Real-World Use of a Decellularized Porcine Placental Extracellular Matrix in Hard-to-Heal Wounds: A Retrospective, Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. PPECM + SoC Application
2.4. Outcomes
2.5. Statistical Methods
3. Results
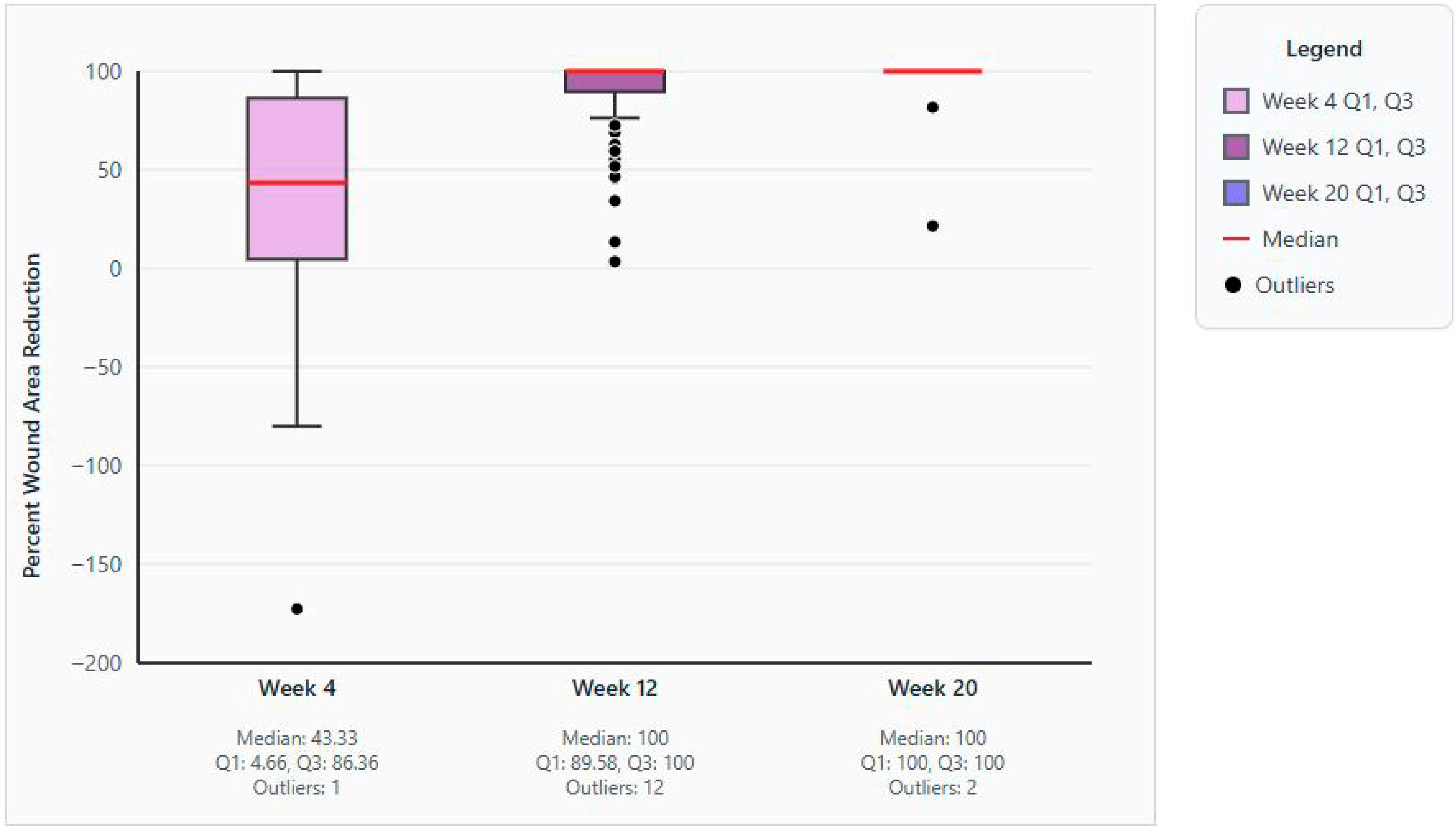
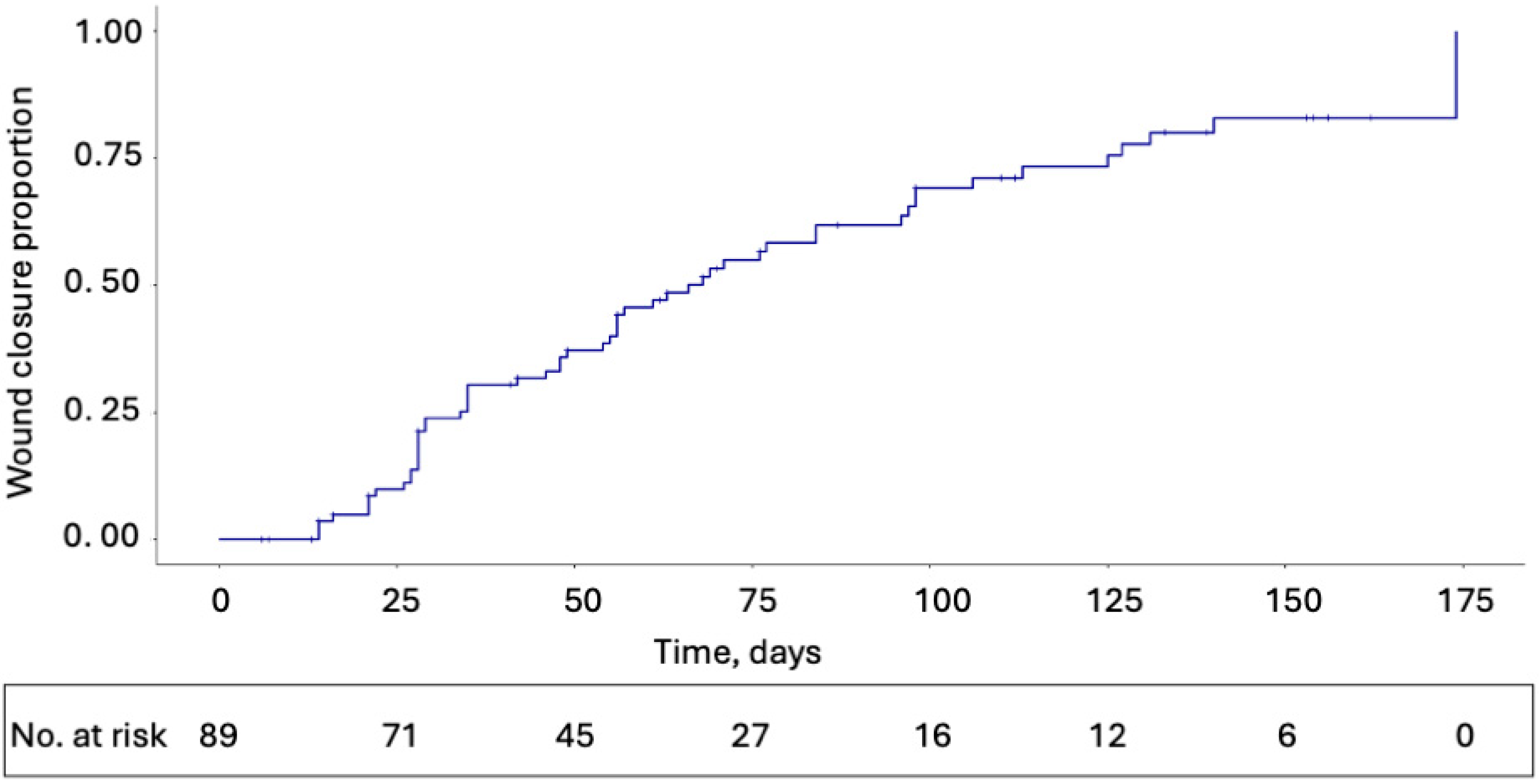
3.1. Primary Outcome
3.2. Secondary and Exploratory Outcomes
3.3. Safety
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| DFU | Diabetic foot ulcer |
| ECM | Extracellular matrix |
| EMR | Electronic medical record |
| HIPAA | Health Insurance Portability and Accountability Act |
| PPECM | Porcine placental extracellular matrix |
| SAE | Serious adverse event |
| SoC | Standard of care |
| VLU | Venous leg ulcer |
References
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Atkin, L.; Bućko, Z.; Conde Montero, E.; Cutting, K.; Moffatt, C.; Probst, A.; Romanelli, M.; Schultz, G.S.; Tettelbach, W. Implementing TIMERS: The race against hard-to-heal wounds. J. Wound Care 2019, 23, S1–S50. [Google Scholar] [CrossRef]
- Carter, M.J.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D.; Fife, C.E. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: Changes between 2014 and 2019. J. Med. Econ. 2023, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Bacci, S. Mast cells and wound healing: Still an open question. Histol. Histopathol. 2025, 40, 21–30. [Google Scholar] [PubMed]
- Grandi, V.; Corsi, A.; Pimpinelli, N.; Bacci, S. Cellular Mechanisms in Acute and Chronic Wounds after PDT Therapy: An Update. Biomedicines 2022, 10, 1624. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Solarte David, V.A.; Güiza-Argüello, V.R.; Arango-Rodríguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized Tissues for Wound Healing: Towards Closing the Gap Between Scaffold Design and Effective Extracellular Matrix Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852. [Google Scholar] [CrossRef]
- Chen, D.; Hao, H.; Fu, X.; Han, W. Insight into Reepithelialization: How Do Mesenchymal Stem Cells Perform? Stem Cells Int. 2016, 2016, 6120173. [Google Scholar] [CrossRef]
- Cramer, M.C.; Badylak, S.F. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef]
- Sicari, B.M.; Dziki, J.L.; Siu, B.F.; Medberry, C.J.; Dearth, C.L.; Badylak, S.F. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 2014, 35, 8605–8612. [Google Scholar] [CrossRef]
- Huleihel, L.; Dziki, J.L.; Bartolacci, J.G.; Rausch, T.; Scarritt, M.E.; Cramer, M.C.; Vorobyov, T.; LoPresti, S.T.; Swineheart, I.T.; White, L.J.; et al. Macrophage phenotype in response to ECM bioscaffolds. Semin. Immunol. 2017, 29, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.T.; Kremer, M.; Landry, J.; Hatfield, S.A.; Wathieu, D.; Brug, A.; Lightell, D.J.; Spiller, K.L.; Woods, T.C. Modulation of inflammation in wounds of diabetic patients treated with porcine urinary bladder matrix. Regen. Med. 2019, 14, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Protzman, N.M.; Mao, Y.; Long, D.; Sivalenka, R.; Gosiewska, A.; Hariri, R.J.; Brigido, S.A. Placental-Derived Biomaterials and Their Application to Wound Healing: A Review. Bioengineering 2023, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Tofigh, A.; Tajik, M. Comparing the standard surgical dressing with dehydrated amnion and platelet-derived growth factor dressings in the healing rate of diabetic foot ulcer: A randomized clinical trial. Diabetes Res. Clin. Pract. 2022, 185, 109775. [Google Scholar] [CrossRef]
- Serena, T.E.; Yaakov, R.; Moore, S.; Cole, W.; Coe, S.; Snyder, R.; Patel, K.; Doner, B.; Kasper, M.A.; Hamil, R.; et al. A randomized controlled clinical trial of a hypothermically stored amniotic membrane for use in diabetic foot ulcers. J. Comp. Eff. Res. 2020, 9, 23–34. [Google Scholar] [CrossRef]
- Lavery, L.A.; Fulmer, J.; Shebetka, K.A.; Regulski, M.; Vayser, D.; Fried, D.; Kashefsky, H.; Owings, T.M.; Nadarajah, J. The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: Results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 2014, 11, 554–560. [Google Scholar] [CrossRef]
- Tettelbach, W.; Cazzell, S.; Reyzelman, A.M.; Sigal, F.; Caporusso, J.M.; Agnew, P.S. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: A prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int. Wound J. 2019, 16, 19–29. [Google Scholar] [CrossRef]
- Tettelbach, W.; Cazzell, S.; Sigal, F.; Caporusso, J.M.; Agnew, P.S.; Hanft, J.; Dove, C. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int. Wound J. 2019, 16, 122–130. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, L.A.; Orgill, D.P.; Galiano, R.D.; Serena, T.E.; Carter, M.J.; Kaufman, J.P.; Young, N.J.; Jacobs, A.M.; Zelen, C.M. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: A prospective, randomised, multi-centre clinical trial in 80 patients. Int. Wound J. 2018, 15, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Orgill, D.P.; Armstrong, D.G.; Galiano, R.D.; Glat, P.M.; Carter, M.J.; Kaufman, J.P.; Li, W.W.; Zelen, C.M. A Multicenter, Randomized, Controlled, Clinical Trial Evaluating Dehydrated Human Amniotic Membrane in the Treatment of Venous Leg Ulcers. Plast. Reconstr. Surg. 2022, 150, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Cazzell, S.; Vayser, D.; Reyzelman, A.M.; Dosluoglu, H.; Tovmassian, G. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix(®)) allograft for the treatment of venous leg ulcers. Int. Wound J. 2018, 15, 114–122. [Google Scholar] [CrossRef]
- Carter, M.J. Dehydrated human amnion and chorion allograft versus standard of care alone in treatment of Wagner 1 diabetic foot ulcers: A trial-based health economics study. J. Med. Econ. 2020, 23, 1273–1283. [Google Scholar] [CrossRef]
- Stetkevich, S.; Gupta, M.; Simman, R.; Jackson, S.E. How to Select an Extracellular Matrix for Wound Repair: A Comprehensive Review. Eplasty 2023, 23, e51. [Google Scholar]
- Coburn, J.; Pandit, A. Development of Naturally-Derived Biomaterials and Optimization of Their Biomechanical Properties. Top. Tissue Eng. 2007, 3, 1–14. [Google Scholar]
- Wu, S.; Carter, M.; Cole, W.; Crombie, R.; Kapp, D.L.; Kim, P.; Milne, C.; Molnar, J.; Niezgoda, J.; Woo, K.; et al. Best practice for wound repair and regeneration use of cellular, acellular and matrix-like products (CAMPs). J. Wound Care 2023, 32, S1–S31. [Google Scholar] [CrossRef]
- Hughes, A.; Zilic, L.; Burgos-Amador, R.; Metcalf, D. The cell migration effects of decellularized porcine placental extracellular matrix in viable wounded human skin ex vivo. In Proceedings of the Symposium on Advanced Wound Care (SAWC) Spring 2024, Orlando, FL, USA, 14–18 May 2024. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bmj 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Lammert, A.; Kiehlmeier, S.; Dissemond, J.; Münter, K.-C.; Schnorpfeil, W.; Pohl, J. Percentage area reduction as surrogate for complete healing of hard-to-heal wounds: A review of clinical trials. J. Wound Care 2024, 33, 737–755. [Google Scholar] [CrossRef]
- Swoboda, L. A Retrospective Analysis of Clinical Use and Outcomes Using Viable Placental Membrane Allografts in Chronic Wounds. Wounds 2021, 33, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Raspovic, K.M.; Wukich, D.K.; Naiman, D.Q.; Lavery, L.A.; Kirsner, R.S.; Kim, P.J.; Steinberg, J.S.; Attinger, C.E.; Danilkovitch, A. Effectiveness of viable cryopreserved placental membranes for management of diabetic foot ulcers in a real world setting. Wound Repair. Regen. 2018, 26, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fife, C.E.; Eckert, K.A.; Carter, M.J. Publicly Reported Wound Healing Rates: The Fantasy and the Reality. Adv. Wound 2018, 7, 77–94. [Google Scholar] [CrossRef]
- Hampton, J.; Meagher, H.; Sharpe, A.; Styche, T.; Hughes, J. Multi-centre, international practice- based evidence using PICOTM single- use negative pressure wound therapy: Challenging current behaviours in wound care practice. Wounds Int. 2022, 13, 46–53. [Google Scholar]
- van de Vyver, M.; Idensohn, P.J.; Niesler, C.U. A regenerative approach to the pharmacological management of hard-to-heal wounds. Biochimie 2022, 196, 131–142. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort study evaluating the burden of wounds to the UK′s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef]
- Queen, D.; Harding, K. What’s the true costs of wounds faced by different healthcare systems around the world? Int. Wound J. 2023, 20, 3935–3938. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Marcinek, B.; Levinson, J.; Nally, S.; Varghese, I.; Sheetz, C.; Kardel, P.; Taylor, C. Comparative effectiveness of porcine placental extracellular matrix against other cellular, acellular and matrix-like products in diabetic foot ulcers from the Medicare database. J. Comp. Eff. Res. 2025, 14, e250092. [Google Scholar] [CrossRef]
- Food and Drug Administration. Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use. U.S. Department of Health and Human Services. July 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed on 3 September 2025).
- di Pompeo, F.S.; Firmani, G.; Paolini, G.; Amorosi, V.; Briganti, F.; Sorotos, M. Immediate prepectoral breast reconstruction using an ADM with smooth round implants: A prospective observational cohort study. J. Plast. Reconstr. Aesthet. Surg. 2023, 80, 56–65. [Google Scholar] [CrossRef]
- Madu, T. Conducting meaningful real-world data analysis in wound care—A guide. J. Wound Care 2021, 30, 93–94. [Google Scholar] [CrossRef]
- Fife, C.; LeBoutillier, B.; Taylor, C.; Marcinek, B.T. Real-World Use and Outcomes of Hard-To-Heal Wounds Managed With Porcine Placental Extracellular Matrix. Cureus 2024, 16, e76262. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fife, C.E.; Walker, D.; Thomson, B. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv. Skin. Wound Care 2009, 22, 316–324. [Google Scholar] [CrossRef]
- Serena, T.E.; Fife, C.E.; Eckert, K.A.; Yaakov, R.A.; Carter, M.J. A new approach to clinical research: Integrating clinical care, quality reporting, and research using a wound care network-based learning healthcare system. Wound Repair. Regen. 2017, 25, 354–365. [Google Scholar] [CrossRef]
| Inclusion criteria |
| Male and female subjects aged 18 years or older at the time the data were reported. |
| Patients with a hard-to-heal wound, defined as those that have a reported history of standard of care * that has failed to show improvement (which is defined as ≥50% reduction in wound area over 4 weeks of standard wound care treatment [2]). |
| Initial wound area that is >0.5 cm2 and <25 cm2 |
| PPECM applied to target wound for a minimum of two consecutive weekly visits inside a 4-week period. |
| Patients were compliant with wound protection strategies through treatment period (offloading, compression, etc.). |
| The target wound is not undergoing active management at the time of data entry. |
| Exclusion criteria |
| Wound area showed a ≥50% reported reduction in 4 weeks preceding initial PPECM application. |
| Cases where PPECM was not applied at a minimum of two consecutive weekly visits inside a 4-week period. |
| Patients who were non-compliant with additional wound protection strategies (offloading, compression, etc.) |
| Wound area <0.5 cm2 or >25 cm2 |
| The target wound is still under active treatment. |
| Any other reason for exclusion at the discretion of the principal investigator. |
| Malignant wounds. |
| Characteristic | Data Value |
|---|---|
| Age, years | |
| Mean (SD) | 75.52 (6.15) |
| Median (IQR) | 79.0 (72.50–80.00) |
| Range | 48–≥80 |
| 18–29 | 0 |
| 30–39 | 0 |
| 40–49 | 1 (1.1) |
| 50–59 | 1 (1.1) |
| 60–69 | 14 (15.7) |
| 70–79 | 30 (33.7) |
| ≥80 | 43 (48.3) |
| Sex, n | |
| Male | 48 (53.9) |
| Female | 41 (46.1) |
| Ethnicity, n | |
| Not Hispanic or Latino | 72 (80.9) |
| Unknown | 14 (15.7) |
| Not reported | 3 (3.4) |
| Race, n | |
| American Indian or Alaskan Native | 2 (2.2) |
| Asian | 1 (1.1) |
| Black of African American | 7 (7.9) |
| Native Hawaiian or Other Pacific Islander | 2 (2.2) |
| White/Caucasian | 64 (71.9) |
| Unknown | 13 (14.6) |
| Wound age at first application of PPECM, weeks | |
| Mean (SD) | 26.28 (58.65) |
| Median (Q1–Q3) | 11.43 (7.29–20.14) |
| Range | 4.0–484.9 |
| Wound etiology or type, n | |
| Arterial ulcer | 2 (2.2) |
| Burn | 2 (2.2) |
| Cellulitis | 1 (1.1) |
| Diabetic ulcer | 9 (10.1) |
| Neuropathic | 2 (2.2) |
| Pressure ulcer | 1 (1.1) |
| Radiation wound | 3 (3.4) |
| Surgical wound | 19 (21.3) |
| Trauma wound | 27 (30.3) |
| Venous ulcer | 23 (25.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soong, E.; Marcinek, B.; Taylor, C.; Michaelis, C. Real-World Use of a Decellularized Porcine Placental Extracellular Matrix in Hard-to-Heal Wounds: A Retrospective, Single-Center Study. J. Clin. Med. 2025, 14, 6823. https://doi.org/10.3390/jcm14196823
Soong E, Marcinek B, Taylor C, Michaelis C. Real-World Use of a Decellularized Porcine Placental Extracellular Matrix in Hard-to-Heal Wounds: A Retrospective, Single-Center Study. Journal of Clinical Medicine. 2025; 14(19):6823. https://doi.org/10.3390/jcm14196823
Chicago/Turabian StyleSoong, Eddie, Brad Marcinek, Cristin Taylor, and Christopher Michaelis. 2025. "Real-World Use of a Decellularized Porcine Placental Extracellular Matrix in Hard-to-Heal Wounds: A Retrospective, Single-Center Study" Journal of Clinical Medicine 14, no. 19: 6823. https://doi.org/10.3390/jcm14196823
APA StyleSoong, E., Marcinek, B., Taylor, C., & Michaelis, C. (2025). Real-World Use of a Decellularized Porcine Placental Extracellular Matrix in Hard-to-Heal Wounds: A Retrospective, Single-Center Study. Journal of Clinical Medicine, 14(19), 6823. https://doi.org/10.3390/jcm14196823





