Eosinophilic Myocarditis Treated with IL-5 Blockade: An Integrated Case Report and Literature Review
Abstract
1. Introduction
1.1. Clinical Course and Pathophysiology of Eosinophilic Myocarditis
1.2. Etiologic Spectrum
- Immune-mediated disorders, chiefly eosinophilic granulomatosis with polyangiitis (EGPA) and hypereosinophilic syndromes (HES) [16,17,18,19,20,21,22,23,24,25,26,27,28]. Cardiac involvement is a leading cause of EGPA-related mortality; estimates vary, but up to approximately 30% of patients are affected, with cardiac disease accounting for nearly half of deaths [29,30]. Other eosinophilic/atopic conditions, such as allergic bronchopulmonary mycosis (ABPM) and eosinophilic asthma, are recognized causes of peripheral eosinophilia and, albeit rarely, can involve the myocardium [31,32,33,34];
- Myeloproliferative and idiopathic entities, including hematologic malignancy-associated eosinophilia [36].
1.3. Diagnostic Work-Up
1.4. Current Therapeutics and Unmet Needs
1.5. Rationale for IL-5—Targeted Therapy
1.6. Knowledge Gap Highlighted by EGPA Cardiac Cohorts
2. Aim and Methods of the Present Review
3. Case Presentation
3.1. Clinical History
3.2. Past Respiratory Course
3.3. Initial Evaluation
- ECG: nonspecific ST-T changes (Figure 2b).
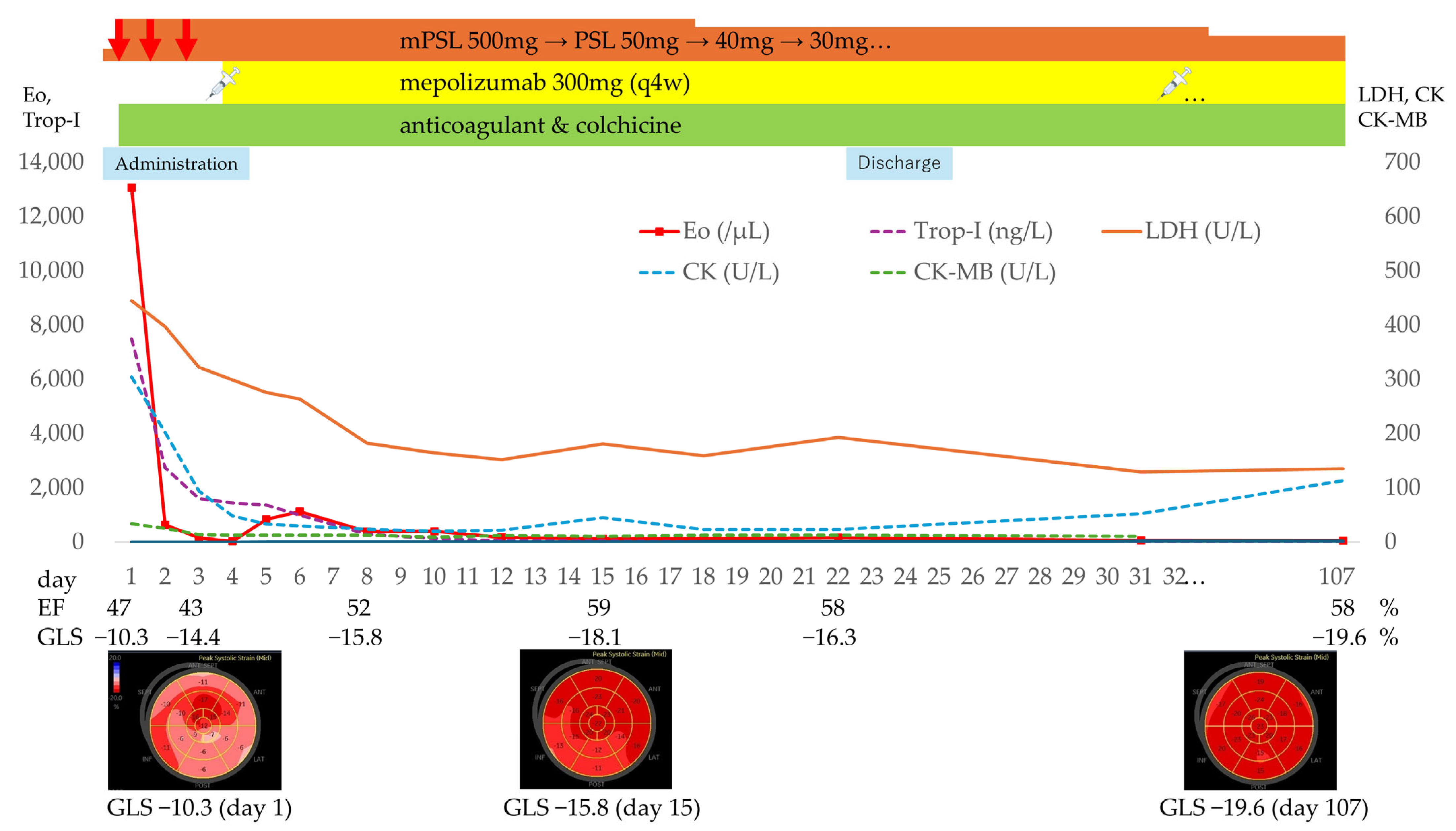
- Laboratory findings: white blood cell count 21,500/μL (eosinophils 13,060/μL), Creatine kinase [CK] 304 U/L, Creatine kinase–MB isoenzyme [CK-MB] 33.6 U/L, high-sensitivity troponin-I 7496 ng/L, BNP 145.9 pg/mL, fibrinogen/fibrin degradation products 4.2 µg/mL, D-dimer 1.8 µg/mL, serum IgE 1402 U/mL and Thymus and activation-regulated chemokine [TARC, also called CCL17] 1372 pg/mL (reference range: 0–450). C-reactive protein was 0.79 mg/dL, IL-6 mildly elevated at 15 pg/mL (reference range: 0–7.9) (Figure 3).
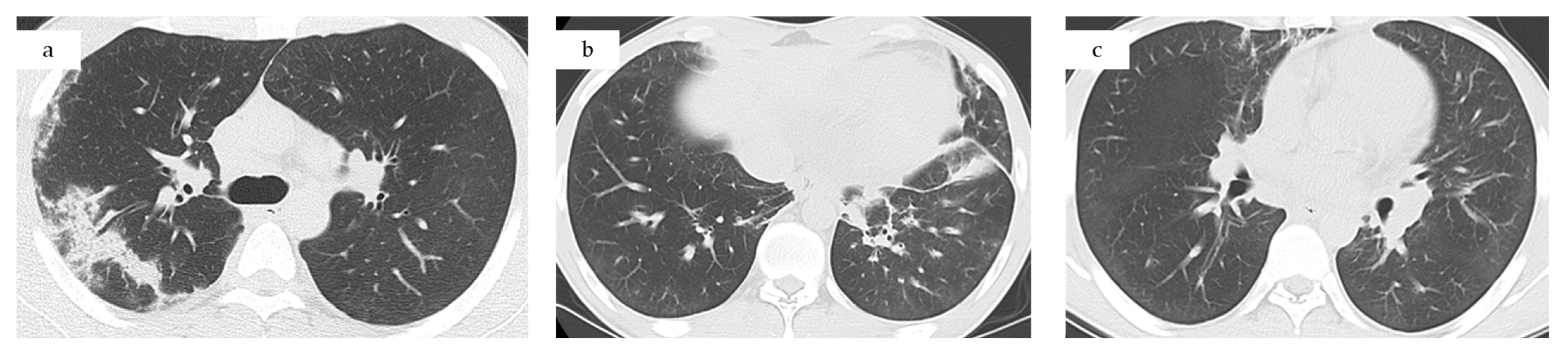
- Computed tomography (CT) chest/abdominal: recurrent bilateral ground-glass opacities and patchy consolidations without pleural effusion or lymphadenopathy, consistent with relapsed CEP. Abdominal and pelvic organs were unremarkable (Figure 1c).
- Fractional exhaled nitric oxide (FeNO): 197 ppb on Day 2 (previously 92 ppb four months earlier).
3.4. Diagnostic Work-Up
3.5. Treatment
3.6. Clinical Course and Follow-Up
3.7. Longitudinal Biomarker Evaluation in This Case
4. Literature Synthesis
4.1. Cohort Characteristics
4.2. Cardiac Presentation
4.2.1. Transthoracic Echocardiography (TTE)
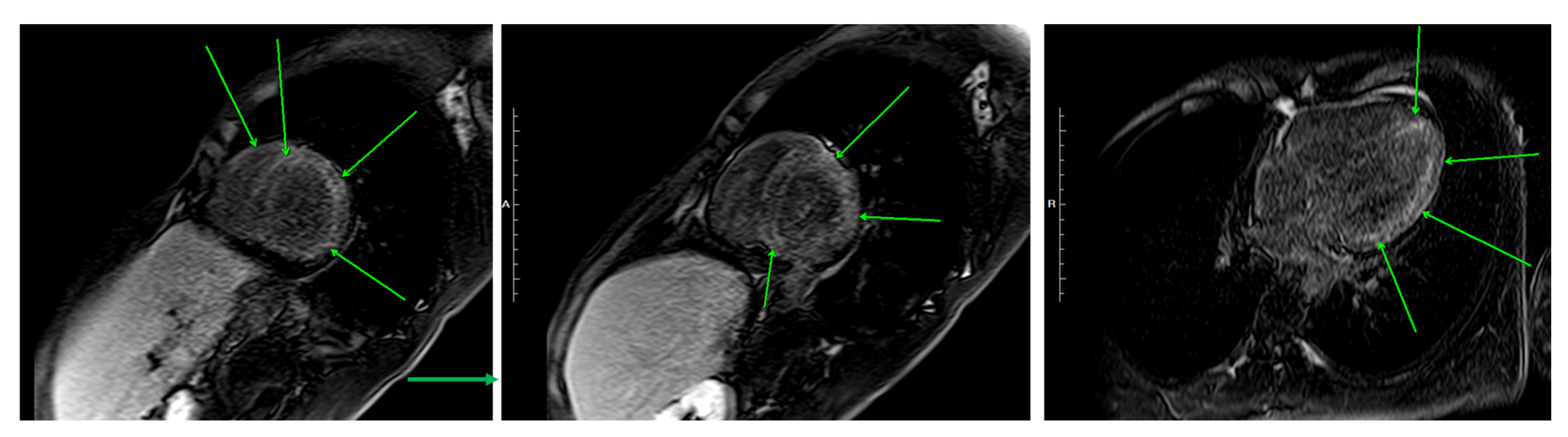
4.2.2. Cardiac Magnetic Resonance Imaging (CMR)
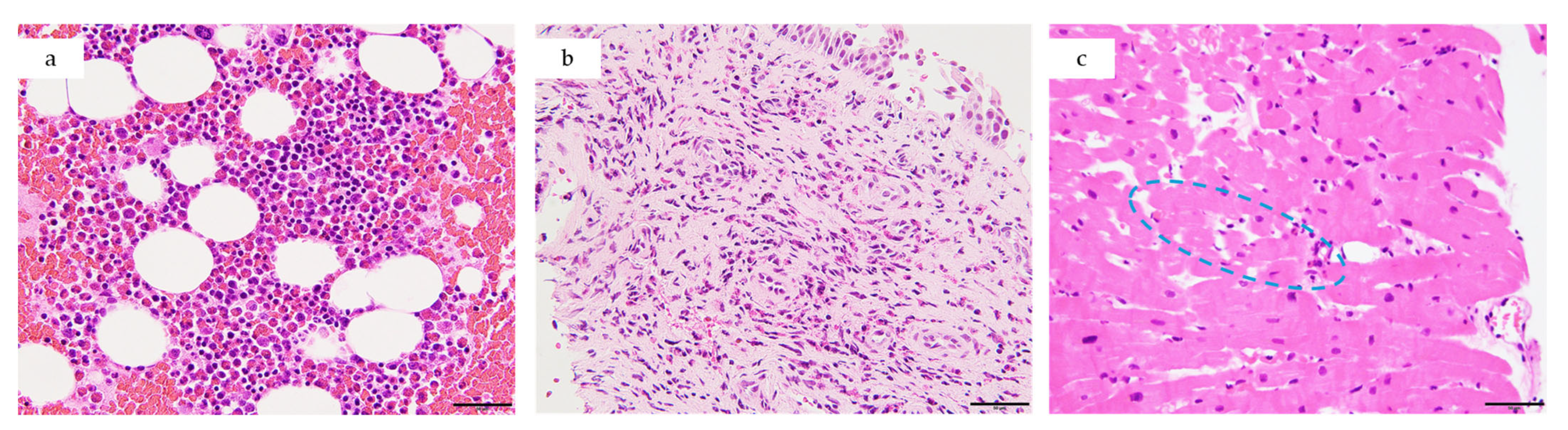
4.2.3. Endomyocardial Biopsy
4.3. Use of IL-5 Inhibitors
- No cases of dose reduction, discontinuation, or switching of IL-5 inhibitors were reported during the treatment course in the reviewed literature.
- Corticosteroids: All 21 patients received high-dose corticosteroids at presentation.
- Conventional immunosuppressants: Eight patients (38%) also received immunosuppressants (rituximab, cyclophosphamide, azathioprine, or methotrexate), either before or concomitantly with IL-5 inhibition.
4.4. Clinical Outcomes
4.4.1. Survival and Safety
4.4.2. Therapeutic Impact
4.4.3. Functional Recovery
5. Discussion
5.1. Significance of Early IL-5 Inhibition in Eosinophilic Myocarditis
5.2. Why Corticosteroids Are Necessary but Not Sufficient
5.3. Complementary Mechanisms of IL-5 Blockade
5.4. Mepolizumab and Benralizumab: Comparative Considerations
5.5. Dose Considerations: 300 mg vs. 100 mg Mepolizumab
5.6. Imaging and Anticoagulation in EM: Thrombosis, Eosinophils, and IL-5 Blockade
5.7. Colchicine and Myocarditis
5.8. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPM | Allergic bronchopulmonary mycosis |
| ACR/EULAR | American College of Rheumatology/European Alliance of Associations for Rheumatology |
| ADCC | Antibody-dependent cellular cytotoxicity |
| ANCA | Antineutrophil cytoplasmic antibody |
| AZA | Azathioprine |
| BNP | B-type natriuretic peptide |
| CEP | Chronic eosinophilic pneumonia |
| CK | Creatine kinase |
| CK-MB | Creatine kinase-MB isoenzyme |
| CLC | Charcot–Leyden crystal(s) |
| CMR | Cardiac magnetic resonance |
| CPA | Cyclophosphamide |
| CT | Computed tomography |
| DRESS | Drug reaction with eosinophilia and systemic symptoms |
| ECG | Electrocardiogram |
| ECMO | Extracorporeal membrane oxygenation |
| ECV | Extracellular volume |
| EGPA | Eosinophilic granulomatosis with polyangiitis |
| EGPA-EM | EGPA-associated eosinophilic myocarditis |
| EM | Eosinophilic myocarditis |
| EMB | Endomyocardial biopsy |
| EETosis | Eosinophil extracellular trap cell death |
| Eos | Eosinophil count per microliter |
| FeNO | Fractional exhaled nitric oxide |
| GLS | Global longitudinal strain |
| H&E | Hematoxylin and Eosin |
| HES | Hypereosinophilic syndrome |
| IgE | Immunoglobulin E |
| iHES | Idiopathic hypereosinophilic syndrome |
| IL | Interleukin |
| IL-1β | Interleukin-1 beta |
| IL-5 | Interleukin-5 |
| IVIG | Intravenous immunoglobulin |
| LGE | Late gadolinium enhancement |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| MANDARA | Phase 3 trial comparing benralizumab with mepolizumab in EGPA |
| MIRRA | Phase 3 trial of mepolizumab in relapsing/refractory EGPA |
| MMF | Mycophenolate mofetil |
| MPO-ANCA | Myeloperoxidase-ANCA |
| MTX | Methotrexate |
| MRI | Magnetic resonance imaging |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| PR3-ANCA | Proteinase 3-ANCA |
| RTX | Rituximab |
| RV | Right ventricle |
| TARC/CCL17 | Thymus and activation-regulated chemokine, also known as CC chemokine ligand 17 |
| TTE | Transthoracic echocardiography |
| V-A ECMO | Veno-arterial extracorporeal membrane oxygenation |
References
- Li, H.; Dai, Z.; Wang, B.; Huang, W. A case report of eosinophilic myocarditis and a review of the relevant literature. BMC Cardiovasc. Disord. 2015, 15, 15. [Google Scholar] [CrossRef]
- Zhong, Z.; Yang, Z.; Peng, Y.; Wang, L.; Yuan, X. Diagnosis and treatment of eosinophilic myocarditis. J. Transl. Autoimmun. 2021, 4, 100118. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Matassini, M.V.; Adler, E.D.; Klingel, K.; Camici, P.G.; Ammirati, E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2363–2375. [Google Scholar] [CrossRef]
- Asada, A.M.; Kahwash, R.; Trovato, V. Eosinophilic Myocarditis: A Concise Review. Curr. Cardiol. Rep. 2025, 27, 38. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Kamide, Y.; Ueki, S.; Miyabe, Y.; Konno, Y.; Oka, N.; Takeuchi, H.; Koyota, S.; Hirokawa, M.; Yamada, T.; et al. Eosinophil ETosis-Mediated Release of Galectin-10 in Eosinophilic Granulomatosis With Polyangiitis. Arthritis Rheumatol. 2021, 73, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, H.; Yamada, Y.; Arima, M.; Miyabe, Y.; Fukuchi, M.; Hikichi, H.; Melo, R.C.N.; Yamada, T.; Ueki, S. Galectin-10 as a Potential Biomarker for Eosinophilic Diseases. Biomolecules 2022, 12, 1385. [Google Scholar] [CrossRef]
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.U. In vivo evidence for extracellular DNA trap formation. Cell Death Dis. 2020, 11, 300. [Google Scholar] [CrossRef]
- Aegerter, H.; Smole, U.; Heyndrickx, I.; Verstraete, K.; Savvides, S.N.; Hammad, H.; Lambrecht, B.N. Charcot-Leyden crystals and other protein crystals driving type 2 immunity and allergy. Curr. Opin. Immunol. 2021, 72, 72–78. [Google Scholar] [CrossRef]
- Rodriguez-Alcazar, J.F.; Ataide, M.A.; Engels, G.; Schmitt-Mabmunyo, C.; Garbi, N.; Kastenmuller, W.; Latz, E.; Franklin, B.S. Charcot-Leyden Crystals Activate the NLRP3 Inflammasome and Cause IL-1beta Inflammation in Human Macrophages. J. Immunol. 2019, 202, 550–558. [Google Scholar] [CrossRef]
- Han, T.; Tang, H.; Lin, C.; Shen, Y.; Yan, D.; Tang, X.; Guo, D. Extracellular traps and the role in thrombosis. Front. Cardiovasc. Med. 2022, 9, 951670. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Su, S.; Liang, L.; Lu, L.; Li, M.; Zhang, X.; Jin, Y.; Wei, W.; Wan, Z. In-Depth Review of Loeffler Endocarditis: What Have We Learned? J. Inflamm. Res. 2024, 17, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Kowtoniuk, R.; Pinninti, M.; Tyler, W.; Doddamani, S. DRESS syndrome-associated acute necrotizing eosinophilic myocarditis with giant cells. BMJ Case Rep. 2018, 2018, bcr-2018226461. [Google Scholar] [CrossRef] [PubMed]
- Truong, K.; Kelly, S.; Bayly, A.; Smith, A. Successful mepolizumab treatment for DRESS-induced refractory eosinophilic myocarditis and concurrent thyroiditis. BMJ Case Rep. 2021, 14, e242240. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Nakamoto, Y.; Aita, T.; Naganuma, T.; Takahashi, S.; Kiko, Y.; Nakagawa, H.; Hamaguchi, S. Fatal Myocarditis in Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Without Mortality-Related Risk Factors: A Case Report and Literature Review. Cureus 2024, 16, e63541. [Google Scholar] [CrossRef]
- Song, T.; Jones, D.M.; Homsi, Y. Therapeutic effect of anti-IL-5 on eosinophilic myocarditis with large pericardial effusion. BMJ Case Rep. 2017, 2017, bcr-2016218992. [Google Scholar] [CrossRef]
- Huynh, R.; Sy, R.W.; Wong, S.J.; Wong, C.C.Y. A unique case report of relapsing eosinophilic myocarditis causing atrial myopathy and persistent sinus arrest. Eur. Heart J. Case Rep. 2022, 6, ytac047. [Google Scholar] [CrossRef]
- Higashitani, K.; Yoshimi, R.; Sato, Y.; Watanabe, T.; Ihata, A. Rituximab and mepolizumab combination therapy for glucocorticoid-resistant myocarditis related to eosinophilic granulomatosis with polyangiitis. Mod. Rheumatol. Case Rep. 2022, 6, 87–92. [Google Scholar] [CrossRef]
- Ulu, K.; Caglayan, S.; Cetemen, A.; Cakan, M.; Oner, T.; Sozeri, B. Mepolizumab therapy in a pediatric patient with eosinophilic granulomatosis with polyangiitis associated with refractory myocarditis. Arch. Rheumatol. 2023, 38, 326–328. [Google Scholar] [CrossRef]
- Wang, C.R.; Tsai, Y.S.; Lee, C.H. Mepolizumab therapy improves endomyocarditis in seropositive eosinophilic granulomatosis with polyangiitis. Arch. Rheumatol. 2023, 38, 159–161. [Google Scholar] [CrossRef]
- Bello, F.; Emmi, G.; Tamburini, C.; Maggi, L.; Annunziato, F.; Cosmi, L.; Prisco, D. Eosinophilic granulomatosis with polyangiitis-related myocarditis during mepolizumab therapy reveals a Th1/Th17-mediated vasculitic response. Clin. Exp. Rheumatol. 2022, 40, 863–864. [Google Scholar] [CrossRef]
- Rao, K.; Arustamyan, M.; Walling, A.; Christodoulidis, G.; Ashwath, M.; Hagedorn, J.; Priya, S. Utility of cardiac magnetic resonance imaging in diagnosing eosinophilic myocarditis in a patient recently recovered from COVID-19: A grand round case report. Eur. Heart J. Case Rep. 2023, 7, ytad090. [Google Scholar] [CrossRef]
- Panina, A.; Ligere, E.; Aleksejeva, E.; Davidsone, Z.; Cebure, E.; Erdmane, I. Eosinophilic Granulomatosis with Polyangiitis in an 8-year-old Girl Manifesting as Hypereosinophilic Syndrome with Myocarditis, Stroke, and Subsequent Orbital Involvement. Acta Med. Litu. 2023, 30, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Trovato, V.; Asada, A.; Fussner, L.; Curtis, C.; Kahwash, R. Interleukin-5 Antagonist Monoclonal Antibody Therapy Improves Symptoms and Reduces Steroid Dependence in Eosinophilic Myocarditis Patients. JACC Case Rep. 2024, 29, 102267. [Google Scholar] [CrossRef] [PubMed]
- Brick, C.; Leet, A.; Tay, H.; Kaye, D.M.; Taylor, A.J. Fulminant eosinophilic myocarditis and refractory ventricular arrhythmias requiring mechanical circulatory support: A case report. Eur. Heart J. Case Rep. 2024, 8, ytae409. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Desai, S.; Leoni, J.; Paghdar, S.; Ruiz, J.; Goswami, R. Case report: Eosinophilic myocarditis in hypereosinophilic syndrome: A journey to heart transplantation. Front. Immunol. 2024, 15, 1418665. [Google Scholar] [CrossRef]
- Colantuono, S.; Pellicano, C.; Leodori, G.; Cilia, F.; Francone, M.; Visentini, M. Early benralizumab for eosinophilic myocarditis in eosinophilic granulomatosis with polyangiitis. Allergol. Int. 2020, 69, 483–484. [Google Scholar] [CrossRef]
- Belfeki, N.; Abroug, S.; Ghriss, N.; Chouchane, I.; Hamrouni, S.; Strazzulla, A.; Zayet, S. Successful benralizumab for eosinophilic myocarditis in eosinophilic granulomatosis with polyangiitis. Clin. Exp. Rheumatol. 2022, 40, 834–837. [Google Scholar] [CrossRef]
- Pakbaz, M.; Pakbaz, M. Cardiac Involvement in Eosinophilic Granulomatosis with Polyangiitis: A Meta-Analysis of 62 Case Reports. J. Tehran Heart Cent. 2020, 15, 18–26. [Google Scholar] [CrossRef]
- Srikantharajah, M.; Gopalan, D.; Wilson-Morkeh, H.; Siddiqui, S.; McAdoo, S.P.; Youngstein, T. Cardiac Involvement in Eosinophilic Granulomatosis with Polyangiitis. Curr. Cardiol. Rep. 2025, 27, 109. [Google Scholar] [CrossRef]
- Matsuda, S.; Yokota, E.; Furugo, I.; Ikematsu, W.; Imamura, Y.; Matsumoto, I.; Ogata, Y.; Ashihara, T.; Fukuyama, T.; Daimaru, Y.; et al. A case of cardiomyopathy due to allergic bronchopulmonary aspergillosis. Kokyu Junkan. 1991, 39, 703–707. [Google Scholar] [PubMed]
- Goyack, L.; Garcha, G.; Shah, R.; Ortiz Gonzalez, Y.; Vollenweider, M.; Salimian, M.; Cheung, W. Rapid effect of benralizumab in fulminant eosinophilic myocarditis in the setting of uncontrolled eosinophilic asthma. J. Cardiol. Cases 2023, 28, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, N.; Nakano, C.; Oshio, T.; Hirouchi, T.; Satou, M.; Moroi, M.; Oharaseki, T.; Matsuse, H. Successful treatment of an elderly patient with severe eosinophilic asthma and eosinophilic myocarditis using benralizumab. Geriatr. Gerontol. Int. 2022, 22, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, F.; Messina, M.R.; Rizzo, S.; Heffler, E.; Panico, C. Anti-interleukin-5 efficacy in an inclisiran-triggered eosinophilic myocarditis: A case report. Eur. Heart J. Case Rep. 2025, 9, ytaf127. [Google Scholar] [CrossRef]
- Luong, T.V.; Hoang, T.A.; Pham, N.N.; Nguyen, S.T.M.; Tran, Q.B.; Nguyen, H.M.; Doan, T.C.; Ho, B.A.; Dang, H.N.N. Eosinophilic myocarditis due to parasitic infection: A case-based minireview. World J. Cardiol. 2025, 17, 107729. [Google Scholar] [CrossRef]
- Reuss, C.S.; Wilansky, S. Images in cardiovascular medicine. Eosinophilic heart disease in acute myeloproliferative disorder. Circulation 2007, 115, e614–e616. [Google Scholar] [CrossRef]
- Meindl, C.; Paulus, M.; Poschenrieder, F.; Zeman, F.; Maier, L.S.; Debl, K. Patients with acute myocarditis and preserved systolic left ventricular function: Comparison of global and regional longitudinal strain imaging by echocardiography with quantification of late gadolinium enhancement by CMR. Clin. Res. Cardiol. 2021, 110, 1792–1800. [Google Scholar] [CrossRef]
- Writing, C.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T.; Aggarwal, N.R.; Basso, C.; Bhave, N.M.; Caforio, A.L.P.; Ferreira, V.M.; Heidecker, B.; et al. 2024 ACC Expert Consensus Decision Pathway on Strategies and Criteria for the Diagnosis and Management of Myocarditis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2025, 85, 391–431. [Google Scholar] [CrossRef]
- Cundari, G.; Galea, N.; De Rubeis, G.; Frustaci, A.; Cilia, F.; Mancuso, G.; Marchitelli, L.; Catapano, F.; Carbone, I.; Catalano, C.; et al. Use of the new Lake Louise Criteria improves CMR detection of atypical forms of acute myocarditis. Int. J. Cardiovasc. Imaging 2021, 37, 1395–1404. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Azzu, A.; Androulakis, E.; Tanking, C.; Papagkikas, P.; Mohiaddin, R.H. Eosinophilic heart disease: Diagnostic and prognostic assessment by cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1273–1284. [Google Scholar] [CrossRef]
- Nagai, T.; Inomata, T.; Kohno, T.; Sato, T.; Tada, A.; Kubo, T.; Nakamura, K.; Oyama-Manabe, N.; Ikeda, Y.; Fujino, T.; et al. JCS 2023 Guideline on the Diagnosis and Treatment of Myocarditis. Circ. J. 2023, 87, 674–754. [Google Scholar] [CrossRef]
- Ammirati, E.; Buono, A.; Moroni, F.; Gigli, L.; Power, J.R.; Ciabatti, M.; Garascia, A.; Adler, E.D.; Pieroni, M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr. Cardiol. Rep. 2022, 24, 597–609. [Google Scholar] [CrossRef]
- Karameh, M.; Meir, K.; Qadan, A.; Pappo, O.; Cohen, D.; Durst, R.; Amir, O.; Asleh, R. Endomyocardial biopsy in clinical practice: The diagnostic yield and insights from a 5-year single-center experience. Hellenic J. Cardiol. 2025, 84, 22–31. [Google Scholar] [CrossRef]
- Martens, P.; Cooper, L.T.; Tang, W.H.W. Diagnostic Approach for Suspected Acute Myocarditis: Considerations for Standardization and Broadening Clinical Spectrum. J. Am. Heart Assoc. 2023, 12, e031454. [Google Scholar] [CrossRef]
- Shiomi, M.; Watanabe, R.; Ishihara, R.; Tanaka, S.; Nakazawa, T.; Hashimoto, M. Comparative Insights on IL-5 Targeting with Mepolizumab and Benralizumab: Enhancing EGPA Treatment Strategies. Biomolecules 2025, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Nair, P.; Terrier, B.; Walz, B.; Bourdin, A.; Jayne, D.R.W.; Jackson, D.J.; Roufosse, F.; Borjesson Sjo, L.; Fan, Y.; et al. Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2024, 390, 911–921. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Li, J.; Guo, T.; Lv, Z.; Zhang, D.; Feng, X.; Zhang, J.; Fang, L.; Tian, X.; et al. Cardiac involvement in eosinophilic granulomatosis with polyangiitis: Acute eosinophilic myocarditis and chronic inflammatory cardiomyopathy. Rheumatology 2025, 64, 722–731. [Google Scholar] [CrossRef]
- Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics 2022, 12, 399. [Google Scholar] [CrossRef]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef]
- Bogacka, J.; Pawlik, K.; Ciapala, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R.; on behalf of the American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Roufosse, F.E.; Johansson, M.W. Editorial: Pathogenic Advances and Therapeutic Perspectives for Eosinophilic Inflammation. Front. Med. 2018, 5, 243. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, K.; Onoue, K.; Terasaki, S.; Nakano, T.; Nakai, M.; Sumita, Y.; Hatakeyama, K.; Terasaki, F.; Kawakami, R.; Iwanaga, Y.; et al. Features and Outcomes of Histologically Proven Myocarditis With Fulminant Presentation. Circulation 2022, 146, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Sato, N.; Legrand, F.; Gadkari, M.; Makiya, M.; Stokes, K.; Howe, K.N.; Yu, S.J.; Linde, N.S.; Clevenger, R.R.; et al. Glucocorticoid-induced eosinopenia results from CXCR4-dependent bone marrow migration. Blood 2020, 136, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Mejia, R.; Nutman, T.B. Evaluation and differential diagnosis of marked, persistent eosinophilia. Semin. Hematol. 2012, 49, 149–159. [Google Scholar] [CrossRef]
- Kamide, Y.; Taniguchi, M. Eosinophilic granulomatosis with polyangiitis: Current status and future perspectives. Respir. Investig. 2025, 63, 639–650. [Google Scholar] [CrossRef]
- Fricker, M.; Harrington, J.; Hiles, S.A.; Gibson, P.G. Mepolizumab depletes inflammatory but preserves homeostatic eosinophils in severe asthma. Allergy 2024, 79, 3118–3128. [Google Scholar] [CrossRef]
- Pouliquen, I.J.; Kornmann, O.; Barton, S.V.; Price, J.A.; Ortega, H.G. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. Int. J. Clin. Pharmacol. Ther. 2015, 53, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Toor, I.S.; Ruckerl, D.; Mair, I.; Ainsworth, R.; Meloni, M.; Spiroski, A.M.; Benezech, C.; Felton, J.M.; Thomson, A.; Caporali, A.; et al. Eosinophil Deficiency Promotes Aberrant Repair and Adverse Remodeling Following Acute Myocardial Infarction. JACC Basic Transl. Sci. 2020, 5, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, L.; Atkins, C.; Kuhlman, L.; Zhao, J.; Jeong, J.M.; Wen, Y.; Moreno, N.; Kim, K.H.; An, Y.A.; et al. Novel IL-4/HB-EGF-dependent crosstalk between eosinophils and macrophages controls liver regeneration after ischaemia and reperfusion injury. Gut 2024, 73, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.A.; Nair, P.K.; Khalidi, N.; Terrier, B.; Hellmich, B.; Bourdin, A.; Jayne, D.R.W.; Jackson, D.J.; Roufosse, F.; Pagnoux, C.; et al. Two-year efficacy and safety of anti-interleukin-5/receptor therapy for eosinophilic granulomatosis with polyangiitis. Ann. Rheum. Dis. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and with Left Ventricular Thrombus: A Scientific Statement from the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef]
- Tan, B.E.; Baqai, F.; Padilla, F.; Nimri, N.; Cheung, J.W.; Kottam, A.; Medina, H.M. Cardiac CT Versus Transesophageal Echocardiography Following Left Atrial Appendage Closure: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2025, 18, e018151. [Google Scholar] [CrossRef]
- Slungaard, A.; Vercellotti, G.M.; Tran, T.; Gleich, G.J.; Key, N.S. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J. Clin. Investig. 1993, 91, 1721–1730. [Google Scholar] [CrossRef]
- Marx, C.; Novotny, J.; Salbeck, D.; Zellner, K.R.; Nicolai, L.; Pekayvaz, K.; Kilani, B.; Stockhausen, S.; Burgener, N.; Kupka, D.; et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 2019, 134, 1859–1872. [Google Scholar] [CrossRef]
- Al-Maimoony, T.; Al-Matari, K.; Al-Habeet, A.; Aljaber, N.N.; Al-Marwala, M.; Al-Hashmi, S. Efficacy and safety of off-label direct oral anticoagulants vs. warfarin for left ventricular thrombus: An inverse probability of treatment weighting analysis. Front. Cardiovasc. Med. 2025, 12, 1465866. [Google Scholar] [CrossRef]
- Johannesdottir, S.A.; Horvath-Puho, E.; Dekkers, O.M.; Cannegieter, S.C.; Jorgensen, J.O.; Ehrenstein, V.; Vandenbroucke, J.P.; Pedersen, L.; Sorensen, H.T. Use of glucocorticoids and risk of venous thromboembolism: A nationwide population-based case-control study. JAMA Intern. Med. 2013, 173, 743–752. [Google Scholar] [CrossRef]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Tresoldi, M.; Dagna, L. Myocarditis: An Interleukin-1-Mediated Disease? Front. Immunol. 2018, 9, 1335. [Google Scholar] [CrossRef]
- Kamide, Y.; Watai, K.; Nakamura, Y.; Iwata, M.; Fukutomi, Y.; Taniguchi, M.; Sekiya, K. Reduction in ANCA levels associated with mepolizumab add-on treatment in eosinophilic granulomatosis with polyangiitis: Case series and literature review. Allergol. Int. 2024, 73, 180–183. [Google Scholar] [CrossRef]

| (a) | ||||||||||
| Author, Year | Age, Sex | Country | Etiological Background | Eos (/μL) | Extra-Cardiac Involvement | Echocardiographic Findings|Cardiac MRI Findings (→ After Treatment) | Cardiac Histopathology | Treatment | Timing of Mepo after EM—Indication | Mepolizumab Effect—Long Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Song, 2017 [16] | 60, M | USA | HES or ANCA-negative EGPA | 7800 | Sinus, asthma | EF 30%, large pericardial effusion, global hypokinesis → 35–40%|Diffuse endomyocardial infiltration | Eosinophilic infiltration with early thrombus; no vasculitis | Steroids → Aza → RTX → mepo (100 mg) | 6 months—Treatment at relapse/worsening | Steroid-sparing, EF stabilization, clinical improvement—stable at 7 months |
| Kowtoniuk, 2018 [13] | 45, F | USA | DRESS (caused by lamotrigine) | 270 | Skin | EF 30–34% → 60% (2 weeks)|Patchy mid-myocardial/subendocardial edema + LGE, improved | Dense eosinophilic infiltrates with necrosis, rare giant cells | Steroids → CPA → mepo (300 mg→500 mg) | 6 weeks—Treatment at relapse/worsening | Steroid-sparing, clinical improvement—remission at 12 months |
| Truong, 2021 [14] | 50 s, M | Australia | DRESS (caused by ciprofloxacin) | 3800 | Thyroid, skin | EF 33% → 65% (within 2 weeks)|Not reported | Mixed lymphohistiocytic + eosinophilic infiltrates, no necrosis | Steroids → mepo (300 mg) + CPA | Day 13—Initial therapy | Steroid-sparing, EF stabilization, clinical improvement—stable at 9 months |
| Huynh, 2022 [17] | 20, M | Australia | HES | 2200 | Bone marrow | EF reduced → normalized (Day 19)|Atrial-predominant fibrosis | Eosinophilic infiltration | Steroids → mepo (300 mg) + CPA | Day 19—Treatment at relapse/worsening | Clinical improvement, EF stabilization—symptom-free at 6 months but persistent sinus arrest |
| Higashitani, 2022 [18] | 46, F | Japan | ANCA-negative EGPA | 3250 | Asthma, mononeuritis multiplex | EF 41%, LV thickening, pericardial effusion → EF 48%|Diffuse edema, high ECV, mid-wall LGE; regressed | Eosinophilic infiltration, no vasculitis | Steroids → mepo (300 mg) + RTX | Day 40—Initial therapy | Steroid-sparing, EF improvement, clinical improvement—stable at 6 months |
| Ulu, 2023 [19] | 17, F | Türkiye | ANCA-negative EGPA | 1500 | Skin, lung, bone marrow | Pericardial effusion 14 mm → resolved|Acute myocarditis with edema → resolved | Not performed | Steroids + IVIG + MTX → CPA → RTX→mepo (100 mg) | 3 months—Treatment at relapse/worsening | Steroid-sparing, clinical improvement, EF stabilization –remission at 12 months |
| Wang, 2023 [20] | 36, F | Taiwan | MPO-ANCA-positive EGPA | 7140 | Asthma, mononeuritis multiplex | EF reduced → normalized (12 months)|Diffuse mid-wall/endocardial LGE + edema → edema resolved, LGE ↓ | Not described | Steroids → mepo (100 mg) | 1 month—Treatment at relapse/worsening | Steroid-sparing, EF normalization, clinical improvement—remission at 12 months |
| Rao, 2023 [22] | 20, M | USA | ANCA-negative EGPA | 10,227 | Sinus, asthma, lung | EF 40% → 45%|Diffuse edema + transmural LGE + mural thrombi → improved, residual LGE | Eosinophilic infiltration with thrombus | Steroids + mepo (300 mg) | Day 3—Initial therapy | EF improvement, clinical improvement—stable at 6 months |
| Panina, 2023 [23] | 8, F | Latvia | ANCA-negative EGPA | 25,530 | Sinus, skin | EF normal; RVH → decreased|Diffuse subendocardial LGE + edema → improved, residual LGE | Not performed (bone marrow: hypercellularity with eosinophilia) | Steroids + mepo (dose not stated) | 10 months—Steroid-sparing as maintenance therapy | EF improvement, clinical improvement—not stated |
| Trovato, 2024 [24] | 34, F | USA | HES | 5260 | Asthma | EF 31% → 46%|Myopericarditis with mural thrombus + fibrosis | Eosinophilic infiltration | Steroids → mepo (300 mg) | 10 months—Treatment at relapse/worsening | Steroid-sparing, EF improvement, clinical improvement—remission at 16 months |
| Trovato, 2024 [24] | 65, M | USA | EGPA (ANCA not reported) | 990 | Asthma | EF n/a → normalized|Acute on chronic myocarditis with patchy LGE + edema (CMR EF 50%) | Not described | Steroids → mepo (300 mg) | shortly after diagnosis—Initial therapy | Steroid-sparing, EF stabilization, clinical improvement—remission at 12 months |
| Trovato, 2024 [24] | 61, F | USA | HES | 11,300 | Asthma | EF 35% → 40–45%|Active inflammation with subendocardial fibrosis | Not described | Steroids → mepo (300 mg) | 3 months—Steroid-sparing as maintenance therapy | Steroid-sparing, EF improvement, clinical improvement—stable at 3 months |
| Watanabe, 2024 [15] | 30, F | Japan | DRESS (caused by phenobarbital) | ND | Liver, skin | EF diffusely impaired (exact value not reported), LVH, pericardial effusion|Not reported | Extensive eosinophilic + macrophage infiltration | Steroids → CPA → mepo (300 mg) | 8 months—Treatment at relapse/worsening | Only transient improvement—fatal course |
| Brick, 2024 [25] | 38, F | Australia | Idiopathic HES (iHES) | 9700 | ND | EF n/a (MRI 41%) → normalized after mechanical support|Biventricular thrombi + transmural LGE→both improved | Biventricular thrombus with transmural necrosis | Steroids → mepo (not stated) | Day 87—Steroid-sparing as maintenance therapy | Steroid-sparing, EF improvement, clinical improvement—stable at 3 months |
| Sharma, 2024 [26] | 51, F | USA | Idiopathic HES (iHES) | 4180 | Asthma, | EF 41%, biventricular apical thickened and thrombi → EF 60% post-transplant|Diffuse subendocardial LGE | No inflammatory infiltrate, granuloma, or fibrosis | Steroids → mepo (300 mg) | 6 weeks—Initial therapy | Reduced disease flares—stable at 3 months |
| Tartaglia, 2025 [34] | 72, M | Italy | ABPM | 880 | Asthma, lung | EF normal → normal|basal anterior and inferior LGE → improved | Eosinophilic infiltration with mural thrombus | Steroids + mepo (300 mg) | 8 months—Treatment at relapse/worsening | Steroid-sparing, EF improvement, clinical improvement—stable at 6 months |
| This case, 2025 | 24, M | Japan | ANCA-negative EGPA | 13,060 | Sinus, asthma, lung, bone marrow | EF 47%, myocardial edema (Figure 2c) → EF 59% (Day 15)|biventricular subendocardial enhancement (Figure 5) | Mixed lymphohistiocytic + eosinophilic infiltrates, no necrosis | Steroids → mepo (300 mg) | Day 4—Initial therapy | Steroid-sparing, EF improvement, clinical improvement—remission at 19 months |
| (b) | ||||||||||
| Author, Year | Age, Sex | Country | Etiological background | Eos (/μL) | Extra-Cardiac Involvement | Echocardiographic Findings|Cardiac MRI Findings (→ After Treatment) | Cardiac Histopathology | Treatment | Timing of Benr After EM—Indication | Benralizumab Effect—Long Outcomes |
| Colantuono, 2020 [27] | 19, M | Italy | ANCA-negative EGPA | 13,470 | Asthma, colitis, neuropathy, skin | EF n/a (MRI 40%) → 60% (2 months)|Diffuse subendocardial edema/fibrosis → edema resolved, fibrosis persisted (12 months) | Eosinophilic myocarditis with subendocardial fibrosis. | Steroids → benra 30 mg | 6 weeks—Initial therapy | Steroid-sparing, EF improvement, clinical improvement—remission at 12 months |
| Kodaka, 2022 [33] | 72, F | Japan | Eosinophilic asthma | 940 | Asthma | EF 41% → 48%|Not reported | Eosinophilic infiltration with interstitial fibrosis | Steroids → benra 30 mg | 3 years after onset—Treatment at relapse/worsening | Steroid-sparing, EF improvement, clinical improvement—remission at 12 months |
| Belfeki, 2022 [28] | 66, M | France | MPO-ANCA-positive EGPA | 2500 | Neuropathy, renal | EF normal → normal|Patchy subepicardial LGE → resolved | Not described. | Steroids + RTX → relapse → benra 30 mg | After 3rd maintenance RTX (≈18 M)—Treatment at relapse/worsening | Steroid-sparing, EF improvement, clinical improvement—stable at 30 months |
| Goyack, 2023 [32] | 51, M | USA | Eosinophilic asthma | 18,150 | Asthma | EF 30–34% → 40–44%|Diffuse subendocardial + septal LGE → improved, residual septal enhancement | Eosinophil degranulation → follow-up biopsy: no eosinophils, mild myocyte hypertrophy | Steroids → benra 30 mg | Day 10—Initial therapy | Steroid-sparing, EF improvement, clinical improvement—stable at 9 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, H.; Awaya, T.; Nagamatsu, H.; Satake, Y.; Hirose, R.; Toba, N.; Toyama-Kousaka, M.; Ota, S.; Morikawa, M.; Tajiri, Y.; et al. Eosinophilic Myocarditis Treated with IL-5 Blockade: An Integrated Case Report and Literature Review. J. Clin. Med. 2025, 14, 6829. https://doi.org/10.3390/jcm14196829
Takahashi H, Awaya T, Nagamatsu H, Satake Y, Hirose R, Toba N, Toyama-Kousaka M, Ota S, Morikawa M, Tajiri Y, et al. Eosinophilic Myocarditis Treated with IL-5 Blockade: An Integrated Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(19):6829. https://doi.org/10.3390/jcm14196829
Chicago/Turabian StyleTakahashi, Hidenori, Toru Awaya, Hiroki Nagamatsu, Yugo Satake, Ryutaro Hirose, Naoya Toba, Mio Toyama-Kousaka, Shinichiro Ota, Miwa Morikawa, Yuta Tajiri, and et al. 2025. "Eosinophilic Myocarditis Treated with IL-5 Blockade: An Integrated Case Report and Literature Review" Journal of Clinical Medicine 14, no. 19: 6829. https://doi.org/10.3390/jcm14196829
APA StyleTakahashi, H., Awaya, T., Nagamatsu, H., Satake, Y., Hirose, R., Toba, N., Toyama-Kousaka, M., Ota, S., Morikawa, M., Tajiri, Y., Agemi, Y., Nakano, N., & Shinkai, M. (2025). Eosinophilic Myocarditis Treated with IL-5 Blockade: An Integrated Case Report and Literature Review. Journal of Clinical Medicine, 14(19), 6829. https://doi.org/10.3390/jcm14196829






