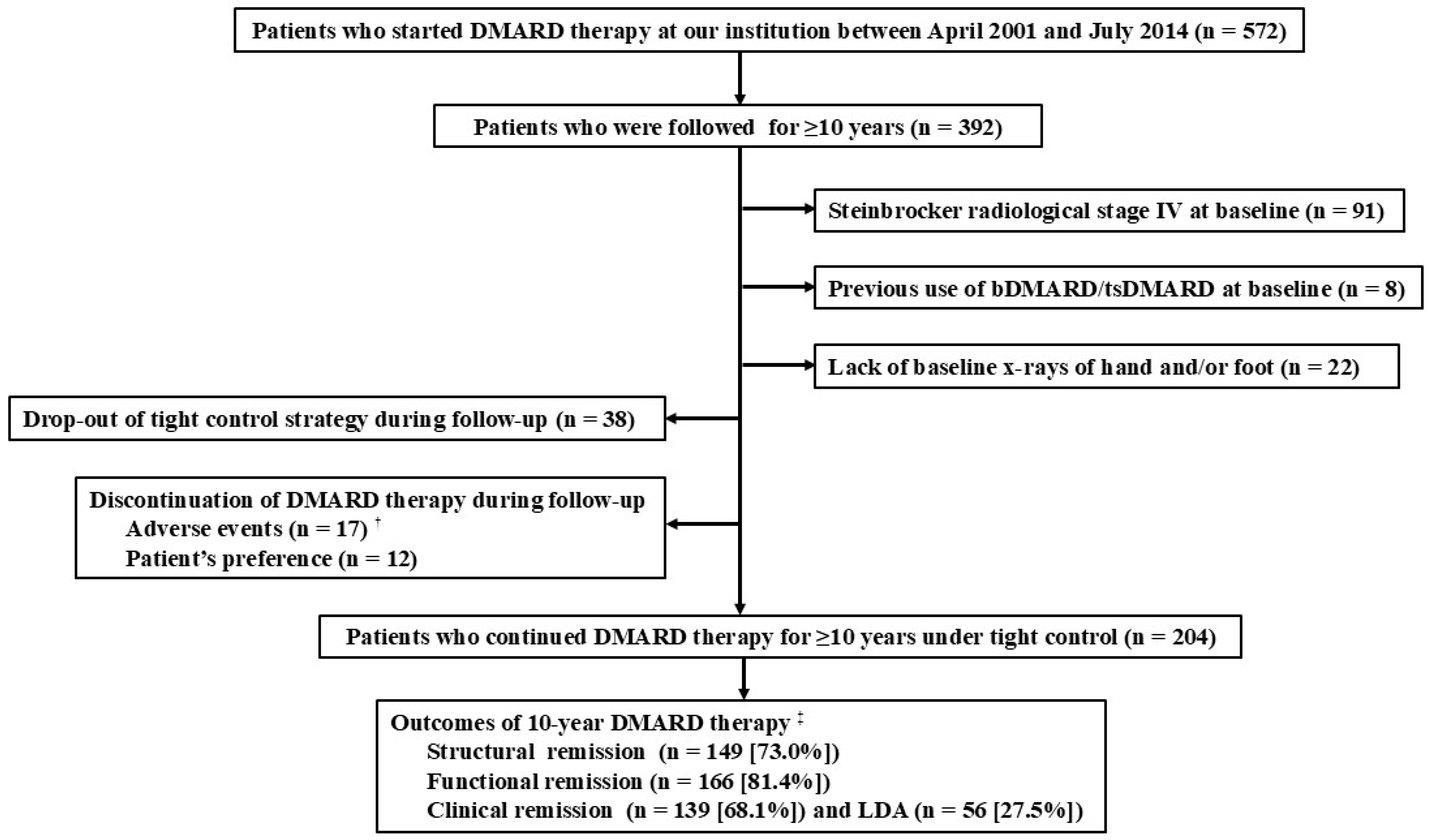
Structural and Functional Outcomes in Rheumatoid Arthritis After 10-Year Therapy with Disease-Modifying Antirheumatic Drugs Under Tight Control: Evidence from Real-World Cohort Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection at Baseline
2.3. Monitoring Clinical Disease Activity During Follow-Up
2.4. Assessment of Joint Destruction
2.5. Outcomes of Interest
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of RA Patients Grouped by Achievement of Structural and Functional Remission After 10-Year DMARD Therapy
3.2. Clinical, Functional, and Structural Outcomes in RA Patients Grouped by Disease Activity Control During 10-Year DMARD Therapy
3.3. DMARD Use for 10 Years in RA Patients Under Tight Control
3.4. Predictors of Structural Remission After 10-Year DMARD Therapy Under Tight Control
3.5. Predictors of Functional Remission After 10-Year DMARD Therapy Under Tight Control
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Pugner, K.; Kaarela, K.; Doyle, D.V.; Woolf, A.; Holmes, J.; Hieke, K. The links between joint damage and disability in rheumatoid arthritis. Rheumatology 2000, 39, 122–132. [Google Scholar] [CrossRef]
- Scott, D.L.; Smith, C.; Kingsley, G. Joint damage and disability in rheumatoid arthritis: An updated systematic review. Clin. Exp. Rheumatol. 2003, 21, S20–S27. [Google Scholar]
- Bombardier, C.; Barbieri, M.; Parthan, A.; Zack, D.J.; Walker, V.; Macarios, D.; Smolen, J.S. The relationship between joint damage and functional disability in rheumatoid arthritis: A systematic review. Ann. Rheum. Dis. 2012, 71, 836–844. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Brown, P.; Pratt, A.G.; Hyrich, K.L. Therapeutic advances in rheumatoid arthritis. BMJ 2024, 384, e070856. [Google Scholar] [CrossRef]
- Bakker, M.F.; Jacobs, J.W.; Verstappen, S.M.; Bijlsma, J.W. Tight control in the treatment of rheumatoid arthritis: Efficacy and feasibility. Ann. Rheum. Dis. 2007, 66 (Suppl. 3), iii56–iii60. [Google Scholar] [CrossRef]
- Mease, P.J. Improving the routine management of rheumatoid arthritis: The value of tight control. J. Rheumatol. 2010, 37, 1570–1578. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): A review of their usefulness and validity in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, S100–S108. [Google Scholar]
- Aletaha, D.; Ward, M.M.; Machold, K.P.; Nell, V.P.; Stamm, T.; Smolen, J.S. Remission and active disease in rheumatoid arthritis: Defining criteria for disease activity states. Arthritis Rheum. 2005, 52, 2625–2636. [Google Scholar] [CrossRef]
- Grigor, C.; Capell, H.; Stirling, A.; McMahon, A.D.; Lock, P.; Vallance, R.; Kincaid, W.; Porter, D. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): A single-blind randomised controlled trial. Lancet 2004, 364, 263–269. [Google Scholar] [CrossRef]
- Goekoop-Ruiterman, Y.P.; de Vries-Bouwstra, J.K.; Allaart, C.F.; van Zeben, D.; Kerstens, P.J.; Hazes, J.M.; Zwinderman, A.H.; Ronday, H.K.; Han, K.H.; Westedt, M.L.; et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2005, 52, 3381–3390. [Google Scholar] [CrossRef]
- Makinen, H.; Kautiainen, H.; Hannonen, P.; Mottonen, T.; Leirisalo-Repo, M.; Laasonen, L.; Korpela, M.; Blafield, H.; Hakola, M.; Sokka, T. Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. J. Rheumatol. 2007, 34, 316–321. [Google Scholar]
- Verstappen, S.M.; Jacobs, J.W.; van der Veen, M.J.; Heurkens, A.H.; Schenk, Y.; ter Borg, E.J.; Blaauw, A.A.; Bijlsma, J.W.; Utrecht Rheumatoid Arthritis Cohort study, g. Intensive treatment with methotrexate in early rheumatoid arthritis: Aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann. Rheum. Dis. 2007, 66, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.; Breedveld, F.C.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; de Wit, M.; Dougados, M.; et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2010, 69, 631–637. [Google Scholar] [CrossRef]
- Schoels, M.; Knevel, R.; Aletaha, D.; Bijlsma, J.W.J.; Breedveld, F.C.; Boumpas, D.T.; Burmester, G.; Combe, B.; Cutolo, M.; Dougados, M.; et al. Evidence for treating rheumatoid arthritis to target: Results of a systematic literature search. Ann. Rheum. Dis. 2010, 69, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Oon, S.; Nikpour, M. Efficacy and safety of treat-to-target strategy studies in rheumatic diseases: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2024, 67, 152465. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Mori, S.; Naito, H.; Ohtani, S.; Yamanaka, T.; Sugimoto, M. Diagnostic utility of anti-cyclic citrullinated peptide antibodies for rheumatoid arthritis in patients with active lung tuberculosis. Clin. Rheumatol. 2009, 28, 277–283. [Google Scholar] [CrossRef]
- Aletaha, D.; Nell, V.P.; Stamm, T.; Uffmann, M.; Pflugbeil, S.; Machold, K.; Smolen, J.S. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: Validation of a clinical activity score. Arthritis Res. Ther. 2005, 7, R796–R806. [Google Scholar] [CrossRef] [PubMed]
- Inoue, E.; Yamanaka, H.; Hara, M.; Tomatsu, T.; Kamatani, N. Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann. Rheum. Dis. 2007, 66, 407–409. [Google Scholar] [CrossRef]
- van der Heijde, D.M.; van Riel, P.L.; Nuver-Zwart, I.H.; Gribnau, F.W.; vad de Putte, L.B. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet 1989, 1, 1036–1038. [Google Scholar] [CrossRef]
- van der Heijde, D. How to read radiographs according to the Sharp/van der Heijde method. J. Rheumatol. 2000, 27, 261–263. [Google Scholar]
- van der Heijde, D. Erosions versus joint space narrowing in rheumatoid arthritis: What do we know? Ann. Rheum. Dis. 2011, 70 (Suppl. 1), I116–I118. [Google Scholar] [CrossRef]
- Lillegraven, S.; van der Heijde, D.; Uhlig, T.; Kvien, T.K.; Haavardsholm, E.A. What is the clinical relevance of erosions and joint space narrowing in RA? Nat. Rev. Rheumatol. 2012, 8, 117–120. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lydersen, S. Statistical review: Frequently given comments. Ann. Rheum. Dis. 2015, 74, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Courvoisier, N.; Dougados, M.; Cantagrel, A.; Goupille, P.; Meyer, O.; Sibilia, J.; Daures, J.P.; Combe, B. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: A prospective study. Arthritis Res. Ther. 2008, 10, R106. [Google Scholar] [CrossRef]
- Odegard, S.; Landewe, R.; van der Heijde, D.; Kvien, T.K.; Mowinckel, P.; Uhlig, T. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: A ten-year, longitudinal observational study in 238 patients. Arthritis Rheum. 2006, 54, 68–75. [Google Scholar] [CrossRef]
- Syversen, S.W.; Gaarder, P.I.; Goll, G.L.; Odegard, S.; Haavardsholm, E.A.; Mowinckel, P.; van der Heijde, D.; Landewe, R.; Kvien, T.K. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: Results from a 10-year longitudinal study. Ann. Rheum. Dis. 2008, 67, 212–217. [Google Scholar] [CrossRef]
- Navarro-Compan, V.; Landewe, R.; Provan, S.A.; Odegard, S.; Uhlig, T.; Kvien, T.K.; Keszei, A.P.; Ramiro, S.; van der Heijde, D. Relationship between types of radiographic damage and disability in patients with rheumatoid arthritis in the EURIDISS cohort: A longitudinal study. Rheumatology 2015, 54, 83–90. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakahashi, H.; Ishii, S.; Sato, E.; Hirose, K.; Ishima, T. Synovial membrane enhancement and bone erosion by magnetic resonance imaging for prediction of radiologic progression in patients with early rheumatoid arthritis. Rheumatol. Int. 2005, 25, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.; Nikiphorou, E.; Sharpe, R.; Norton, S.; Rennie, K.; Bunn, F.; Scott, D.L.; Dixey, J.; Young, A. Have radiographic progression rates in early rheumatoid arthritis changed? A systematic review and meta-analysis of long-term cohorts. Rheumatology 2016, 55, 1053–1065. [Google Scholar] [CrossRef]
- Markusse, I.M.; Akdemir, G.; Dirven, L.; Goekoop-Ruiterman, Y.P.; van Groenendael, J.H.; Han, K.H.; Molenaar, T.H.; Le Cessie, S.; Lems, W.F.; van der Lubbe, P.A.; et al. Long-Term Outcomes of Patients With Recent-Onset Rheumatoid Arthritis After 10 Years of Tight Controlled Treatment: A Randomized Trial. Ann. Intern. Med. 2016, 164, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Allaart, C.F.; Markusse, I.M.; Lems, W.F. What have we learned from BeSt? Clin. Immunol. 2018, 186, 74–78. [Google Scholar] [CrossRef]
- Heimans, L.; Wevers-de Boer, K.V.; Visser, K.; Goekoop, R.J.; van Oosterhout, M.; Harbers, J.B.; Bijkerk, C.; Speyer, I.; de Buck, M.P.; de Sonnaville, P.B.; et al. A two-step treatment strategy trial in patients with early arthritis aimed at achieving remission: The IMPROVED study. Ann. Rheum. Dis. 2014, 73, 1356–1361. [Google Scholar] [CrossRef]
- Heimans, L.; Akdemir, G.; Boer, K.V.; Goekoop-Ruiterman, Y.P.; Molenaar, E.T.; van Groenendael, J.H.; Peeters, A.J.; Steup-Beekman, G.M.; Lard, L.R.; de Sonnaville, P.B.; et al. Two-year results of disease activity score (DAS)-remission-steered treatment strategies aiming at drug-free remission in early arthritis patients (the IMPROVED-study). Arthritis Res. Ther. 2016, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, G.; Heimans, L.; Bergstra, S.A.; Goekoop, R.J.; van Oosterhout, M.; van Groenendael, J.; Peeters, A.J.; Steup-Beekman, G.M.; Lard, L.R.; de Sonnaville, P.B.J.; et al. Clinical and radiological outcomes of 5-year drug-free remission-steered treatment in patients with early arthritis: IMPROVED study. Ann. Rheum. Dis. 2018, 77, 111–118. [Google Scholar] [CrossRef]
- Keystone, E.C.; Breedveld, F.C.; van der Heijde, D.; Landewe, R.; Florentinus, S.; Arulmani, U.; Liu, S.; Kupper, H.; Kavanaugh, A. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J. Rheumatol. 2014, 41, 5–14. [Google Scholar] [CrossRef]
- Keystone, E.C.; van der Heijde, D.; Kavanaugh, A.; Kupper, H.; Liu, S.; Guerette, B.; Mozaffarian, N. Clinical, functional, and radiographic benefits of longterm adalimumab plus methotrexate: Final 10-year data in longstanding rheumatoid arthritis. J. Rheumatol. 2013, 40, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Ruyssen-Witrand, A.; Guernec, G.; Dupont, J.; Lapuyade, D.; Liote, F.; Vittecoq, O.; Degboe, Y.; Constantin, A. Ten-year radiographic and functional outcomes in rheumatoid arthritis patients in remission compared to patients in low disease activity. Arthritis Res. Ther. 2023, 25, 207. [Google Scholar] [CrossRef] [PubMed]
- Bergstra, S.A.; Landewe, R.B.M.; Huizinga, T.W.J.; Allaart, C.F. Rheumatoid arthritis patients with continued low disease activity have similar outcomes over 10 years, regardless of initial therapy. Rheumatology 2017, 56, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Markusse, I.M.; Dirven, L.; Gerards, A.H.; van Groenendael, J.H.; Ronday, H.K.; Kerstens, P.J.; Lems, W.F.; Huizinga, T.W.; Allaart, C.F. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res. Ther. 2015, 17, 232. [Google Scholar] [CrossRef]
- Markatseli, T.E.; Voulgari, P.V.; Alamanos, Y.; Drosos, A.A. Prognostic factors of radiological damage in rheumatoid arthritis: A 10-year retrospective study. J. Rheumatol. 2011, 38, 44–52. [Google Scholar] [CrossRef]
- Smolen, J.S.; van der Heijde, D.M.; Aletaha, D.; Xu, S.; Han, J.; Baker, D.; St Clair, E.W. Progression of radiographic joint damage in rheumatoid arthritis: Independence of erosions and joint space narrowing. Ann. Rheum. Dis. 2009, 68, 1535–1540. [Google Scholar] [CrossRef]
- Heckert, S.L.; Maassen, J.M.; Nevins, I.; Baudoin, P.; Steup-Beekman, G.M.; Huizinga, T.W.J.; Bergstra, S.A.; Allaart, C.F. Long-term clinical outcomes in early rheumatoid arthritis that was treated-to-target in the BeSt and IMPROVED studies. Rheumatology 2025, 64, 1052–1059. [Google Scholar] [CrossRef]
- Welsing, P.M.; van Gestel, A.M.; Swinkels, H.L.; Kiemeney, L.A.; van Riel, P.L. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001, 44, 2009–2017. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D. Patients with rheumatoid arthritis in clinical care. Ann. Rheum. Dis. 2004, 63, 221–225. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.; Ward, M.M. Measuring function in rheumatoid arthritis: Identifying reversible and irreversible components. Arthritis Rheum. 2006, 54, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Funovits, J.; Smolen, J.S. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann. Rheum. Dis. 2011, 70, 733–739. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, J.A.; Allaart, C.F.; Huizinga, T.W.J.; Bergstra, S.A. Are poor prognostic factors a realistic basis for treatment decisions in patients with rheumatoid arthritis? Lessons from the IMPROVED study. RMD Open 2024, 10, e004382. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Total | Structural Remission ¶ | p * | Functional Remission ¶ | p * | ||
|---|---|---|---|---|---|---|---|
| (n = 204) | Yes | No | Yes | No | |||
| (n = 149) | (n = 55) | (n = 166) | (n = 38) | ||||
| Age, years, mean (SD) | 57.5 (10.4) | 57.9 (10.8) | 56.4 (9.3) | 0.34 | 56.7 (9.9) | 61.3 (11.7) | 0.013 |
| >65 years, number (%) | 48 (23.5) | 38 (25.5) | 10 (18.2) | 0.35 | 32 (19.0) | 16 (44.4) | 0.005 |
| Male, number (%) | 50 (24.5) | 43 (28.9) | 7 (12.7) | 0.018 | 46 (27.4) | 4 (11.1) | 0.035 |
| Anti-CCP positive, number (%) | 179 (87.7) | 127 (85.2) | 52 (94.5) | 0.092 | 144 (86.7) | 35 (92.1) | 0.58 |
| RF positive, number (%) | 162 (79.4) | 114 (76.5) | 48 (87.3) | 0.12 | 131 (78.9) | 31 (81.6) | 0.83 |
| DAS28-ESR, mean (SD) | 4.8 (1.2) | 4.7 (1.2) | 5.1 (1.1) | 0.030 | 4.7 (1.1) | 5.4 (1.2) | <0.001 |
| High (>5.1) number (%) | 86 (42.2) | 58 (38.9) | 28 (50.9) | 0.15 | 63 (38.0) | 23 (60.5) | 0.017 |
| Disease duration †, years, mean (SD) | 1.8 (3.5) | 1.4 (2.9) | 2.8 (4.7) | 0.020 | 1.7 (3.5) | 2.4 (3.5) | 0.23 |
| ≤6 months, number (%) | 126 (61.8) | 94 (63.1) | 32 (58.2) | 0.52 | 106 (63.9) | 20 (52.6) | 0.20 |
| Year of the first visit ‡, number (%) | |||||||
| 2001–2008 | 75 (36.8) | 58 (38.9) | 17 (30.9) | 0.33 | 62 (37.3) | 13 (34.2) | 0.85 |
| 2009–2011 | 77 (37.7) | 55 (36.9) | 22 (40.0) | 0.75 | 63 (38.0) | 14 (36.8) | 1.00 |
| 2012–2014 | 52 (25.5) | 36 (24.2) | 16 (29.1) | 0.47 | 41 (24.7) | 11 (28.9) | 0.68 |
| Previous csDMARD use, number (%) | |||||||
| No use | 173 (84.8) | 129 (86.6) | 44 (80) | 0.27 | 141 (84.9) | 32 (84.2) | 1.00 |
| Smoking history > 30 PYs, number (%) | 31 (15.2) | 27 (18.1) | 4 (7.3) | 0.077 | 28 (16.9) | 3 (7.9) | 0.21 |
| BMI > 25, number (%) | 32 (15.7) | 19 (12.8) | 13 (23.6) | 0.081 | 24 (14.5) | 8 (21.1) | 0.13 |
| mTSS (range 0–448) §, mean (SD) | 8.4 (13.7) | 5.8 (10.7) | 15.5 (18.0) | <0.001 | 6.6 (11.6) | 16.5 (18.7) | <0.001 |
| 0, number (%) | 63 (30.9) | 57 (38.3) | 6 (10.9) | <0.001 | 60 (36.1) | 3 (7.9) | <0.001 |
| >0 and ≤5, number (%) | 66 (32.4) | 50 (33.6) | 16 (29.1) | 0.62 | 53 (31.9) | 13 (34.2) | 0.85 |
| >5 and ≤25, number (%) | 55 (27.0) | 33 (22.1) | 22 (40) | 0.013 | 41 (24.5) | 14 (36.8) | 0.16 |
| >25, number (%) | 20 (9.8) | 9 (6.0) | 11 (20) | 0.006 | 12 (7.2) | 8 (21.1) | 0.016 |
| Erosion score, mean (SD) | 3.2 (6.0) | 2.4 (4.6) | 5.5 (8.3) | <0.001 | 2.6 (4.6) | 6.1 (9.6) | <0.001 |
| No erosion, number (%) | 94 (46.1) | 75 (50.3) | 19 (34.5) | 0.057 | 85 (51.2) | 9 (23.7) | 0.002 |
| >0 and ≤3, number (%) | 63 (30.9) | 46 (30.9) | 17 (30.9) | 1.00 | 46 (27.7) | 17 (44.7) | 0.051 |
| >3 and ≤10, number (%) | 26 (12.7) | 19 (12.8) | 7 (12.7) | 1.00 | 22 (13.3) | 4 (10.5) | 0.79 |
| >10, number (%) | 21 (10.3) | 9 (6.0) | 12 (21.8) | 0.003 | 13 (7.8) | 8 (21.1) | 0.033 |
| Only erosion, number (%) | 33 (16.2) | 20 (13.4) | 13 (23.6) | 0.089 | 28 (16.9) | 5 (13.2) | 0.79 |
| JSN score, mean (SD) | 5.2 (8.7) | 3.5 (6.6) | 10.0 (11.5) | <0.001 | 4.0 (7.7) | 10.4 (10.9) | <0.001 |
| Normal joint space, number (%) | 97 (47.5) | 83 (55.7) | 14 (25.5) | <0.001 | 89 (53.6) | 8 (21.1) | <0.001 |
| >0 and ≤3, number (%) | 33 (16.2) | 26 (17.4) | 7 (12.7) | 0.52 | 28 (16.9) | 5 (13.2) | 0.81 |
| >3 and ≤10, number (%) | 35 (17.2) | 23 (15.4) | 12 (21.8) | 0.30 | 27 (16.3) | 8 (21.1) | 0.48 |
| >10, number (%) | 39 (19.1) | 17 (11.4) | 22 (40) | <0.001 | 22 (13.3) | 17 (44.7) | <0.001 |
| Only JSN, number (%) | 36 (17.6) | 28 (18.8) | 8 (14.5) | 0.54 | 30 (18.1) | 6 (15.8) | 0.61 |
| Outcomes at 10 Years | Total | Good Control † | Moderate Control † | Poor Control † | p * |
|---|---|---|---|---|---|
| (n = 204) | (n = 134) | (n = 52) | (n = 18) | ||
| Clinical disease activity at 10 years | |||||
| CDAI, mean (SD) | 2.8 (3.1) | 1.9 (2.1) | 3.5 (3.5) | 7.6 (3.6) | <0.001 |
| Remission (<2.8), number (%) | 139 (68.1) | 109 (81.3) | 28 (53.8) | 2 (11.1) | <0.001 |
| Low (>2.8 and ≤10), number (%) | 56 (27.5) | 23 (17.2) | 20 (38.5) | 13 (72.2) | <0.001 |
| Physical function at 10 years | |||||
| HAQ-DI, mean (SD) | 0.26 (0.49) | 0.12 (0.34) | 0.35 (0.45) | 1.05 (0.69) | <0.001 |
| HAQ-DI ≤ 0.5 (remission), number (%) | 166 (81.4) | 126 (94.0) | 37 (71.2) | 3 (16.6) | <0.001 |
| Structural changes for 10 years | |||||
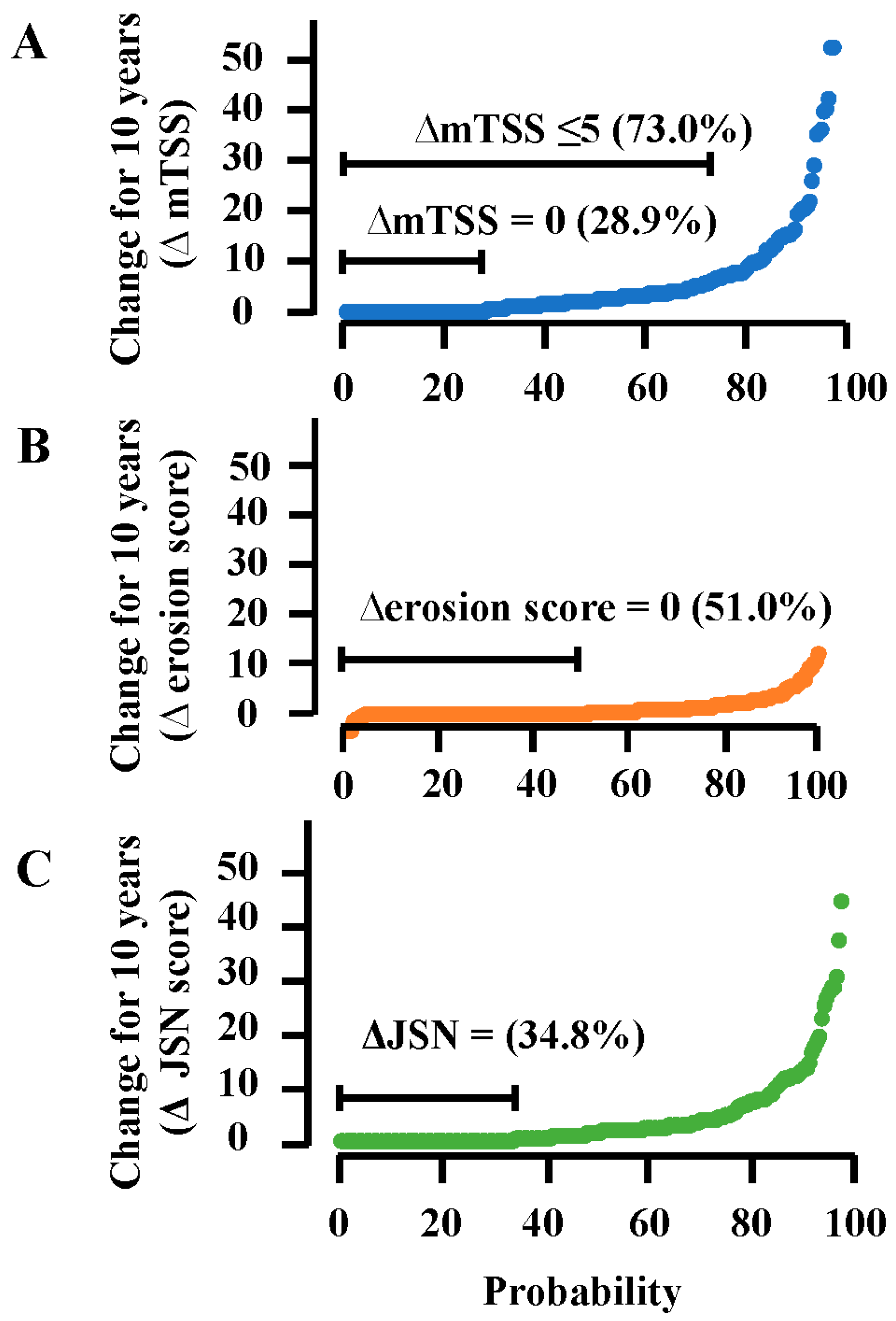
| mTSS at 10 years, mean (SD) | 13.8 (17.8) | 7.8 (12.0) | 22.5 (20.6) | 34.1 (20.9) | <0.001 |
| ∆mTSS, mean (SD) | 5.4 (9.0) | 1.7 (2.2) | 8.6 (9.3) | 22.9 (14.6) | <0.001 |
| ∆mTSS, median (IQR) | 2.0 (0, 6) | 1.0 (0, 3.0) | 5.3 (2.5, 13) | 20.3 (9.9, 36.4) | <0.001 |
| ∆mTSS ≤ 5 (remission), number (%) | 149 (73.0) | 121 (90.3) | 26 (50.0) | 2 (11.1) | <0.001 |
| ∆mTSS = 0 (no progression), number (%) | 59 (28.9) | 55 (41.0) | 4 (7.7) | 0 | <0.001 |
| ∆mTSS ≥ 25, number (%) | 10 (4.9) | 0 | 2 (3.8) | 8 (44.4) | <0.001 |
| Changes in bone erosion for 10 years | |||||
| Erosion score at 10 years, mean (SD) | 4.4 (6.9) | 2.8 (4.9) | 7.5 (9.8) | 7.5 (6.0) | <0.001 |
| ∆erosion score, mean (SD) | 1.2 (2.4) | 0.4 (1.0) | 2.0 (2.9) | 4.8 (3.7) | <0.001 |
| ∆erosion score ≤ 3, number (%) | 180 (88.2) | 131 (97.8) | 41 (78.8) | 8 (44.4) | <0.001 |
| ∆erosion score = 0, number (%) | 104 (51.0) | 85 (63.4) | 17 (32.7) | 2 (11.1) | <0.001 |
| Changes in joint space for 10 years | |||||
| JSN score at 10 years, mean (SD) | 9.4 (12.4) | 4.9 (7.8) | 15.0 (13.0) | 26.5 (17.1) | <0.001 |
| ΔJSN score, mean (SD) | 4.2 (7.3) | 1.3 (1.9) | 6.8 (7.2) | 18.1 (12.5) | <0.001 |
| ΔJSN score ≤ 3, number (%) | 143 (70.1) | 105 (91.3) | 34 (51.5) | 4 (17.4) | <0.001 |
| ΔJSN score = 0, number (%) | 71 (34.8) | 58 (50.4) | 13 (19.7) | 0 | <0.001 |
| Total | Structural Remission †† | p * | Functional Remission †† | p * | |||
|---|---|---|---|---|---|---|---|
| (n = 204) | Yes | No | Yes | No | |||
| (n = 149) | (n = 55) | (n = 166) | (n = 38) | ||||
| MTX use, number (%) | 197 (96.6) | 142 (95.3) | 55 (100) | 0.19 | 161 (97.0) | 36 (94.7) | 0.62 |
| Use as monotherapy, number (%) | 49 (24.0) | 41 (27.5) | 8 (14.5) | 0.065 | 47 (28.3) | 2 (5.3) | 0.001 |
| Exposure, months, mean (SD) † | 104.3 (28.0) | 109.1 (22.7) | 92.0 (35.9) | <0.001 | 107.4 (24.9) | 89.7 (37.0) | <0.001 |
| b/tsDMARD use ‡, number (%) | 155 (76.0) | 108 (72.5) | 47 (85.5) | 0.065 | 119 (71.7) | 36 (94.7) | 0.001 |
| Use of 1 class, number (%) | 79 (38.7) | 61 (40.9) | 18 (32.7) | 0.33 | 69 (41.6) | 10 (26.3) | 0.10 |
| Use of 2 classes, number (%) | 40 (19.6) | 29 (19.5) | 11 (20.0) | 1.00 | 30 (18.1) | 10 (26.3) | 0.26 |
| Use of ≥3 classes, number (%) | 36 (17.6) | 18 (12.1) | 18 (32.7) | 0.001 | 20 (12.0) | 16 (42.1) | <0.001 |
| Number of classes used per patient, mean (SD) | 1.4 (1.1) | 1.2 (1.0) | 1.9 (1.3) | <0.001 | 1.2 (1.0) | 2.3 (1.3) | <0.001 |
| Exposure, months, mean (SD) § | 79.6 (36.1) | 76.9 (37.7) | 85.8 (31.4) | 0.16 | 77.4 (36.8) | 86.8 (32.9) | 0.17 |
| Time to the first use, months, mean (SD) § | 23.3 (28.6) | 23.7 (29.9) | 22.3 (25.4) | 0.79 | 24.0 (29.2) | 21.0 (26.4) | 0.58 |
| b/tsDMARD failure per patient, number, mean (SD) §¶ | 0.7 (1.1) | 0.5 (1.0) | 1.2 (1.3) | <0.001 | 0.5 (0.9) | 1.5 (1.4) | <0.001 |
| No failure, number (%) | 95 (61.3) | 75 (69.4) | 20 (42.6) | 0.002 | 84 (70.6) | 11 (30.6) | <0.001 |
| Failure of 1 class, number (%) | 26 (16.8) | 15 (13.9) | 11 (23.4) | 0.16 | 17 (14.3) | 9 (25.0) | 0.14 |
| Failure of 2 classes, number (%) | 19 (12.3) | 14 (13.0) | 5 (10.6) | 0.79 | 13 (10.9) | 6 (16.7) | 0.39 |
| Failure of ≥3 classes, number (%) | 15 (9.7) | 4 (3.7) | 11 (23.4) | <0.001 | 5 (4.2) | 10 (27.8) | <0.001 |
| Variables at Baseline | Univariable Analysis | Multivariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|
| (Model 1) | (Model 2) | |||||
| Unadjusted OR | p * | Adjusted OR | p * | Adjusted OR | p * | |
| (95% CI) | (95% CI) | (95% CI) | ||||
| Age per additional year | 1.02 (0.99–1.05) | 0.34 | – | – | – | – |
| Age > 65 years, yes vs. no | 1.54 (0.71–3.35) | 0.28 | – | – | – | – |
| Male vs. female | 2.78 (1.17–6.63) | 0.020 | 3.57 (0.97–13.16) | 0.056 | 3.13 (0.83–11.77) | 0.091 |
| Anti-CCP, positive vs. negative | 0.33 (0.10–1.16) | 0.080 | 0.35 (0.09–1.43) | 0.14 | 0.30 (0.07–1.29) | 0.11 |
| RF, positive vs. negative | 0.48 (0.20–1.14) | 0.097 | – | – | – | – |
| RF > 200 U/mL, yes or no | 0.49 (0.23–1.02) | 0.060 | 0.44 (0.18–1.09) | 0.076 | 0.43 (0.17–1.08) | 0.073 |
| DAS28-ESR per additional unit | 0.74 (0.57–10.97) | 0.030 | 0.74 (0.53–1.02) | 0.066 | 0.74 (0.53–1.04) | 0.083 |
| High (>5.1), yes vs. no | 0.62 (0.33–1.15) | 0.13 | – | – | – | – |
| Disease duration per additional day | 1.00 (1.00–1.00) | 0.030 | 1.00 (1.00–1.00) | 0.71 | 1.00 (1.00–1.00) | 0.98 |
| ≤6 months, yes vs. no | 0.92 (0.49–1.75) | 0.81 | – | – | – | – |
| Year of the first visit | ||||||
| 2001–2008 | 1 (reference) | – | – | – | – | – |
| 2009–2011 | 1.99 (0.97–4.06) | 0.060 | 2.18 (0.95–5.00) | 0.066 | 2.45 (1.04–5.77) | 0.040 |
| 2012–2014 | 2.10 (0.93–4.74) | 0.080 | 1.64 (0.63–4.24) | 0.31 | 1.94 (0.73–5.17) | 0.18 |
| Previous csDMARD use, yes vs. no | 0.62 (0.28–1.40) | 0.25 | – | – | – | – |
| Smoking history > 30 PYs, yes vs. no | 2.82 (0.94–8.48) | 0.060 | 2.57 (0.51–12.92) | 0.25 | 2.58 (0.49–13.50) | 0.26 |
| BMI > 25, yes vs. no | 0.47 (0.22–1.04) | 0.060 | 0.56 (0.22–1.47) | 0.24 | 0.49 (0.18–1.32) | 0.16 |
| mTSS | ||||||
| 0 (no erosion/normal joint space) | 1 (reference) | – | 1 (reference) | – | – | – |
| >0 and ≤5 | 0.33 (0.12–0.91) | 0.030 | 0.38 (0.13–1.10) | 0.074 | – | – |
| >5 and ≤25 | 0.16 (0.06–0.43) | <0.001 | 0.15 (0.05–0.46) | <0.001 | – | – |
| >25 | 0.09 (0.03–0.29) | <0.001 | 0.12 (0.03–0.48) | 0.003 | – | – |
| Erosion score | ||||||
| 0 (no erosion) | 1 (reference) | – | – | – | 1 (reference) | – |
| >0 and ≤3 | 0.69 (0.32–1.45) | 0.32 | – | – | 1.16 (0.48–2.82) | 0.75 |
| >3 and ≤10 | 0.69 (0.25–1.87) | 0.46 | – | – | 2.18 (0.57–8.37) | 0.26 |
| >10 | 0.19 (0.07–0.52) | 0.001 | – | – | 0.76 (0.19–3.09) | 0.70 |
| JSN score | ||||||
| 0 (normal joint space) | 1 (reference) | – | – | – | 1 (reference) | – |
| >0 and ≤3 | 0.63 (0.23–1.72) | 0.36 | – | – | 0.73 (0.23–2.24) | 0.58 |
| >3 and ≤10 | 0.32 (0.13–0.79) | 0.014 | – | – | 0.29 (0.10–0.83) | 0.021 |
| >10 | 0.13 (0.06–0.31) | <0.001 | – | – | 0.14 (0.04–0.47) | 0.002 |
| Variables at Baseline | Univariable Analysis | Multivariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|
| (Model 1) | (Model 2) | |||||
| Unadjusted OR | p * | Adjusted OR | p * | Adjusted OR | p * | |
| (95% CIs) | (95% CI) | (95% CI) | ||||
| Age per additional year | 0.96 (0.92–0.99) | 0.015 | – | – | – | – |
| Age > 65 years, yes vs. no | 0.33 (0.16–0.70) | 0.004 | 0.29 (0.12–0.70) | 0.006 | 0.27 (0.10–0.69) | 0.006 |
| Male vs. female | 3.26 (1.10–9.70) | 0.034 | 6.66 (1.91–23.15) | 0.003 | 6.15 (1.66–22.74) | 0.006 |
| Anti-CCP, positive vs. negative | 0.56 (0.16–1.98) | 0.37 | – | – | – | – |
| RF, positive vs. negative | 0.85 (0.34–2.08) | 0.71 | – | – | – | – |
| RF > 200 U/mL, yes or no | 1.27 (0.49–3.31) | 0.62 | – | – | – | – |
| DAS28-ESR per additional unit | 0.58 (0.42–0.80) | <0.001 | 0.55 (0.38–0.80) | 0.002 | 0.57 (0.39–0.85) | 0.006 |
| High (>5.1), yes vs. no | 0.40 (0.19–0.82) | 0.013 | – | – | – | – |
| Disease duration per additional day | 1.00 (1.00–1.00) | 0.24 | – | – | – | – |
| ≤6 months, yes vs. no | 1.59 (0.78–3.24) | 0.20 | – | – | – | – |
| Year of the first visit | ||||||
| 2001–2008 | 1 (reference) | – | – | – | – | – |
| 2009–2011 | 1.59 (0.70–3.60) | 0.27 | – | – | – | – |
| 2012–2014 | 1.40 (0.57–3.44) | 0.46 | – | – | – | – |
| Previous csDMARD use, yes vs. no | 0.95 (0.36–2.50) | 0.91 | – | – | – | – |
| Smoking history > 30 PYs, yes vs. no | 2.37 (0.68–8.24) | 0.18 | – | – | – | – |
| BMI > 25, yes vs. no | 0.63 (0.26–1.55) | 0.32 | – | – | – | – |
| mTSS | ||||||
| 0 (no erosion/normal joint space) | 1 (reference) | – | 1 (reference) | – | – | – |
| >0 and ≤5 | 0.20 (0.055–0.75) | 0.017 | 0.17 (0.04–0.70) | 0.014 | – | – |
| >5 and ≤10 | 0.15 (0.04–0.54) | 0.004 | 0.10 (0.02–0.43) | 0.002 | – | – |
| >10 | 0.08 (0.02–0.32) | <0.001 | 0.07 (0.01–0.34) | 0.001 | – | – |
| Erosion score | ||||||
| 0 (no erosion) | 1 (reference) | – | – | 1 (reference) | – | |
| >0 and ≤3 | 0.29 (0.12–0.69) | 0.006 | – | 0.46 (0.17–1.25) | 0.13 | |
| >3 and ≤10 | 0.58 (0.16–2.07) | 0.40 | – | 2.00 (0.38–10.54) | 0.42 | |
| >10 | 0.17 (0.06–0.53) | 0.002 | – | 0.84 (0.17–4.19) | 0.83 | |
| JSN score | ||||||
| 0 (normal joint space) | 1 (reference) | – | – | 1 (reference) | – | |
| >0 and ≤3 | 0.50 (0.15–1.66) | 0.26 | – | 0.52 (0.14–1.94) | 0.33 | |
| >3 and ≤10 | 0.30 (0.10–0.89) | 0.029 | – | 0.17 (0.05–0.58) | 0.005 | |
| >10 | 0.12 (0.04–0.30) | <0.001 | – | 0.09 (0.02–0.38) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, S.; Okada, A.; Shimizu, T.; Takatani, A.; Koga, T. Structural and Functional Outcomes in Rheumatoid Arthritis After 10-Year Therapy with Disease-Modifying Antirheumatic Drugs Under Tight Control: Evidence from Real-World Cohort Data. J. Clin. Med. 2025, 14, 6832. https://doi.org/10.3390/jcm14196832
Mori S, Okada A, Shimizu T, Takatani A, Koga T. Structural and Functional Outcomes in Rheumatoid Arthritis After 10-Year Therapy with Disease-Modifying Antirheumatic Drugs Under Tight Control: Evidence from Real-World Cohort Data. Journal of Clinical Medicine. 2025; 14(19):6832. https://doi.org/10.3390/jcm14196832
Chicago/Turabian StyleMori, Shunsuke, Akitomo Okada, Toshimasa Shimizu, Ayuko Takatani, and Tomohiro Koga. 2025. "Structural and Functional Outcomes in Rheumatoid Arthritis After 10-Year Therapy with Disease-Modifying Antirheumatic Drugs Under Tight Control: Evidence from Real-World Cohort Data" Journal of Clinical Medicine 14, no. 19: 6832. https://doi.org/10.3390/jcm14196832
APA StyleMori, S., Okada, A., Shimizu, T., Takatani, A., & Koga, T. (2025). Structural and Functional Outcomes in Rheumatoid Arthritis After 10-Year Therapy with Disease-Modifying Antirheumatic Drugs Under Tight Control: Evidence from Real-World Cohort Data. Journal of Clinical Medicine, 14(19), 6832. https://doi.org/10.3390/jcm14196832






