Feasibility of a Green Breast Surgery Protocol to Reduce Carbon Footprint of Care: BuGS Trial Interim Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization and Treatment
2.3.1. Randomization
2.3.2. BuGS Group
2.3.3. Control Group
2.4. Data Collection
2.4.1. Demographic Data and Clinical Outcome
2.4.2. Environmental Outcome
2.4.3. Quality of Life and Satisfaction Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BC | Breast Cancer |
| BCS | Breast Conserving Surgery |
| BMI | Body Mass Index |
| BuGS Trial | Breast Green Surgery Trial |
| CCI | Charlson Comorbidity Index |
| CMW | Contaminated Medical Waste |
| CET | Comitato Etico Territoriale (Territorial Ethical Committee) |
| CONSORT | Consolidated Standards of Reporting Trials |
| EBC | Early Breast Cancer |
| EPT | Estrogen–Progestin Therapy |
| ERAS | Enhanced Recovery After Surgery |
| EROM | Environment Reported Outcome Measures |
| GHG | Greenhouse Gas |
| Hr | Hour |
| LOS | Length of Stay |
| MAC | Monitored Anesthesia Care |
| MW | Medical Waste |
| NCMW | Non-Contaminated Medical Waste |
| NPRS | Numeric Pain Rating Scale |
| PaMW | Paper Medical Waste |
| Pl | Plastic Medical Waste |
| PF | Physical Functioning |
| PPR | Postoperative Pain at Rest |
| PPD | Postoperative Pain During Movement |
| PROM | Patients’ Reported Outcome Measures |
| PSQ-18 | Short-Form Patient Satisfaction Questionnaire |
| QoL | Quality of Life |
| RCT | Randomized Clinical Trial |
| SF | Short-Form |
| SoC | Standard of Care |
| TSQ | Telemedicine Satisfaction Questionnaire |
| TIVA | Total Intravenous Anesthesia |
| VGA | Volatile General Anesthesia |
Appendix A
Appendix B
| Item | BuGS Group | Control Group |
|---|---|---|
| Preadmission | ||
| Preadmission information, education, and counseling | Preoperative and detailed preoperative counseling. | Preoperative and detailed preoperative counseling. |
| Preadmission information, participants’ and hospital optimization | For daily smokers, 1 mo of abstinence prior to surgery is beneficial. | For daily smokers, 1 mo of abstinence prior to surgery is beneficial. |
| For obese patients, weight reduction to achieve a BMI ≤ 30 kg/m2 prior to surgery is beneficial. | For obese patients, weight reduction to achieve a BMI ≤ 30 kg/m2 prior to surgery is beneficial. | |
| For alcohol abusers, 1 mo of abstinence prior to surgery is beneficial. | For alcohol abusers, 1 mo of abstinence prior to surgery is beneficial. | |
| For specific patients, referral should be made to resources that can support these behavior changes. | For specific patients, referral should be made to resources that can support these behavior changes. | |
| Surgical room schedule optimization to avoid unnecessary hospitalization. | Surgical room schedule optimization. | |
| Outpatient examination. | Preoperative admitted patient examination. | |
| Preoperative | ||
| Admission | Admission of the participants in Breast Unit Outpatient clinic. | Hospitalization. |
| Hospital staff should not wear non-sterile gloves to move patients unless they come in contact with mucous membranes, biological fluids, or wounds. | Not clearly stated. | |
| Reduce energy consumption, air conditioning, and lighting in unused rooms. | Reduce energy consumption, air conditioning, and lighting in unused rooms. | |
| Preoperative fasting | Preoperative fasting should be minimized, and patients should be allowed to drink clear fluids up to 2 h prior to surgery. | Preoperative fasting from midnight regardless of the surgical schedule. |
| Preoperative carbohydrate loading | Preoperative maltodextrin-based drinks should be given to patients 2 h prior to surgery. | None. |
| Venous thromboembolism prophylaxis | Participants should be assessed for venous thromboembolism risk. Unless contraindicated, and balanced by the risk of bleeding; patients at a higher risk should receive low-molecular-weight heparin or unfractionated heparin until ambulatory. Mechanical methods should be added. | Participants should be assessed for venous thromboembolism risk. Unless contraindicated, and balanced by the risk of bleeding; patients at a higher risk should receive low-molecular-weight heparin or unfractionated heparin until ambulatory. Mechanical methods should be added. |
| Intraoperative | ||
| Antimicrobial prophilaxis | Clorexidine skin preparation should be performed. Intravenous antibiotics should be given within 1 h prior to surgery if needed (e.g., contaminate surgery, postoperative surgical drain, frail patients). | Clorexidine skin preparation should be performed. Intravenous antibiotics should be given within 1 h prior to surgery if needed (e.g., contaminate surgery, postoperative surgical drain, frail patients). |
| Postoperative nausea and vomiting prophylaxis | Participants should receive preoperative and intraoperative medications to mitigate postoperative nausea and vomiting. | Participants should receive preoperative and intraoperative medications to mitigate postoperative nausea and vomiting. |
| Preoperative and intraoperative analgesia | Participants should receive multimodal analgesia to mitigate pain. | Participants should receive multimodal analgesia to mitigate pain. |
| Participants should receive preoperative locoregional inter-fascial plane blocks, if clinically safe. | According to the anesthesiologist’s preference. | |
| Preparing for surgery | Reusable textiles. | Reusable textiles. |
| Reduce water and energy consumption. | Reduce water and energy consumption. | |
| Reduce invasive intervention (e.g., antibiotics, surgical drains, catheter). | No clear indication. | |
| Standard anesthesia protocol | Local anesthesia or awake multimodal protocol as an opioid-free MAC where local anesthesia, inter-fascial plane blocks, with or without mild sedation, with target RAS > −3 and <1, without use of airway management devices. | According to the anesthesiologist’s preference. |
| Targeted O2 delivery only if necessary. | According to the anesthesiologist’s preference. | |
| Minimize drug waste (“Don’t open it unless you need it”, pre-empt propofol use). | According to the anesthesiologist’s preference. | |
| Preventing intraoperative hypothermia | Preoperative and intraoperative measures, such as forced air, to prevent hypothermia should be instituted. Temperature monitoring is required to ensure the patient’s body temperature is maintained above 36 °C. | According to the anesthesiologist’s preference. |
| Perioperative intravenous fluid management | Overresuscitation or underresuscitation of fluids should be avoided, and water and electrolyte balance should be maintained. Goal-directed therapy is a useful method of achieving these goals. Balanced crystalloid solutions are recommended. Vasopressors are recommended to support fluid management. | According to the anesthesiologist’s preference. |
| Intraoperative personnel and equipment | Monitor the number of staff in the room. | Monitor the number of staff in the room. |
| Create an instrument preference list for each operation: separate essential vs. optional items to have ready ad hoc. | According to the surgeon’s preference. | |
| Reduce single-use surgical instruments and integrated reusable items. | According to the surgeon’s preference. | |
| Minimize item waste (“Don’t open it unless you need it”). | According to the surgeon’s preference. | |
| Hospital staff should not wear non-sterile gloves to move patients unless they come in contact with mucous membranes, biological fluids, or wounds. | According to the surgeon’s preference. | |
| Waste segregation | Recycle or use lowest carbon waste streams as appropriate (use domestic or recycling waste streams for all packaging). | SoC. |
| Ensure only appropriate contents in sharps bins (sharps/drugs). | SoC. | |
| Arrange metals/battery collection where possible. | SoC. | |
| Postoperative | ||
| Postoperative analgesia | Multimodal postoperative pain management regimens are opioid-sparing and should be used with locoregional peripheral nerve block, if not already performed in the preoperative setting. | According to the anesthesiologist’s preference. |
| Early feeding | Patients should be encouraged to intake fluids and food orally as soon as possible, preferably within 3 h after surgery if awake surgery protocol was maintained. | According to the anesthesiologist’s preference. |
| Postoperative wound management | For incisional closure, conventional sutures are recommended. Complex wounds following skin necrosis are treatable with debridement and negative pressure wound therapy. | According to the surgeon’s preference. |
| Postoperative | ||
| Postoperative analgesia | Multimodal postoperative pain management regimens are opioid-sparing and should be used with locoregional peripheral nerve block, if not already performed in the preoperative setting. | According to the anesthesiologist’s preference. |
| Early feeding | Patients should be encouraged to intake fluids and food orally as soon as possible, preferably within 3 h after surgery if awake surgery protocol was maintained. | According to the anesthesiologist’s preference. |
| Postoperative wound management | For incisional closure, conventional sutures are recommended. Complex wounds following skin necrosis are treatable with debridement and negative pressure wound therapy. | According to the surgeon’s preference. |
| Early mobilization and discharge | Patients should be mobilized within 2 h after surgery. Early physiotherapy, supervised exercise programs, and other supportive care initiatives should be instituted after discharge. | According to the surgeon’s and anesthesiologist’s preference. |
| Manage in the outpatient clinic and discharge after 3 h from surgery. | Hospitalization. | |
| Post-discharge home support and physiotherapy | Encourage telehealth and admission to GP for postoperative care. | Hospital admission. |
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- De Boniface, J.; Szulkin, R.; Johansson, A.L.V. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg. 2021, 156, 628. [Google Scholar] [CrossRef] [PubMed]
- Millen, J.-C.; Sibia, U.; Jackson, K.; Stern, S.L.; Orozco, J.I.J.; Fancher, C.E.; Grumley, J. Comparing Costs: Does Extreme Oncoplastic Breast-Conserving Surgery Confer a Cost Benefit When Compared with Mastectomy and Reconstruction? Ann. Surg. Oncol. 2024, 31, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Costanzo, G.; Pellicciaro, M.; Materazzo, M.; Buonomo, C.; Federico, T.; Giacobbi, E.; Servadei, F.; Anemona, L.; Noce, A.; et al. Awake Breast Surgery: A Systematic Review. In Vivo 2023, 37, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Rajaee, A.N.; Olson, D.W.; Freelove, D.; Velupillai, N.; Buro, K.; Sondekoppam, R.V.; Özelsel, T.J.-P. Comparison of the Quality of Recovery-15 Score in Patients Undergoing Oncoplastic Breast-Conserving Surgery under Monitored Anesthesia Care versus General Anesthesia: A Prospective Quality Improvement Study. Can. J. Anesth./J. Can. D’anesthésie 2023, 70, 1928–1938. [Google Scholar] [CrossRef]
- Buonomo, O.C.; Vinci, D.; De Carolis, G.; Pellicciaro, M.; Petracca, F.; Sadri, A.; Buonomo, C.; Dauri, M.; Vanni, G. Role of Breast-Conserving Surgery on the National Health System Economy From and to SARS-COVID-19 Era. Front. Surg. 2022, 8, 705174. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Paital, B.; Naghdali, Z.; Mortezania, Z.; Hashemi, M.; Karamati Niaragh, E.; Aghababaei, M.; Ghorbankhani, M.; Lichtfouse, E.; Sillanpää, M.; et al. Positive Environmental Effects of the Coronavirus 2020 Episode: A Review. Environ. Dev. Sustain. 2021, 23, 12738–12760. [Google Scholar] [CrossRef]
- Vanni, G.; Materazzo, M.; Chemello, S.; Pellicciaro, M.; Marsella, V.; Calenda, F.; Eskiu, D.; Lisi, G.; Pistilli, G.; Bastone, S.A.; et al. Revolutionizing Women’s Health Care with a Sustainable Change: Breast Green Surgery (BuGS) Protocol. World Cancer Res. J. 2024, 11, e2830. [Google Scholar] [CrossRef]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between Air Pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef]
- Rume, T.; Islam, S.M.D.-U. Environmental Effects of COVID-19 Pandemic and Potential Strategies of Sustainability. Heliyon 2020, 6, e04965. [Google Scholar] [CrossRef]
- Ihsan, F.R.; Bloomfield, J.G.; Monrouxe, L.V. Triple Planetary Crisis: Why Healthcare Professionals Should Care. Front. Med. 2024, 11, 1465662. [Google Scholar] [CrossRef]
- Rizan, C.; Steinbach, I.; Nicholson, R.; Lillywhite, R.; Reed, M.; Bhutta, M.F. The Carbon Footprint of Surgical Operations: A Systematic Review. Ann. Surg. 2020, 272, 986–995. [Google Scholar] [CrossRef]
- Rizan, C.; Reed, M.; Mortimer, F.; Jones, A.; Stancliffe, R.; Bhutta, M. Using Surgical Sustainability Principles to Improve Planetary Health and Optimise Surgical Services Following the COVID-19 Pandemic. Bull. R. Coll. Surg. Engl. 2020, 102, 177–181. [Google Scholar] [CrossRef]
- Vanni, G.; Materazzo, M.; Pellicciaro, M.; Marino, D.; Buonomo, O.C. From Patient Reported Outcome Measure (PROM) to Environment Related Outcome Measure (EROM): Towards “Green Breast Surgery”. In Vivo 2023, 37, 1867–1872. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated Guideline for Reporting Randomised Trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef] [PubMed]
- Robb, H.D.; Pegna, V. The Intercollegiate Green Theatre Checklist. Bull. R. Coll. Surg. Engl. 2023, 105, 64–67. [Google Scholar] [CrossRef]
- Temple-Oberle, C.; Shea-Budgell, M.A.; Tan, M.; Semple, J.L.; Schrag, C.; Barreto, M.; Blondeel, P.; Hamming, J.; Dayan, J.; Ljungqvist, O. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast. Reconstr. Surg. 2017, 139, 1056e–1071e. [Google Scholar] [CrossRef]
- Royal College of Pathologists. Guidelines for Non-Operative Diagnostic Procedures and Reporting in Breast Cancer Screening; The Royal College of Pathologists: London, UK, 2021. [Google Scholar]
- Khatun, A.; Ghosh, A.; Acyatan, M.; Khokher, A.; Cooke, L.; Helme, S.; Mansfield, L.; Agrawal, A.; St John, E.; Alder, L. Assessing the Efficacy of Clavien-Dindo Classification and Comprehensive Complication Index (CCI) in Reporting Post Operative Complications Following Breast Surgery. Do We Need a Breast Specific Complication System? Eur. J. Surg. Oncol. 2024, 50, 109448. [Google Scholar] [CrossRef]
- Tan, E.; Lim, D. Carbon Footprint of Dermatologic Surgery. Australas. J. Dermatol. 2021, 62, e170–e177. [Google Scholar] [CrossRef]
- Rizan, C.; Bhutta, M.F.; Reed, M.; Lillywhite, R. The Carbon Footprint of Waste Streams in a UK Hospital. J. Clean. Prod. 2021, 286, 125446. [Google Scholar] [CrossRef]
- Manfredi, S.; Tonini, D.; Christensen, T.H.; Scharff, H. Landfilling of Waste: Accounting of Greenhouse Gases and Global Warming Contributions. Waste Manag. Res. J. Sustain. Circ. Econ. 2009, 27, 825–836. [Google Scholar] [CrossRef]
- DEFRA. Experimental Statistics on the Carbon Impact of Waste from Households Managed by Local Authorities in England. Available online: https://www.gov.uk/government/statistics/experimental-statistics-on-the-carbon-impact-of-waste-from-households-managed-by-local-authorities-in-england (accessed on 29 July 2025).
- Institute for Applied Ecology. Climate Impact of Pyrolysis of Waste Plastic Packaging in Comparison with Reuse and Mechanical Recycling; Institute for Applied Ecology: Corvallis, OR, USA, 2022. [Google Scholar]
- European Commission. Joint Research Centre. Environmental Effects of Plastic Waste Recycling; Publications Office: Luxembourg, 2021. [Google Scholar]
- Pinar, U.; Anract, J.; Perrot, O.; Tabourin, T.; Chartier-Kastler, E.; Parra, J.; Vaessen, C.; De La Taille, A.; Roupret, M. Preliminary Assessment of Patient and Physician Satisfaction with the Use of Teleconsultation in Urology during the COVID-19 Pandemic. World J. Urol. 2021, 39, 1991–1996. [Google Scholar] [CrossRef]
- Bizot, A.; Karimi, M.; Rassy, E.; Heudel, P.E.; Levy, C.; Vanlemmens, L.; Uzan, C.; Deluche, E.; Genet, D.; Saghatchian, M.; et al. Multicenter Evaluation of Breast Cancer Patients’ Satisfaction and Experience with Oncology Telemedicine Visits during the COVID-19 Pandemic. Br. J. Cancer 2021, 125, 1486–1493. [Google Scholar] [CrossRef]
- Treanor, C.; Donnelly, M. A Methodological Review of the Short Form Health Survey 36 (SF-36) and Its Derivatives among Breast Cancer Survivors. Qual. Life Res. 2015, 24, 339–362. [Google Scholar] [CrossRef]
- Marshall, G.; Hays, R. The Patient Satisfaction Questionnaire Short-Form (PSQ-18); Rand: Arlington, VA, USA, 1994; Volume 7865. [Google Scholar]
- Shubeck, S.P.; Morrow, M.; Dossett, L.A. De-Escalation in Breast Cancer Surgery. Npj Breast Cancer 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-MacGregor, M.; Freedman, G.; Houssami, N.; et al. Society of Surgical Oncology–American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery with Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. Ann. Surg. Oncol. 2014, 21, 704–716. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Noguchi, N.; Morrow, M.; Houssami, N. Changes in Reoperation After Publication of Consensus Guidelines on Margins for Breast-Conserving Surgery: A Systematic Review and Meta-Analysis. JAMA Surg. 2020, 155, e203025. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918. [Google Scholar] [CrossRef]
- Donker, M.; Van Tienhoven, G.; Straver, M.E.; Meijnen, P.; Van De Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or Surgery of the Axilla after a Positive Sentinel Node in Breast Cancer (EORTC 10981-22023 AMAROS): A Randomised, Multicentre, Open-Label, Phase 3 Non-Inferiority Trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Whitrock, J.N.; Pratt, C.G.; Long, S.-A.; Carter, M.M.; Lewis, J.D.; Heelan, A.A. Implementation of Choosing Wisely Guidelines: Omission of Lymph Node Surgery. Surgery 2025, 179, 108843. [Google Scholar] [CrossRef] [PubMed]
- Park, K.U.; Somerfield, M.R.; Anne, N.; Brackstone, M.; Conlin, A.K.; Couto, H.L.; Dengel, L.T.; Eisen, A.; Harvey, B.E.; Hawley, J.; et al. Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2025, 43, 1720–1741. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, O.D.; Botteri, E.; Sangalli, C.; Galimberti, V.; Porpiglia, M.; Agresti, R.; Luini, A.; Viale, G.; Cassano, E.; Peradze, N.; et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients with Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1557. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N. Engl. J. Med. 2023, 388, 585–594. [Google Scholar] [CrossRef]
- Ivascu, R.; Torsin, L.I.; Hostiuc, L.; Nitipir, C.; Corneci, D.; Dutu, M. The Surgical Stress Response and Anesthesia: A Narrative Review. J. Clin. Med. 2024, 13, 3017. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, X.; Wang, S.; Sheng, F.; Liu, C.; Wang, Y.; Jiang, L.; Wang, J.; Feng, W. Opioid-Free Anesthesia Improves Postoperative Recovery Quality of Small and Medium-Sized Surgery: A Prospective, Randomized Controlled Study. Minerva Anestesiol. 2024, 90, 759–768. [Google Scholar] [CrossRef]
- Choi, H.; Hwang, W. Anesthetic Approaches and Their Impact on Cancer Recurrence and Metastasis: A Comprehensive Review. Cancers 2024, 16, 4269. [Google Scholar] [CrossRef]
- Enlund, M.; Berglund, A.; Ahlstrand, R.; Walldén, J.; Lundberg, J.; Wärnberg, F.; Ekman, A.; Sjöblom Widfeldt, N.; Enlund, A.; Bergkvist, L. Survival after Primary Breast Cancer Surgery Following Propofol or Sevoflurane General Anesthesia—A Retrospective, Multicenter, Database Analysis of 6305 Swedish Patients. Acta Anaesthesiol. Scand. 2020, 64, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, L.; Meng, F.; Wang, H. Regional Anesthesia Might Reduce Recurrence and Metastasis Rates in Adult Patients with Cancers after Surgery: A Meta-Analysis. BMC Anesthesiol. 2024, 24, 19. [Google Scholar] [CrossRef]
- Van Den Heuvel, S.A.; Van Der Wal, S.E.; Bronkhorst, E.M.; Warlé, M.C.; Ronday, M.; Plat, J.; Van Alfen, N.; Joosten, L.A.; Lerou, J.G.; Vissers, K.C.; et al. Acute Cytokine Response During Breast Cancer Surgery: Potential Role of Dexamethasone and Lidocaine and Relationship with Postoperative Pain and Complications—Analysis of Three Pooled Pilot Randomized Controlled Trials. J. Pain Res. 2020, 13, 1243–1254. [Google Scholar] [CrossRef]
- Freys, S.M.; Pogatzki-Zahn, E. Pain Therapy to Reduce Perioperative Complications. Innov. Surg. Sci. 2019, 4, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, D.J.; Looha, M.A.; Akbari, M.E.; Akbari, A. Predictors of Postoperative Pain Six Months after Breast Surgery. Sci. Rep. 2023, 13, 8302. [Google Scholar] [CrossRef]
- Rajan, K.K.; Fairhurst, K.; Birkbeck, B.; Novintan, S.; Wilson, R.; Savović, J.; Holcombe, C.; Potter, S. Overall Survival after Mastectomy versus Breast-Conserving Surgery with Adjuvant Radiotherapy for Early-Stage Breast Cancer: Meta-Analysis. BJS Open 2024, 8, zrae040. [Google Scholar] [CrossRef]
- Magnoni, F.; Sacchini, V.; Veronesi, P.; Bianchi, B.; Bottazzoli, E.; Tagliaferri, V.; Mazzotta, E.; Castelnovo, G.; Deguidi, G.; Rossi, E.M.C.; et al. Surgical Management of Inherited Breast Cancer: Role of Breast-Conserving Surgery. Cancers 2022, 14, 3245. [Google Scholar] [CrossRef]
- Mohamedahmed, A.Y.Y.; Zaman, S.; Zafar, S.; Laroiya, I.; Iqbal, J.; Tan, M.L.H.; Shetty, G. Comparison of Surgical and Oncological Outcomes between Oncoplastic Breast-Conserving Surgery versus Conventional Breast-Conserving Surgery for Treatment of Breast Cancer: A Systematic Review and Meta-Analysis of 31 Studies. Surg. Oncol. 2022, 42, 101779. [Google Scholar] [CrossRef]
- Pradere, B.; Mallet, R.; De La Taille, A.; Bladou, F.; Prunet, D.; Beurrier, S.; Bardet, F.; Game, X.; Fournier, G.; Lechevallier, E.; et al. Climate-Smart Actions in the Operating Theatre for Improving Sustainability Practices: A Systematic Review. Eur. Urol. 2023, 83, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Nielsen, C.J. Global Warming Potential of Inhaled Anesthetics: Application to Clinical Use. Anesth. Analg. 2010, 111, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, R.S.; Hirschi, M.; Alford, B.; Evans, T.; Kiluk, J.V.; Patel, S.Y. Enhanced REVENUE After Surgery? A Cost-Standardized Enhanced Recovery Pathway for Mastectomy Decreases Length of Stay. World J. Surg. 2019, 43, 839–845. [Google Scholar] [CrossRef]
- Wiener, A.A.; Hanlon, B.M.; Schumacher, J.R.; Vande Walle, K.A.; Wilke, L.G.; Neuman, H.B. Reexamining Time from Breast Cancer Diagnosis to Primary Breast Surgery. JAMA Surg. 2023, 158, 485. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Choi, J.; Lee, J.; Kim, J.-Y.; Kwon, S.; Kim, J.; Lee, S.; Jeon, S.; Lee, C.; Lee, S.; et al. Time to Surgery and Survival in Breast Cancer. BMC Surg. 2022, 22, 388. [Google Scholar] [CrossRef]
- Vanni, G.; Materazzo, M.; Perretta, T.; Meucci, R.; Anemona, L.; Buonomo, C.; Dauri, M.; Granai, A.V.; Rho, M.; Ingallinella, S.; et al. Impact of Awake Breast Cancer Surgery on Postoperative Lymphocyte Responses. In Vivo 2019, 33, 1879–1884. [Google Scholar] [CrossRef]
- Tevlin, R.; Panton, J.A.; Fox, P.M. Greening Hand Surgery: Targeted Measures to Reduce Waste in Ambulatory Trigger Finger and Carpal Tunnel Decompression. HAND 2023, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Caspi, J.; Facchini, B.A.; Noce, A.; Lisi, G.; Tacconi, F.; Eskiu, D.; Cervelli, V.; et al. Awake Breast Surgery and De-Escalation Treatment: Strategies for Frail and Elderly Breast Cancer Patients. World Cancer Res. J. 2023, 10, e2656. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, J.-M.; Martinez-Ubieto, J.; Muñoz-Rodes, J.-L.; Rodriguez-Fraile, J.-R.; Garcia-Erce, J.-A.; Blanco-Gonzalez, J.; Del Valle-Hernandez, E.; Abad-Gurumeta, A.; Centeno-Robles, E.; Martinez-Perez, C.; et al. Surgical Treatment for Colorectal Cancer: Analysis of the Influence of an Enhanced Recovery Programme on Long-Term Oncological Outcomes—A Study Protocol for a Prospective, Multicentre, Observational Cohort Study. BMJ Open 2020, 10, e040316. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Groups | p-Value | |
|---|---|---|---|
| BuGS Group (n = 18) | Control Group (n = 27) | ||
| Age, yrs (IQR) | 44.18 (40.28–45.91) | 46.74 (43.92–50.47) | 0.122 |
| Menopausal status n (%) | |||
| Yes | 2 (11.1%) | 8 (29.6%) | 0.272 |
| No | 16 (88.9%) | 19 (71.4%) | |
| BMI, Kg/m2 (IQR) | 23.73 (21.53–26.21) | 26.04 (21.66–27.69) | 0.555 |
| Smoke habits, yes (%) | 9 (37.5%) | 15 (63.5%) | 0.714 |
| EPT, yes (%) | 7 (58.3%) | 5 (41.7%) | 0.130 |
| CCI (IQR) | 2.50 (2–3) | 2.93 (2–3) | 0.09 |
| Distance of hospital from home, km (%) | 21.50 (7.72–29.65) | 12.00 (7.9–21.8) | 0.365 |
| Preoperative diagnosis n (%) | |||
| B2 | 3 (16.7%) | 3 (11.1%) | 0.393 |
| B3 | 10 (55.6%) | 11(40.7%) | |
| B5 | 3 (16.7%) | 11(40.7%) | |
| NA | 2 (11.1%) | 2(7.4%) | |
| Characteristics | Groups | p-Value | |
|---|---|---|---|
| BuGS Group (n = 18) | Control Group (n = 27) | ||
| Surgical room occupation min. (IQR) | 80.03 (64.84–101.87) | 133.23 (95.47–144.25) | 0.002 |
| Surgical procedure min. (IQR) | 44.72 (24.64–61.56) | 87.23 (44.68–91.34) | 0.0003 |
| Intrument list n (%) | |||
| Essential | 1 (5.6%) | 0 (0%) | 0.003 |
| Complete | 3 (16.7%) | 0 (0%) | |
| Essential + Oncoplastic | 13 (72.2%) | 16 (59.3%) | |
| Complete + Oncoplastic | 1 (5.5%) | 11 (40.7%) | |
| Anesthesia n (%) | |||
| LA | 2 (11.1%) | 0 (0%) | <0.0001 |
| Locoregional | 16 (88.9%) | 3 (11.1%) | |
| TIVA | 0 (0%) | 21 (77.8%) | |
| VGA | 0 (0%) | 3 (11.1%) | |
| Waste segregation kg (IQR) | |||
| CMW | 3.09 (2.86–3.26) | 3.95 (3.4–4.38) | <0.0001 |
| NCMW | 0.43 (0.38–0.49) | 0.94 (0.86–1.04) | <0.0001 |
| PlMW | 0.25 (0.24–0.30) | - | |
| PaMW | 0.12 (0.11–0.15) | - | |
| Waste disposal, KgCO2e (IQR) | |||
| CMW | 3.31 (3.07–3.51) | 4.24 (3.65–4.69) | <0.0001 |
| NCMW | 0.13 (0.11–0.15) | 0.28 (0.26–0.31) | <0.0001 |
| Waste disposal + recycling + secondary raw material production, KgCO2e (IQR) | |||
| NCMW | 0.19 (10.16–0.21) | 2.15 (2.07–2–44) | <0.0001 |
| Characteristics | Groups | p-Value | |||||
|---|---|---|---|---|---|---|---|
| BuGs Group (n = 18) | Control Group (n = 27) | ||||||
| Outpatient hospital admission n (IQR) | 1 (1–1.75) | 2 (2–2) | 0.002 | ||||
| KgCO2e car single route km (IQR) | 5.53 (1.99–7.63) | 3.09 (2.033–5.61) | 0.365 | ||||
| KgCO2e car all routes km (IQR) | 7.26 (1.99–13.35) | 6.07 (4.07–11.22) | 0.933 | ||||
| Sick leave | |||||||
| >1 day | 1 (5.9%) | 4 (23.5%) | 0.001 | ||||
| 1 day | 13 (76.5%) | 16 (94.1%) | |||||
| 0 days | 4 (36.6%) | 7 (63.4%) | |||||
| Clavien Dindò complication | |||||||
| 0–2 | 17 (94.4%) | 25 (92.6%) | 0.615 | ||||
| >2 | 1 (5.6%) | 2 (7.4%) | |||||
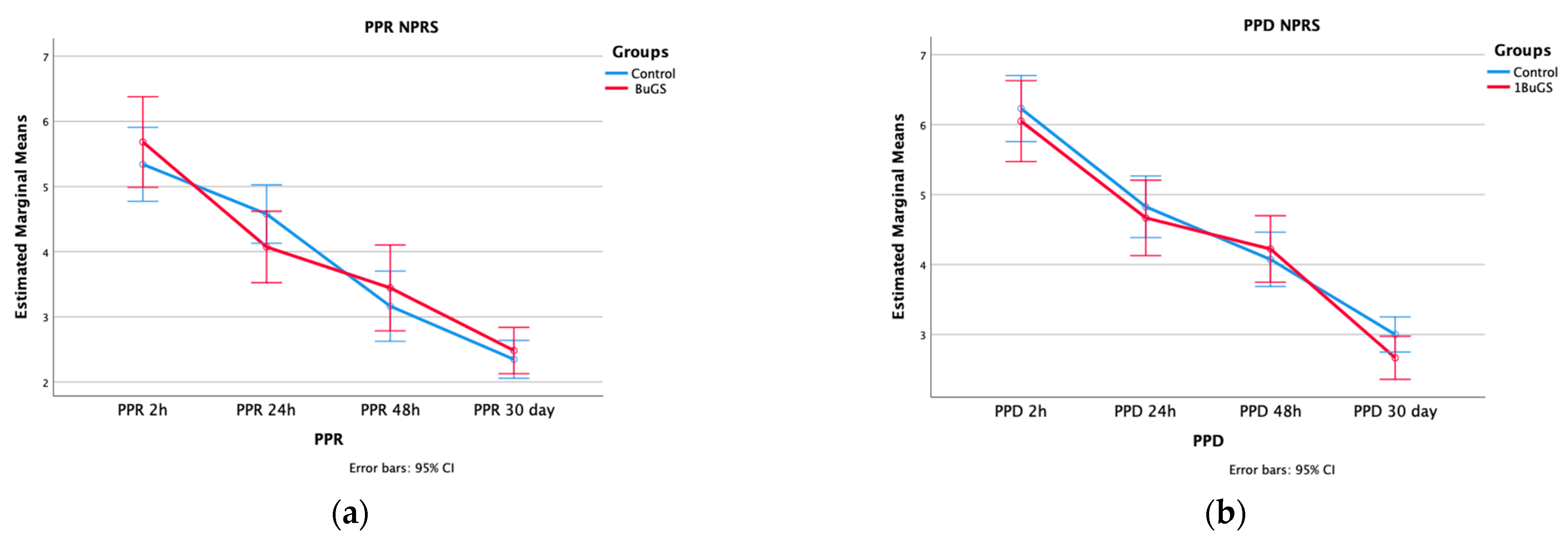
| Postoperative Pain | Wilcoxon–Mann–Whitney | ANOVA | |||||
| p-Value | Time | Time × Group | Between-Group Effect | ||||
| PPR (IQR) | |||||||
| 2 h | 6.2 (4.10–6.57) | 5 (4.40–6.15) | 0.562 | <0.001 | 0.287 | 0.779 | |
| 24 h | 4 (3.10–4.75) | 4.6 (3.90–5.2) | 0.130 | ||||
| 48 h | 3.10 (2.5–4.45) | 3.30 (2.00–4.00) | 0.907 | ||||
| 30 days | 6.20 (4.10–6.50) | 5 (4.40–6.15) | 0.665 | ||||
| PPD (IQR) | |||||||
| 2 h | 6.2 (5.1–7.15) | 6.1 (5.25–7.15) | 0.618 | <0.001 | 0.534 | 0.515 | |
| 24 h | 4.75 (3.725–5.875) | 4.5 (4.15–5.35) | 0.626 | ||||
| 48 h | 4.00 (3.25–5.00) | 4.00 (3.00–5.00) | 0.628 | ||||
| 30 days | 3.00 (2.00–3.00) | 3.00 (2.50–3.50) | 0.120 | ||||
| Hospitalization | |||||||
| Y | 9 (50%) | 27 (100%) | <0.001 | ||||
| N | 9 (50%) | 0 (0%) | |||||
| LOS | 0 (0–1) | 1 (1–2) | 0.0001 | ||||
| Groups | p-Value | |||
|---|---|---|---|---|
| BuGs Group (n = 18) | Control Group (n = 27) | |||
| SF-36 | Physical Functioning | 64.45 (56.96–70.13) | 66.05 (61.97–72.41) | 0.122 |
| Role Physical | 61.73 (57.29–69.01) | 67.04 (58.74–77.58) | 0.075 | |
| Bodily Pain | 68.39 (53.78–72.17) | 67.04 (57.95–73.35) | 0.391 | |
| General Health | 66.70 (56.02–72.79) | 66.83 (54.70–72.44) | 0.832 | |
| Vitality | 67.26 (58.70–71.64) | 67.51 (60.02–76.01) | 0.334 | |
| Social Functioning | 64.74 (54.30–72.46) | 65.26 (58.08–72.97) | 0.465 | |
| Role Emotional | 69.72 (56.20–78.91) | 71.20(67.05–78.13) | 0.188 | |
| Mental Health | 62.23 (51.95–71.11) | 66.21 (60.77–73.32) | 0.072 | |
| PSQ-18 | 4.06 (3.32–4.41) | 4.01 (3.61–4.38) | 0.871 | |
| TSQ, (IQR) | 43.00 (32.25–53.00) | - | N.A. | |
| >40 score n (%) | 12 (63.16%) | - | N.A. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, G.; Materazzo, M.; Pellicciaro, M.; Caspi, J.; Di Mauro, G.; Tacconi, F.; Eskiu, D.; Longo, B.; Cervelli, V.; Berretta, M.; et al. Feasibility of a Green Breast Surgery Protocol to Reduce Carbon Footprint of Care: BuGS Trial Interim Results. J. Clin. Med. 2025, 14, 6881. https://doi.org/10.3390/jcm14196881
Vanni G, Materazzo M, Pellicciaro M, Caspi J, Di Mauro G, Tacconi F, Eskiu D, Longo B, Cervelli V, Berretta M, et al. Feasibility of a Green Breast Surgery Protocol to Reduce Carbon Footprint of Care: BuGS Trial Interim Results. Journal of Clinical Medicine. 2025; 14(19):6881. https://doi.org/10.3390/jcm14196881
Chicago/Turabian StyleVanni, Gianluca, Marco Materazzo, Marco Pellicciaro, Jonathan Caspi, Giordana Di Mauro, Federico Tacconi, Denisa Eskiu, Benedetto Longo, Valerio Cervelli, Massimiliano Berretta, and et al. 2025. "Feasibility of a Green Breast Surgery Protocol to Reduce Carbon Footprint of Care: BuGS Trial Interim Results" Journal of Clinical Medicine 14, no. 19: 6881. https://doi.org/10.3390/jcm14196881
APA StyleVanni, G., Materazzo, M., Pellicciaro, M., Caspi, J., Di Mauro, G., Tacconi, F., Eskiu, D., Longo, B., Cervelli, V., Berretta, M., Buonomo, O. C., & on behalf of BuGS Study Group. (2025). Feasibility of a Green Breast Surgery Protocol to Reduce Carbon Footprint of Care: BuGS Trial Interim Results. Journal of Clinical Medicine, 14(19), 6881. https://doi.org/10.3390/jcm14196881









