Timing of Neoadjuvant Immunotherapy in Relation to Surgery Is Crucial for Outcome. OncoImmunology 2019, 8, e1581530. [Google Scholar] [CrossRef]
- Fatima, S.; Hosein, M.; Butt, S.A.; Baig, F.; Siddiqui, R.A.; Abidi, F. Immunohistochemical Analysis of Expression of Cyclin D1 in Different Grades of Oral Squamous Cell Carcinoma. J. Pharm. Res. Int. 2022, 34, 7–12. [Google Scholar] [CrossRef]
- Chaukar, D.; Dandekar, M.; Kane, S.; Arya, S.; Purandare, N.; Rangarajan, V.; D’Cruz, A. Invasion of the Mandible in Gingivobuccal Complex Cancers: Histopathological Analysis of Routes of Tumour Entry and Correlation with Preoperative Assessment. Oral Oncol. 2018, 86, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Egloff, A.M.; Afeyan, A.B.; Wolff, J.O.; Zeng, Z.; Chernock, R.D.; Zhou, L.; Messier, C.; Lizotte, P.; Pfaff, K.L.; et al. Preexisting Tumor-Resident T Cells with Cytotoxic Potential Associate with Response to Neoadjuvant Anti–PD-1 in Head and Neck Cancer. Sci. Immunol. 2023, 8, eadf4968. [Google Scholar] [CrossRef] [PubMed]


| Clinical Trial Identifier | Phase | Study Design | Disease | Treatment Regimen | Primary Endpoint | Status |
|---|---|---|---|---|---|---|
| NCT05798793 | III | Multicentre Randomized | Resectable locally advanced OSCC | Camrelizumab combined with docetaxel and cisplatin chemotherapy vs. docetaxel and cisplatin chemotherapy | Event-free survival | Active |
| NCT06277791 | Exploratory | Single arm | Stage IVB OSCC | Adrelimab + docetaxel and cisplatin Chemoradiation or radiation depending on functional outcomes after resection | pCR and MPR | Active |
| NCT06219980 | II | Single arm | Locally advanced OSCC and oropharyngeal squamous cell carcinoma | Stereotactic body radiotherapy (SBRT) + sindilizumab + docetaxel and cisplatin | pCR and Safety | Active |
| NCT06353685 | II | Single arm | Locoregionally advanced OSCC | Neoadjuvant immunotherapy + chemotherapy + adjuvant Continuous hyperfractionated accelerated radiotherapy (CHART) | 2-year progression-free survival for patients who achieve pCR and MPR | Active |
| NCT05125055 (Illuminate-2) | II/II | Randomized | Locally advanced resectable OSCC | Neoadjuvant TTP vs. TPF chemotherapy | MPR | Active |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siafa, L.; Ali, A.; Kerr, P.; Pathak, A.; Viallet, N.; Lane, C.; Sayed, S. Neoadjuvant Treatment Approaches to Oral Cancer. J. Clin. Med. 2025, 14, 6883. https://doi.org/10.3390/jcm14196883
Siafa L, Ali A, Kerr P, Pathak A, Viallet N, Lane C, Sayed S. Neoadjuvant Treatment Approaches to Oral Cancer. Journal of Clinical Medicine. 2025; 14(19):6883. https://doi.org/10.3390/jcm14196883
Chicago/Turabian StyleSiafa, Lyna, Aisha Ali, Paul Kerr, Alok Pathak, Norbert Viallet, Ciaran Lane, and Suhail Sayed. 2025. "Neoadjuvant Treatment Approaches to Oral Cancer" Journal of Clinical Medicine 14, no. 19: 6883. https://doi.org/10.3390/jcm14196883
APA StyleSiafa, L., Ali, A., Kerr, P., Pathak, A., Viallet, N., Lane, C., & Sayed, S. (2025). Neoadjuvant Treatment Approaches to Oral Cancer. Journal of Clinical Medicine, 14(19), 6883. https://doi.org/10.3390/jcm14196883






