Added Value of MAPSE to Assess LV Systolic Function in Conventional Cardiac Pacing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Screening, Enrollment and Follow-Up
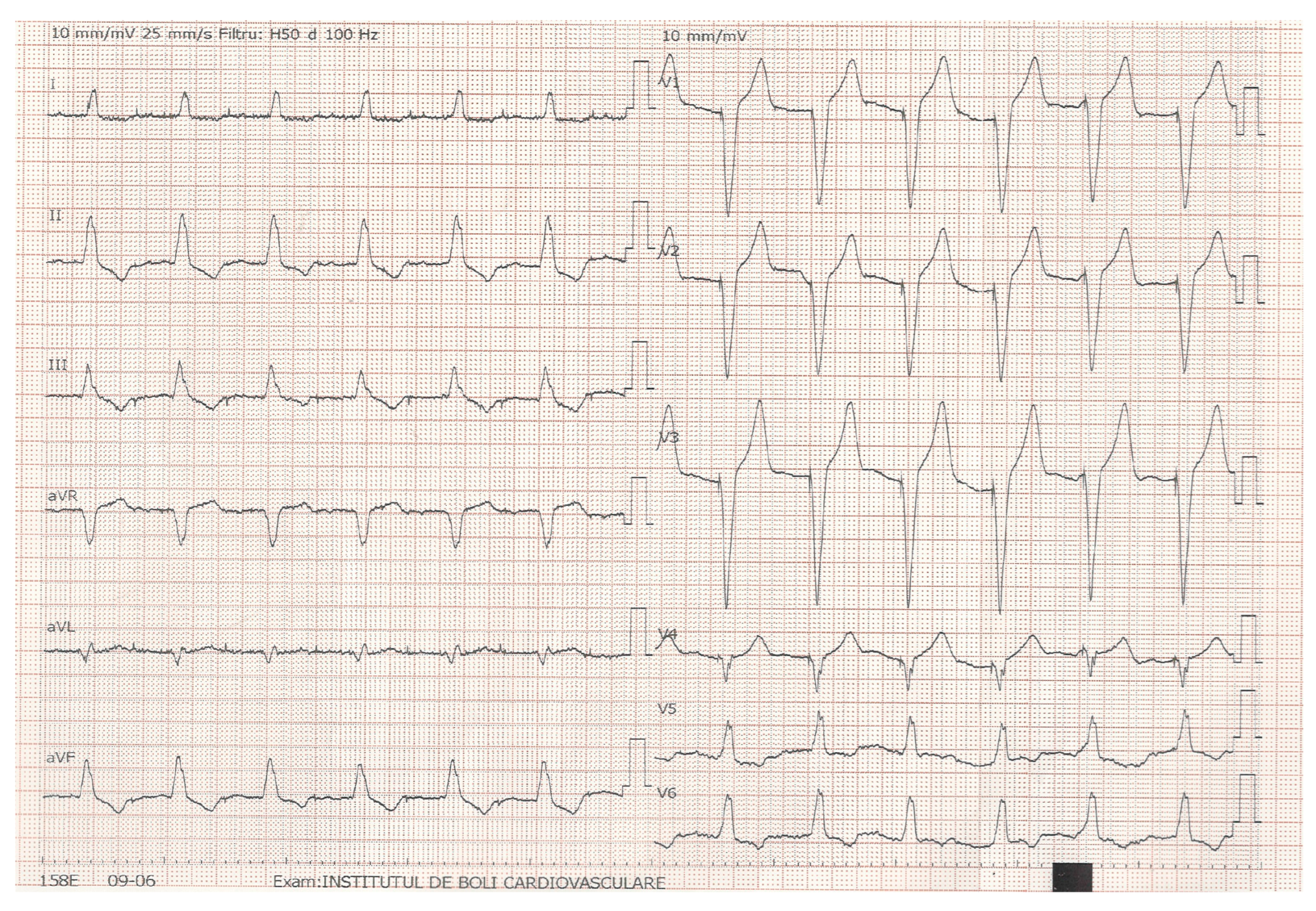
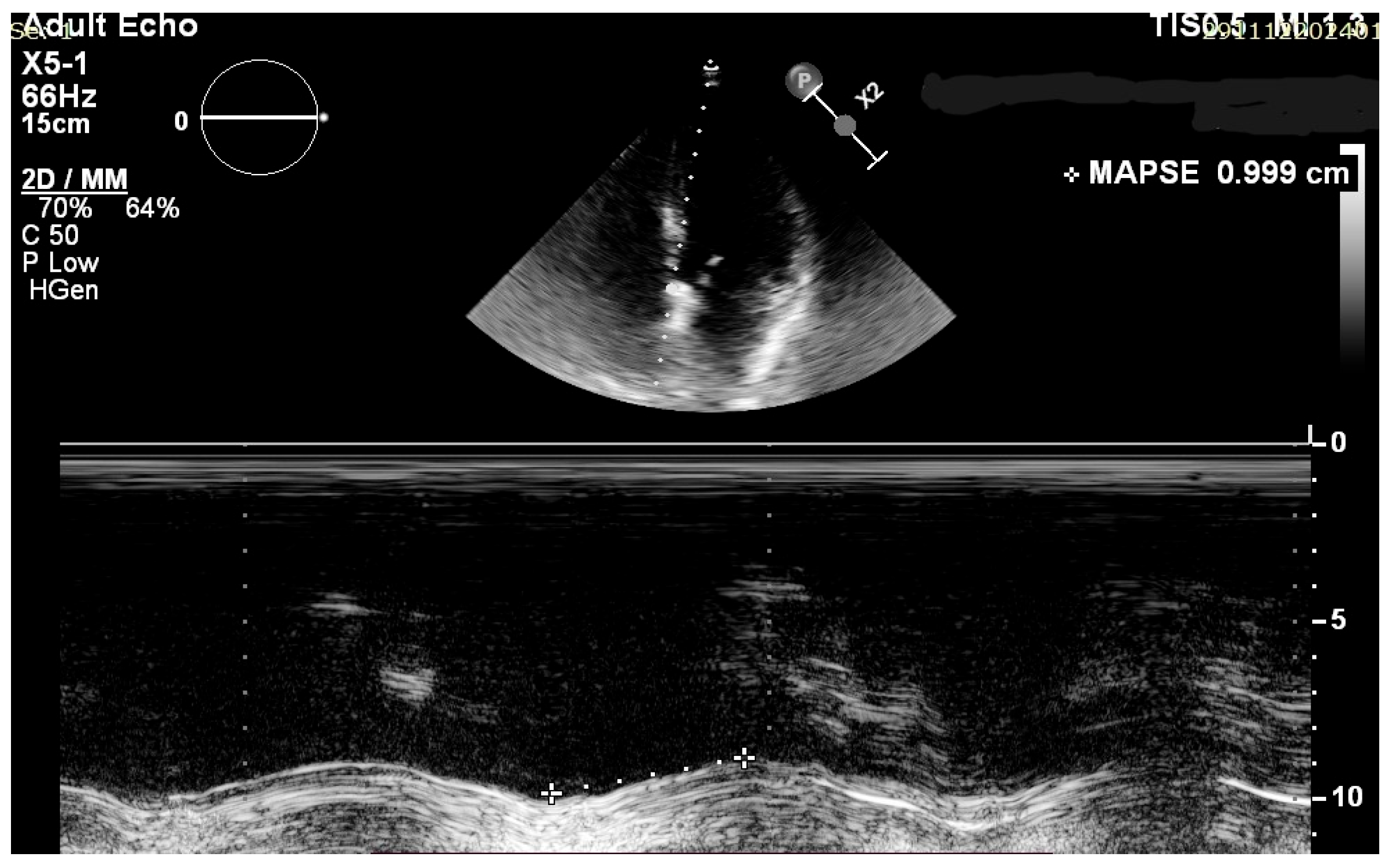
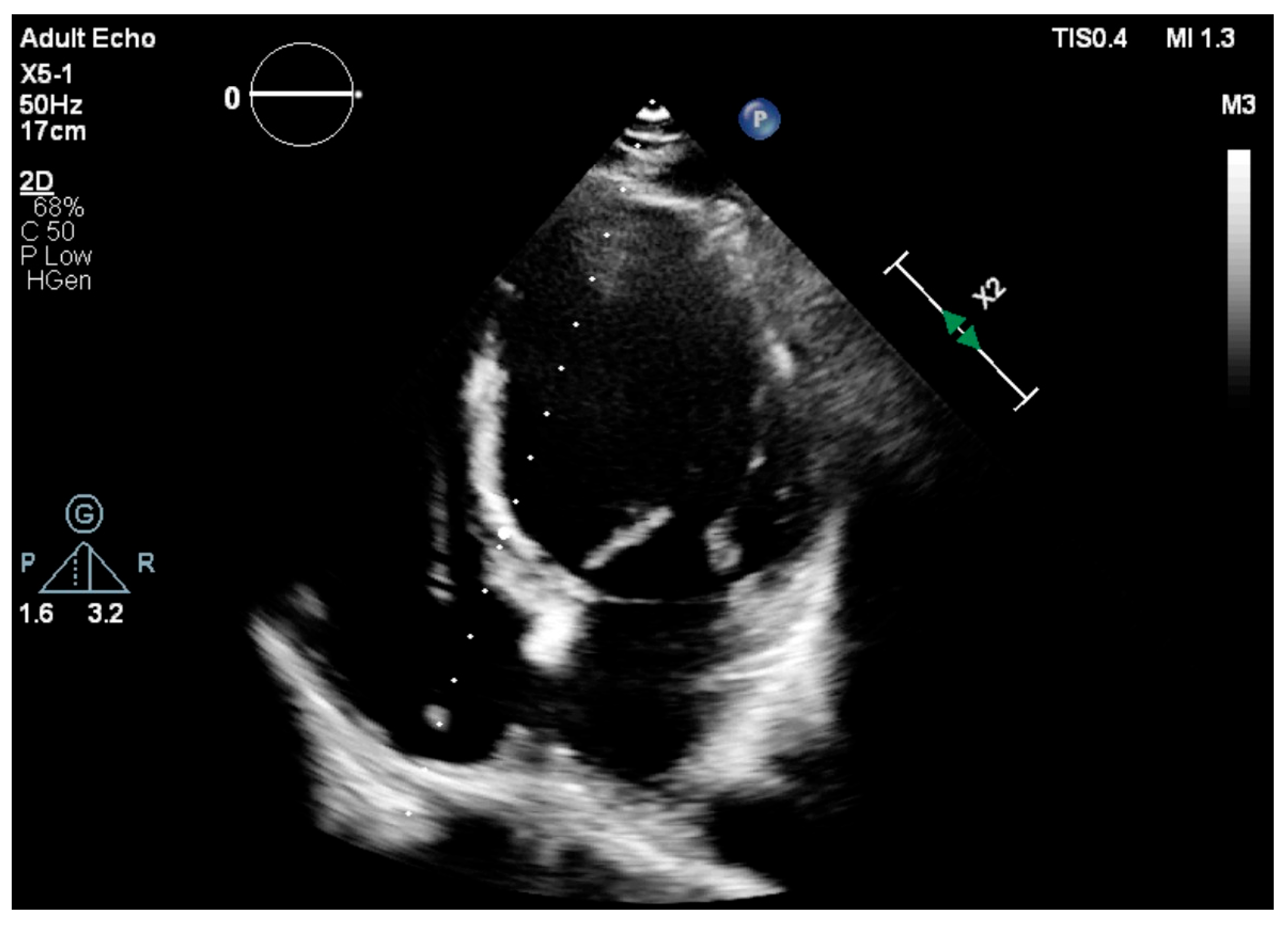
2.3. Echocardiographic Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Future Direction
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAPSE | Mitral annular plane systolic excursion |
| RV | Right ventricle |
| LV | Left ventricle |
| HF | Heart failure |
| PiCM | Pacing-induced cardiomyopathy |
| AVB | Atrioventricular block |
| CSP | Conduction system pacing |
| LVEF | Left ventricular ejection fraction |
| CRT | Cardiac resynchronization therapy |
| RAA | Right atrial appendage |
| RVA | Right ventricular apex |
| IVS | Interventricular septum |
| TTE | Transthoracic echocardiography |
| A4C | Apical 4 chamber |
| LAVi | Left atrium volume index |
| HFpEF | Heart failure with preserved ejection fraction |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| PSM | Paradoxical septal motion |
References
- Khurshid, S.; Epstein, A.E.; Verdino, R.J.; Lin, D.; Goldberg, L.R.; Marchlinski, F.E.; Frankel, D.S. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm 2014, 11, 1619–1625. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016, 13, 2272–2278. [Google Scholar] [CrossRef]
- Tayal, B.; Fruelund, P.; Sogaard, P.; Riahi, S.; Polcwiartek, C.; Atwater, B.D.; Gislason, G.; Risum, N.; Torp-Pedersen, C.; Kober, L.; et al. Incidence of heart failure after pacemaker implantation: A nationwide Danish Registry-based follow-up study. Eur. Heart J. 2019, 40, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Frankel, D.S. Pacing-Induced Cardiomyopathy. Cardiol. Clin. 2023, 41, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Somma, V.; Ha, F.J.; Palmer, S.; Mohamed, U.; Agarwal, S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm 2023, 20, 282–290. [Google Scholar] [CrossRef]
- Cirin, L.; Crișan, S.; Luca, C.-T.; Buzaș, R.; Lighezan, D.F.; Văcărescu, C.; Cozgarea, A.; Tudoran, C.; Cozma, D. Mitral Annular Plane Systolic Excursion (MAPSE): A Review of a Simple and Forgotten Parameter for Assessing Left Ventricle Function. J. Clin. Med. 2024, 13, 5265. [Google Scholar] [CrossRef]
- Brault, C.; Zerbib, Y.; Mercado, P.; Diouf, M.; Michaud, A.; Tribouilloy, C.; Maizel, J.; Slama, M. Mitral annular plane systolic excursion for assessing left ventricular systolic dysfunction in patients with septic shock. BJA Open 2023, 7, 100220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asgeirsson, D.; Hedström, E.; Jögi, J.; Pahlm, U.; Steding-Ehrenborg, K.; Engblom, H.; Arheden, H.; Carlsson, M. Longitudinal shortening remains the principal component of left ventricular pumping in patients with chronic myocardial infarction even when the absolute atrioventricular plane displacement is decreased. BMC Cardiovasc. Disord. 2017, 17, 208. [Google Scholar] [CrossRef]
- Matos, J.; Kronzon, I.; Panagopoulos, G.; Perk, G. Mitral annular plane systolic excursion as a surrogate for left ventricular ejection fraction. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2012, 25, 969–974. [Google Scholar] [CrossRef]
- van Everdingen, W.; Schipper, J.; Sant, J.v.; Misier, K.R.; Meine, M.; Cramer, M. Echocardiography and cardiac resynchronisation therapy, friends or foes? Neth. Heart J. 2016, 24, 25–38, Erratum in Neth. Heart J. 2016, 24, 221–224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vătășescu, R.G.; Târtea, G.C.; Iorgulescu, C.; Cojocaru, C.; Deaconu, A.; Badiul, A.; Goanță, E.-V.; Bogdan, Ș.; Cozma, D. Predictors for Super-Responders in Cardiac Resynchronization Therapy. Am. J. Ther. 2024, 31, e13–e23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Y.; Hua, W.; Shen, F.; Zou, J.; Tang, B.; Chen, K.; Liang, Y.; He, L.; Zhou, X.; Zhang, X.; et al. Left ventricular-only fusion pacing versus cardiac resynchronization therapy in heart failure patients: A randomized controlled trial. Clin. Cardiol. 2021, 44, 1225–1232. [Google Scholar] [CrossRef]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). EP Eur. 2023, 25, 1208–1236. [Google Scholar] [CrossRef]
- Domenichini, G.; Le Bloa, M.; Castillo, C.T.; Graf, D.; Carroz, P.; Ascione, C.; Porretta, A.P.; Pascale, P.; Pruvot, E. Conduction System Pacing versus Conventional Biventricular Pacing for Cardiac Resynchronization Therapy: Where Are We Heading? J. Clin. Med. 2023, 12, 6288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tok, Ö.Ö.; Bingol, G.; Unlu, S.; Boyuk, F. A Cardiac Magnetic Resonance Study: Comparison of Biventricular Longitudinal Function in Hypertrophic Cardiomyopathy Patients and Normal Individuals. Cureus 2023, 15, e34165. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Chang, Y.; Wu, F.; Yang, M.; Dai, S.; Zhang, J.; Zhang, Y. Evaluation of the prognostic value of lateral MAPSE in patients with suspected coronary artery disease. Int. J. Cardiol. Heart Vasc. 2024, 56, 101567. [Google Scholar] [CrossRef]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Weidemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; Mclean, A.; Slama, M.; Orde, S.R. Paradoxical septal motion: A diagnostic approach and clinical relevance. Australas. J. Ultrasound Med. 2018, 21, 79–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tasolar, H.; Mete, T.; Cetin, M.; Altun, B.; Balli, M.; Bayramoglu, A.; Otlu, Y.O. Mitral annular plane systolic excursion in the assessment of left ventricular diastolic dysfunction in obese adults. Anatol. J. Cardiol. 2015, 15, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Hensel, K.O.; Roskopf, M.; Wilke, L.; Heusch, A. Intraobserver and interobserver reproducibility of M-mode and B-mode acquired mitral annular plane systolic excursion (MAPSE) and its dependency on echocardiographic image quality in children. PLoS ONE 2018, 13, e0196614. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | n (%)/SD |
|---|---|
| Male | 225 (55.01%) |
| Female | 184 (44.99%) |
| Age | 68.77 ± 12.69 |
| BMI | 27.5 ± 3.71 |
| HF diagnosed | 95 (23.2%) |
| HFrEF HFmrEF HFpEF | 42 (44.2%) 28 (29.5%) 25 (26.3%) |
| T2DM | 112 (27,38%) |
| CHD | 70 (17.11%) |
| HTN | 233 (56.9%) |
| NTproBNP (mean) | 1723 ± 1023 (pg/mL) |
| Parameter | ||
|---|---|---|
| Mean | SD | |
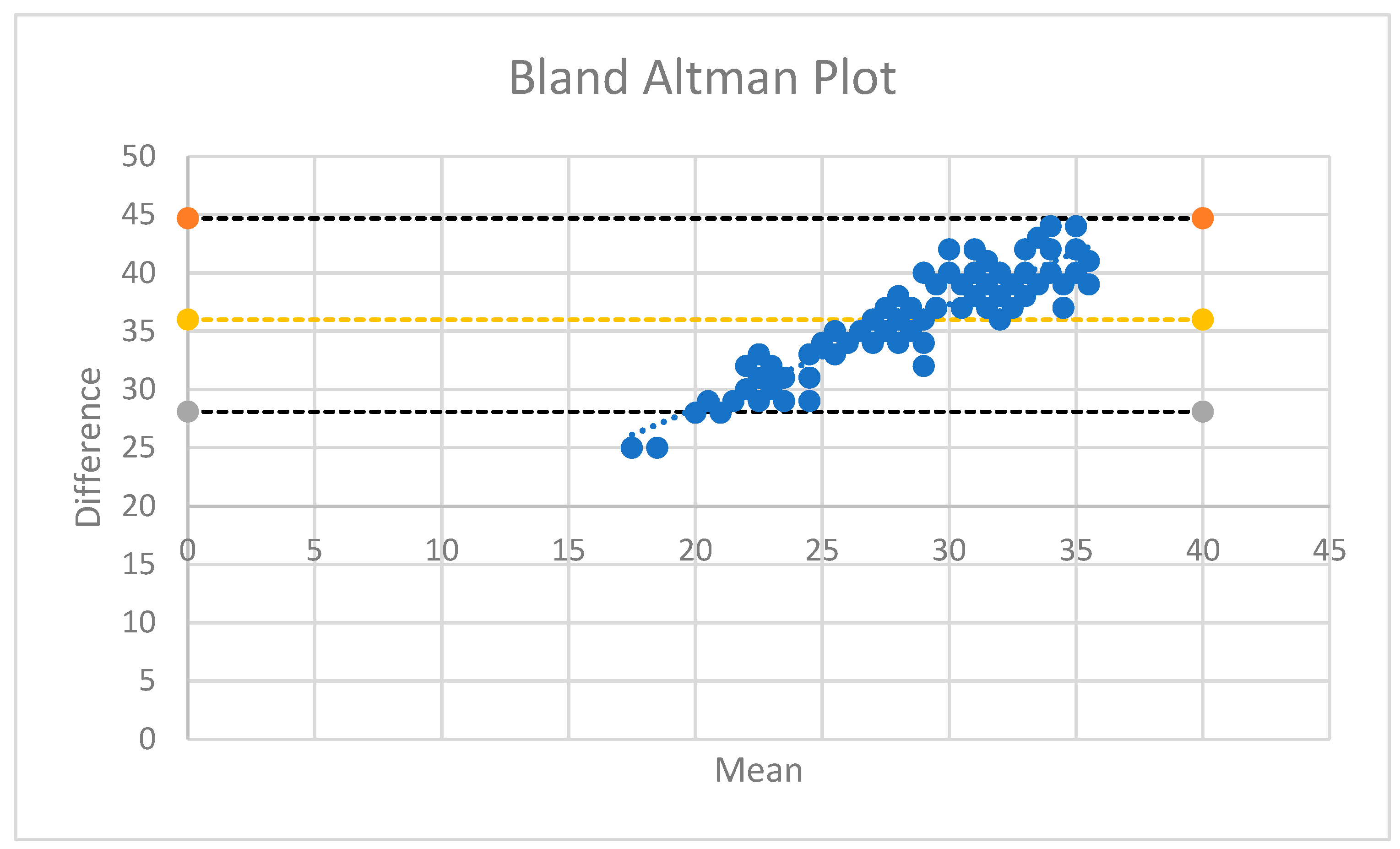
| LVEF (%) (Simpson’s) | 50.23 | 5.07 |
| Avg MAPSE (mm) | 13.65 | 2.05 |
| Septal MAPSE (mm) | 13.15 | 2.02 |
| Lateral MAPSE (mm) | 14.16 | 1.97 |
| LA volume (mL) | 78.51 | 30.55 |
| LA surface (cm2) | 30.55 | 6.57 |
| Parameters | n (%) |
|---|---|
| PM type | 245 (59.9%) dual-chamber |
| Lead position | 295 (72.1%) RVA pacing |
| Mean values | |
| Paced QRS axis (°) | −45.38 RVA pacing 52.26 IVS pacing |
| Paced QRSd (msec) | 152.37 IVS pacing |
| 159.12 RVA pacing |
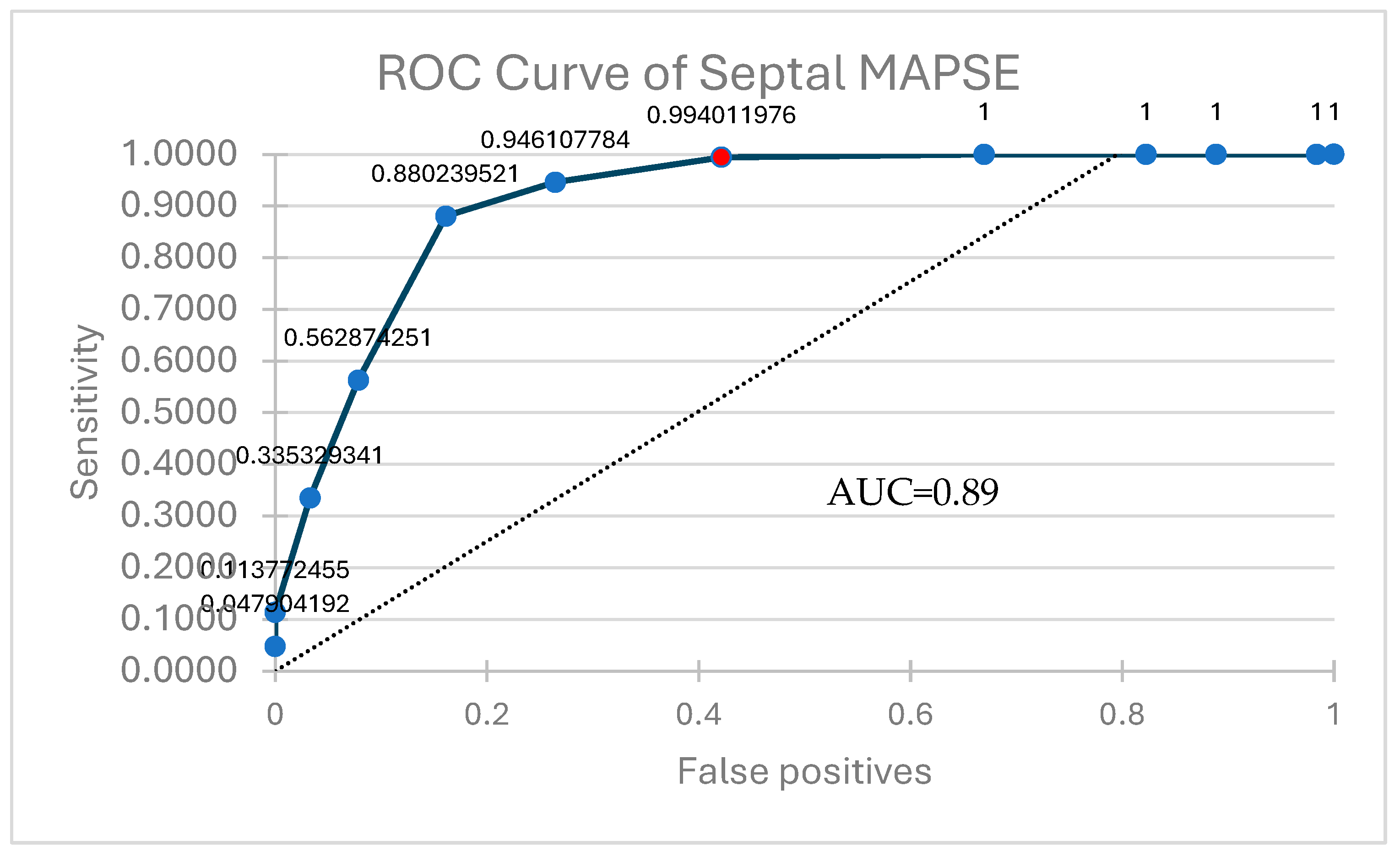
| Parameter | Correlation (r Value) | p |
|---|---|---|
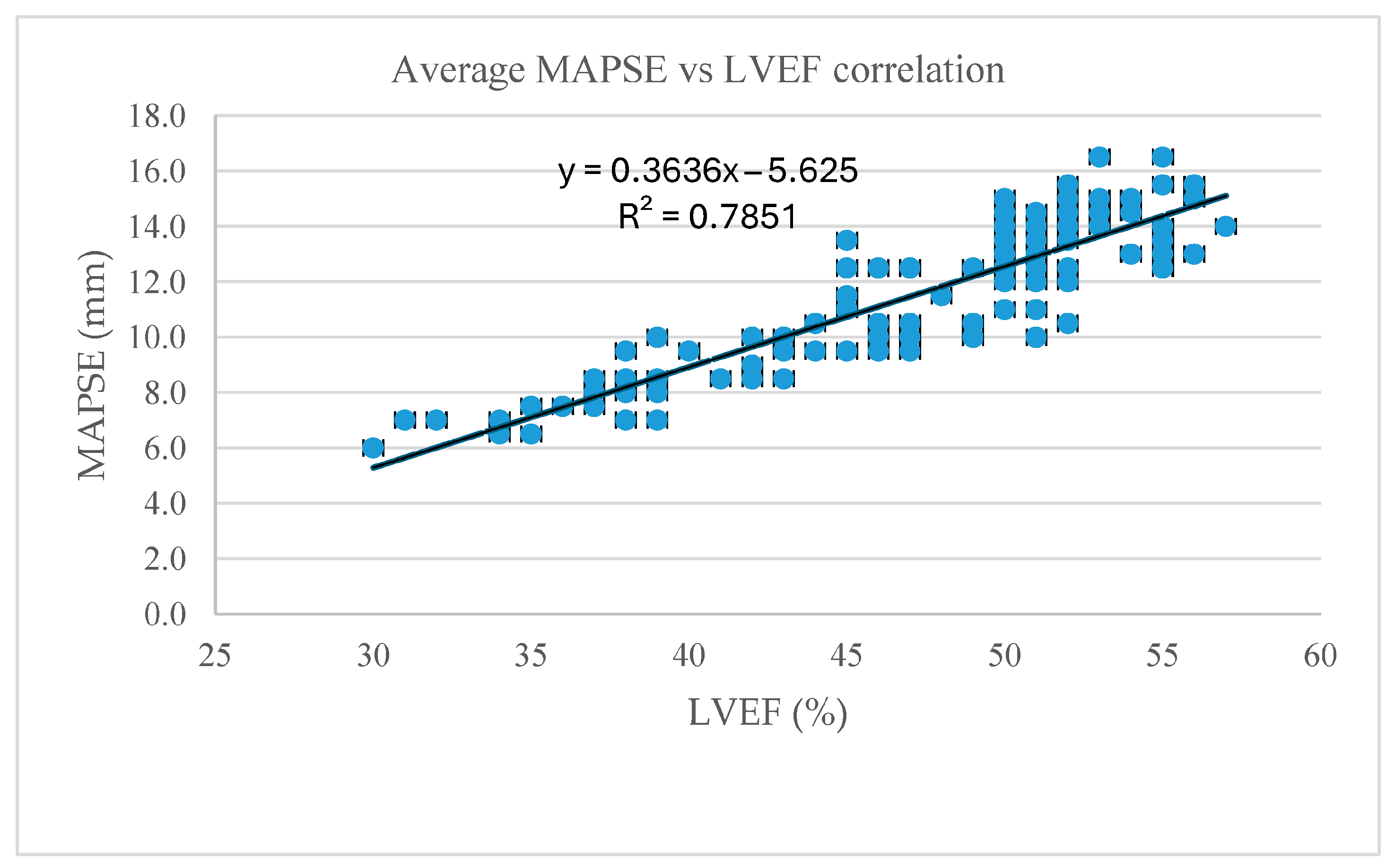
| Average MAPSE | 0.89 | <0.001 |
| Septal MAPSE | 0.90 | |
| Lateral MAPSE | 0.88 |
| Predicted + | Predicted − | |
|---|---|---|
| Actual + | 249 (TP) | 1 (FN) |
| Actual − | 92 (FP) | 67 (TN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirin, L.; Luca, C.T.; Văcărescu, C.; Faur-Grigori, A.A.; Ivan, V.S.; Dima, C.; Buzas, R.; Lighezan, D.-F.; Crișan, S.; Cozma, D. Added Value of MAPSE to Assess LV Systolic Function in Conventional Cardiac Pacing. J. Clin. Med. 2025, 14, 6880. https://doi.org/10.3390/jcm14196880
Cirin L, Luca CT, Văcărescu C, Faur-Grigori AA, Ivan VS, Dima C, Buzas R, Lighezan D-F, Crișan S, Cozma D. Added Value of MAPSE to Assess LV Systolic Function in Conventional Cardiac Pacing. Journal of Clinical Medicine. 2025; 14(19):6880. https://doi.org/10.3390/jcm14196880
Chicago/Turabian StyleCirin, Liviu, Constantin Tudor Luca, Cristina Văcărescu, Adelina Andreea Faur-Grigori, Vlad Sabin Ivan, Ciprian Dima, Roxana Buzas, Daniel-Florin Lighezan, Simina Crișan, and Dragos Cozma. 2025. "Added Value of MAPSE to Assess LV Systolic Function in Conventional Cardiac Pacing" Journal of Clinical Medicine 14, no. 19: 6880. https://doi.org/10.3390/jcm14196880
APA StyleCirin, L., Luca, C. T., Văcărescu, C., Faur-Grigori, A. A., Ivan, V. S., Dima, C., Buzas, R., Lighezan, D.-F., Crișan, S., & Cozma, D. (2025). Added Value of MAPSE to Assess LV Systolic Function in Conventional Cardiac Pacing. Journal of Clinical Medicine, 14(19), 6880. https://doi.org/10.3390/jcm14196880








