Effectiveness and Utility of Genetic Testing in Establishing a Diagnosis of Hereditary Transthyretin Amyloidosis †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Analysis
3. Results
3.1. TTR Positivity
3.2. Baseline Characteristics
3.3. Presenting Signs and Symptoms
3.4. Test Provider Specialties and Types of Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, D.; Koike, H.; Slama, M.; Coelho, T. Hereditary transthyretin amyloidosis: A model of medical progress for a fatal disease. Nat. Rev. Neurol. 2019, 15, 387–404. [Google Scholar] [CrossRef]
- Maurer, M.S.; Hanna, M.; Grogan, M.; Kristen, A.V.; Damy, T.; Dispenzieri, A.; Dorbala, S.; Drachman, B.M.; Jakobsen, R.; Johnson, M.R.; et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J. Am. Coll. Cardiol. 2016, 68, 161–172. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Maurer, M.S. Cardiac amyloidosis due to transthyretin protein: A review. JAMA 2024, 331, 778–791. [Google Scholar] [CrossRef]
- Gonzalez-Duarte, A.; Ulloa-Aguirre, A. A brief journey through protein misfolding in transthyretin amyloidosis (ATTR amyloidosis). Int. J. Mol. Sci. 2021, 22, 13158. [Google Scholar] [CrossRef] [PubMed]
- Poli, L.; Labella, B.; Cotti Piccinelli, S.; Caria, F.; Risi, B.; Damioli, S.; Padovani, A.; Filosto, M. Hereditary transthyretin amyloidosis: A comprehensive review with a focus on peripheral neuropathy. Front. Neurol. 2023, 14, 1242815. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Maurer, M.S.; Suhr, O.B. THAOS—The Transthyretin Amyloidosis Outcomes Survey: Initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr. Med. Res. Opin. 2013, 29, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Coelho, T.; Dispenzieri, A.; Conceição, I.; Waddington-Cruz, M.; Kristen, A.; Wixner, J.; Diemberger, I.; Gonzalez-Moreno, J.; Cariou, E.; et al. A 15-year consolidated overview of data in over 6000 patients from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Orphanet J. Rare Dis. 2023, 18, 350. [Google Scholar] [CrossRef]
- Vera-Llonch, M.; Reddy, S.R.; Chang, E.; Tarbox, M.; Pollock, M. The patient journey toward a diagnosis of hereditary transthyretin (ATTRv) amyloidosis. Orphanet J. Rare Dis. 2021, 16, 25. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Large, S.; Bhambri, R.; Stewart, M.; Whelan, J.; van Doornewaard, A.; Dasgupta, N.; Masri, A.; Nativi-Nicolau, J. Impact of delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR-CM): A targeted literature review. Cardiol. Ther. 2021, 10, 141–159. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac amyloidosis: Evolving diagnosis and management: A scientific statement from the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef]
- Adams, D.; Algalarrondo, V.; Echaniz-Laguna, A. Hereditary transthyretin amyloidosis in the era of RNA interference, antisense oligonucleotide and CRISPR-Cas9 treatments. Blood 2023, 142, 1600–1612. [Google Scholar] [CrossRef]
- Maurer, M.S.; Miller, E.J.; Ruberg, F.L. Addressing health disparities—The case for variant transthyretin cardiac amyloidosis grows stronger. JAMA 2024, 331, 1809–1811. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Grazzini Frantz, J.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: A report of the American College of Cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Reilly, M.M.; Coats, C.J.; Cooper, R.; Cox, H.; Coyne, M.R.E.; Green, A.J.; McGowan, R.; Moody, W.E.; Hawkins, P.N. Clinical and genetic evaluation of people with or at risk of hereditary ATTR amyloidosis: An expert opinion and consensus on best practice in Ireland and the UK. Adv. Ther. 2022, 39, 2292–2301. [Google Scholar] [CrossRef]
- Brito, D.; Castro Albrecht, F.; Perez de Arenaza, D.; Bart, N.; Better, N.; Carvajal-Juarez, I.; Conceição, I.; Damy, T.; Dorbala, S.; Fidalgo, J.-C. World Heart Federation consensus on transthyretin amyloidosis cardiomyopathy (ATTR-CM). Glob. Heart 2023, 18, 59. [Google Scholar] [CrossRef]
- Karam, C.; Mauermann, M.L.; Gonzalez-Duarte, A.; Kaku, M.C.; Ajroud-Driss, S.; Brannagan, T.H., III; Polydefkis, M. Diagnosis and treatment of hereditary transthyretin amyloidosis with polyneuropathy in the United States: Recommendations from a panel of experts. Muscle Nerve 2024, 69, 273–287. [Google Scholar] [CrossRef]
- Gertz, M.; Adams, D.; Ando, Y.; Beirão, J.M.; Bokhari, S.; Coelho, T.; Comenzo, R.L.; Damy, T.; Dorbala, S.; Drachman, B.M. Avoiding misdiagnosis: Expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Fam. Pract. 2020, 21, 198. [Google Scholar] [CrossRef]
- Vogt, B.; Chahin, N.; Wiszniewski, W.; Ragole, T.; Karam, C. Screening for genetic mutations in patients with neuropathy without definite etiology is useful. J. Neurol. 2020, 267, 2648–2654. [Google Scholar] [CrossRef] [PubMed]
- Merino-Merino, A.-M.; Labrador-Gomez, J.; Sanchez-Corral, E.; Delgado-Lopez, P.-D.; Perez-Rivera, J.-A. Utility of genetic testing in patients with transthyretin amyloid cardiomyopathy: A brief review. Biomedicines 2023, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Alnylam Pharmaceuticals Inc. Genetic Testing and Counseling Program. 2024. Available online: https://www.alnylam.com/medical-professional-resources/genetic-testing-counseling (accessed on 30 June 2025).
- Skrahina, V.; Grittner, U.; Beetz, C.; Skripuletz, T.; Juenemann, M.; Krämer, H.H.; Hahn, K.; Rieth, A.; Schaechinger, V.; Patten, M. Hereditary transthyretin-related amyloidosis is frequent in polyneuropathy and cardiomyopathy of no obvious aetiology. Ann. Med. 2021, 53, 1787–1796. [Google Scholar] [CrossRef]
- Fargeot, G.; Echaniz-Laguna, A.; Labeyrie, C.; Svahn, J.; Camdessanché, J.-P.; Cintas, P.; Chanson, J.-B.; Esselin, F.; Piedvache, C.; Verstuyft, C.; et al. Hereditary transthyretin amyloidosis in middle-aged and elderly patients with idiopathic polyneuropathy: A nationwide prospective study. Amyloid 2024, 31, 62–69. [Google Scholar] [CrossRef]
- Rösner, S.; Pardo, L.M.; Bertoli-Avella, A.M.; Skrahina, V.; Engel, P.; Schröder, S.; Zielske, S.; Bonke, V.; Kreth, J.; Westphal, G.; et al. Hereditary transthyretin-related amyloidosis ongoing observational study: A baseline report of the first 3167 participants. J. Clin. Med. 2024, 13, 6197. [Google Scholar] [CrossRef]
- Auer-Grumbach, M.; Rettl, R.; Ablasser, K.; Agis, H.; Beetz, C.; Duca, F.; Gattermeier, M.; Glaser, F.; Hacker, M.; Kain, R.; et al. Hereditary ATTR amyloidosis in Austria: Prevalence and epidemiological hot spots. J. Clin. Med. 2020, 9, 2234. [Google Scholar] [CrossRef]
- Aung, N.; Nicholls, H.L.; Chahal, C.A.A.; Khanji, M.Y.; Rauseo, E.; Chadalavada, S.; Petersen, S.E.; Munroe, P.B.; Elliott, P.M.; Lopes, L.R. Prevalence, cardiac phenotype, and outcomes of transthyretin variants in the UK biobank population. JAMA Cardiol. 2024, 9, 964–972. [Google Scholar] [CrossRef]
- Carry, B.J.; Young, K.; Fielden, S.; Kelly, M.A.; Sturm, A.C.; Avila, J.D.; Martin, C.L.; Kirchner, H.L.; Fornwalt, B.K.; Haggerty, C.M. Genomic screening for pathogenic transthyretin variants finds evidence of underdiagnosed amyloid cardiomyopathy from health records. JACC CardioOncol. 2021, 3, 550–561. [Google Scholar] [CrossRef]
- Maestro-Benedicto, A.; Vela, P.; de Frutos, F.; Mora, N.; Pomares, A.; Gonzalez-Vioque, E.; Briceño, A.; Cabrera, E.; Cobo-Marcos, M.; Dominguez, F.; et al. Frequency of hereditary transthyretin amyloidosis among elderly patients with transthyretin cardiomyopathy. Eur. J. Heart Fail. 2022, 24, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.K.; Grodin, J.L.; Maurer, M.S.; Ruberg, F.L.; Patel, A.R.; Khouri, M.G.; Roth, L.R.; Aras, M.A.; Bhardwaj, A.; Bhattacharya, P.; et al. The Cardiac Amyloidosis Registry Study (CARS): Rationale, design and methodology. J. Card. Fail. 2024, 30, 669–678. [Google Scholar] [CrossRef]
- Rowczenio, D.; Quarta, C.C.; Fontana, M.; Whelan, C.J.; Martinez-Naharro, A.; Trojer, H.; Baginska, A.; Ferguson, S.M.; Gilbertson, J.; Rezk, T.; et al. Analysis of the TTR gene in the investigation of amyloidosis: A 25-year single UK center experience. Hum. Mutat. 2019, 40, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Claggett, B.; Shah, S.H.; Mentz, R.J.; Khouri, M.G.; Manichaikul, A.W.; Khan, S.S.; Rich, S.S.; Mosley, T.H.; Levitan, E.B.; et al. Cardiovascular burden of the V142I transthyretin variant. JAMA 2024, 331, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Madhani, A.; Sabogal, N.; Massillon, D.; Paul, L.D.; Rodriguez, C.; Fine, D.; Helmke, S.; Winburn, M.; Kurian, D.; Raiszadeh, F.; et al. Clinical penetrance of the transthyretin V122I variant in older black patients with heart failure: The SCAN-MP (Screening for Cardiac Amyloidosis with Nuclear Imaging in Minority Populations) study. J. Am. Heart Assoc. 2023, 12, e028973. [Google Scholar] [CrossRef]
- Selvaraj, S.; Claggett, B.; Minamisawa, M.; Windham, B.G.; Chen, L.Y.; Inciardi, R.M.; Buxbaum, J.N.; Mosley, T.H.; Shah, A.M.; Solomon, S.D. Atrial fibrillation and ischemic stroke with the amyloidogenic V122I transthyretin variant among black Americans. J. Am. Coll. Cardiol. 2021, 78, 89–91. [Google Scholar] [CrossRef]
- Hewitt, K.; Starr, N.; Togher, Z.; Sulong, S.; Morris, J.P.; Alexander, M.; Coyne, M.; Murphy, K.; Giblin, G.; Murphy, S.M.; et al. Spectrum of hereditary transthyretin amyloidosis due to T60A (p.Thr80Ala) variant in an Irish Amyloidosis Network. Open Heart 2024, 11, e002906. [Google Scholar] [CrossRef]
- Carr, A.S.; Pelayo-Negro, A.L.; Evans, M.R.B.; Laurà, M.; Blake, J.; Stancanelli, C.; Iodice, V.; Wechalekar, A.D.; Whelan, C.J.; Gillmore, J.D.; et al. A study of the neuropathy associated with transthyretin amyloidosis (ATTR) in the UK. J. Neurol. Neurosurg. Psychiatry 2016, 87, 620–627. [Google Scholar] [CrossRef]
- Sattianayagam, P.T.; Hahn, A.F.; Whelan, C.J.; Gibbs, S.D.J.; Pinney, J.H.; Stangou, A.J.; Rowczenio, D.; Pflugfelder, P.W.; Fox, Z.; Lachmann, H.J.; et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur. Heart J. 2012, 33, 1120–1127. [Google Scholar] [CrossRef]
- Obici, L.; Kuks, J.B.; Buades, J.; Adams, D.; Suhr, O.B.; Coelho, T. Recommendations for presymptomatic genetic testing and management of individuals at risk for hereditary transthyretin amyloidosis. Curr. Opin. Neurol. 2016, 29 (Suppl. S1), S27–S35. [Google Scholar] [CrossRef] [PubMed]
- Grandis, M.; Obici, L.; Luigetti, M.; Briani, C.; Benedicenti, F.; Bisogni, G.; Canepa, M.; Cappelli, F.; Danesino, C.; Fabrizi, G.M.; et al. Recommendations for pre-symptomatic genetic testing for hereditary transthyretin amyloidosis in the era of effective therapy: A multicenter Italian consensus. Orphanet J. Rare Dis. 2020, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Péréon, Y.; Adams, D.; Camdessanché, J.-P.; Chanson, J.-B.; Cintas, P.; Magy, L.; Signaté, A.; Solé, G.; Svahn, J.; Tard, C.; et al. Diagnosis of hereditary transthyretin amyloidosis in patients with suspected chronic inflammatory demyelinating polyneuropathy unresponsive to intravenous immunoglobulins: Results of a retrospective study. Orphanet J. Rare Dis. 2025, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Highlights of Prescribing Information—ATTRUBY. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/216540s000lbl.pdf (accessed on 30 June 2025).
- AstraZeneca. US Prescribing Information: WAINUA™ (Eplontersen) Injection, for Subcutaneous Use. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217388s002lbl.pdf (accessed on 30 June 2025).
- European Medicines Agency. Summary of Product Characteristics: Tegsedi 284 mg Solution for Injection in PRE-Filled Syringe. 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tegsedi (accessed on 30 June 2025).
- Alnylam Pharmaceuticals Inc. US Prescribing Information: ONPATTRO (Patisiran) Lipid Complex Injection, for Intravenous use. 2022. Available online: https://www.alnylam.com/sites/default/files/pdfs/ONPATTRO-Prescribing-Information.pdf (accessed on 30 June 2025).
- Alnylam Pharmaceuticals Inc. US Prescribing Information: AMVUTTRA (Vutrisiran) Injection, for Subcutaneous Use. 2025. Available online: https://www.alnylam.com/sites/default/files/pdfs/amvuttra-us-prescribing-information.pdf (accessed on 30 June 2025).
- Singh, A. Utility of Genetic Testing for Diagnosing hATTR Patients: Results from a European and Middle East Genetic Testing Programme. In Proceedings of the Heart Failure 2025 Congress, Belgrade, Serbia, 17–20 May 2025. [Google Scholar]
- Roblin, S. Alnylam Act®: Effectiveness of Genetic Testing in Establishing a Diagnosis in Patients with Suspicion of Hereditary Transthyretin Amyloidosis. In Proceedings of the American Association of Neuromuscular and Electrodiagnostic Medicine 2024, Savannah, GA, USA, 15–18 October 2024. [Google Scholar]
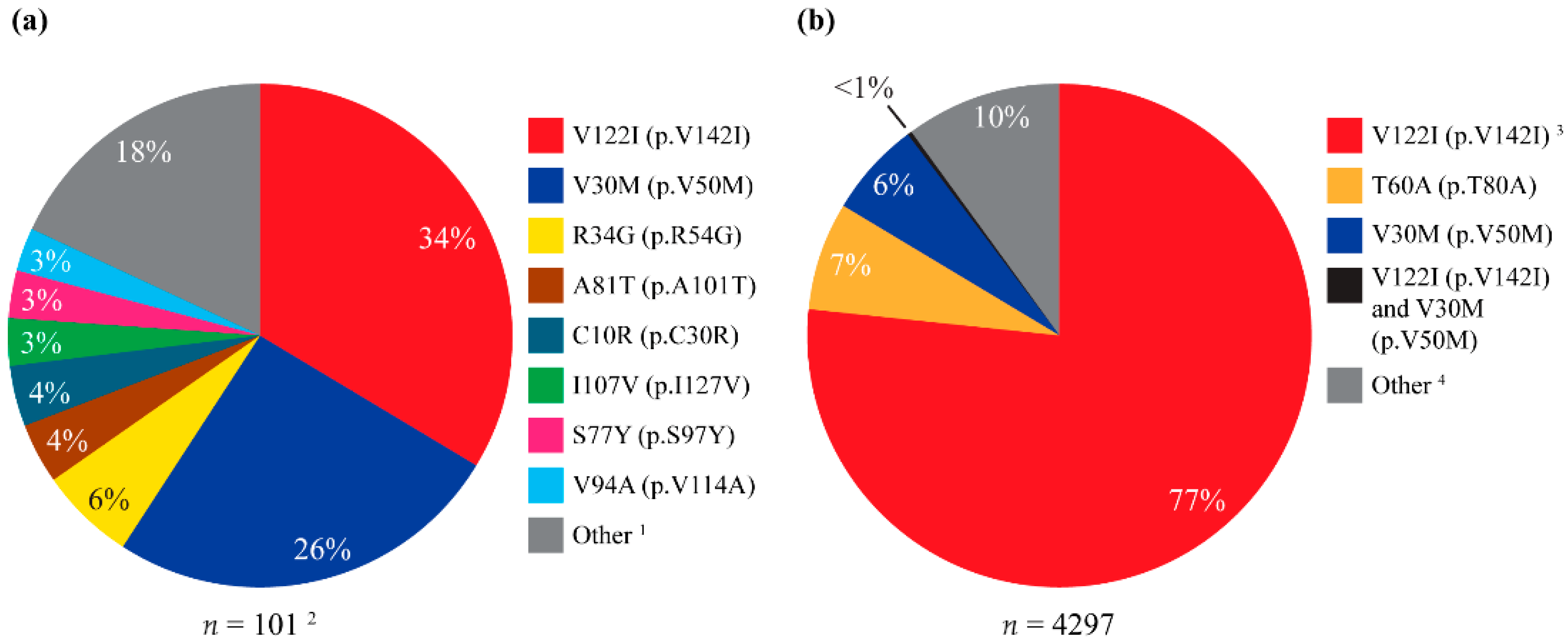

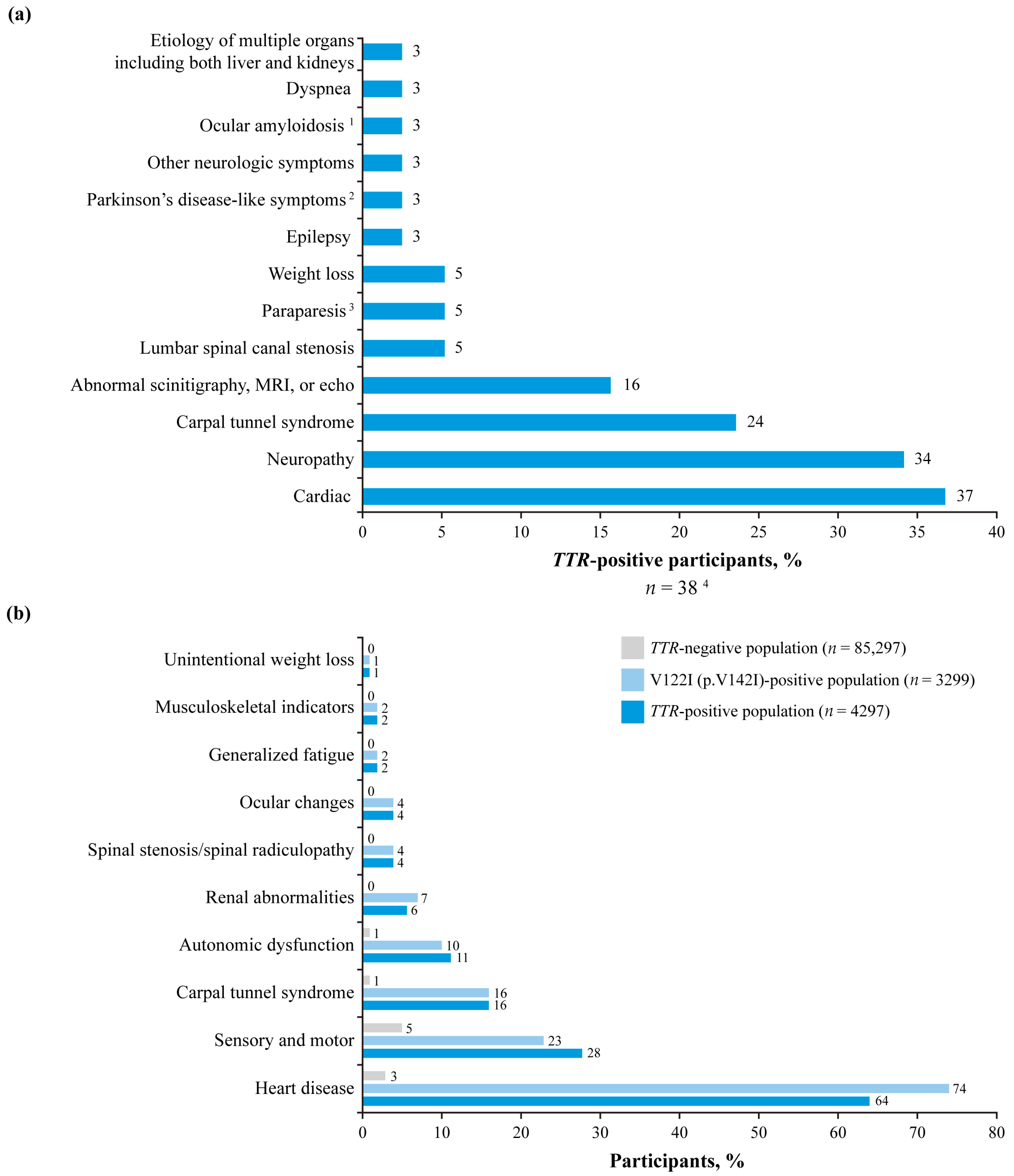

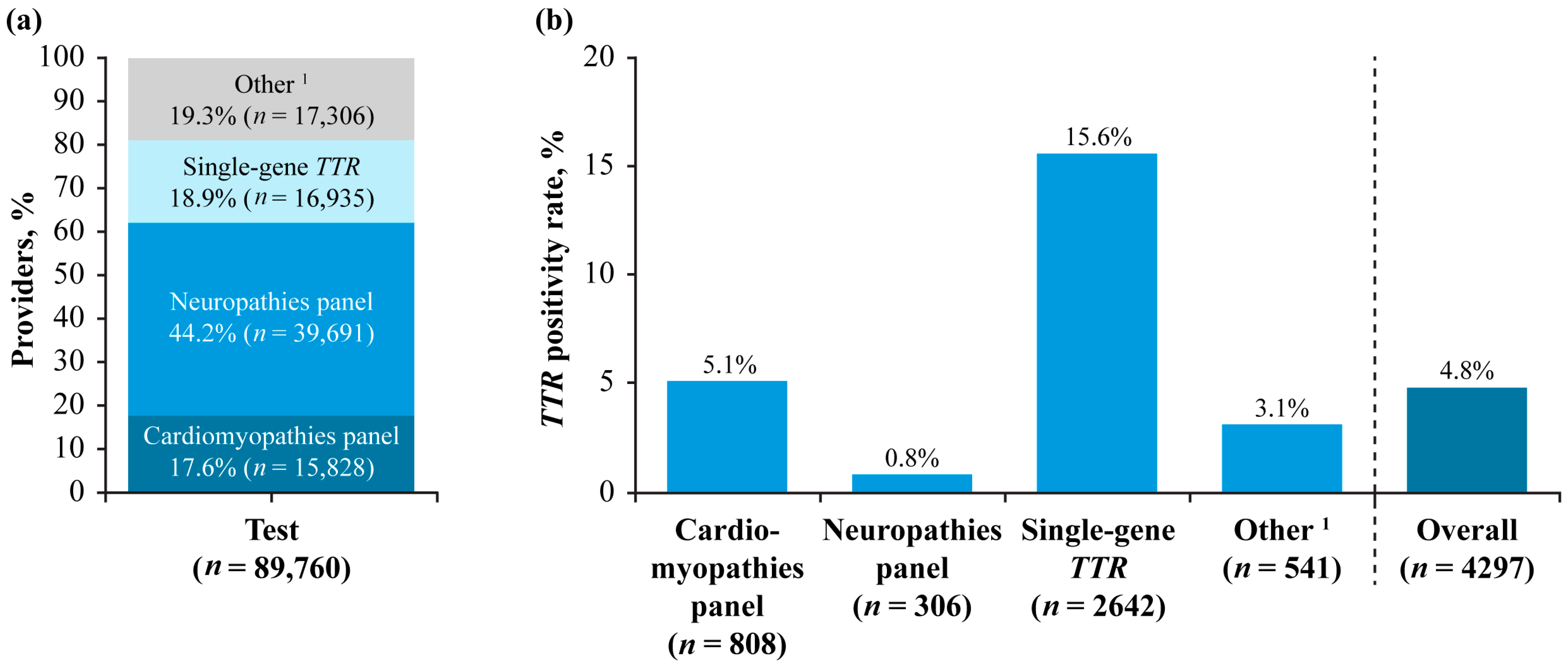
| Total Participants | TTR-Positive Participants | |
|---|---|---|
| GeneAct® 1 | ||
| Participant number | n = 2713 1 | n = 101 2 |
| Age 3, years, mean (SD) | 65.0 (15.9) | 57.2 (18.2) |
| Males 4, n (%) | 1730 (63.8) | 57 (56.4) |
| Females 4, n (%) | 971 (35.8) | 44 (43.6) |
| Alnylam Act® | ||
| Participant number | n = 89,760 | n = 4297 5 |
| Age, years, mean (SD) | 60.5 (16.5) | 65.6 (14.8) |
| Males 6, n (%) | 51,219 (57.1) | 2482 (57.8) |
| Race 7, n (%) | ||
| White | 57,384 (63.9) | 866 (20.2) |
| Black/African American | 12,816 (14.3) | 2640 (61.4) |
| Hispanic | 3099 (3.5) | 183 (4.3) |
| Confirmed family history, n (%) | 2372 (2.6) | 764 (17.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Wyatt, J.; Théaudin, M.; Karam, C.; Kasper, D.; Streubel, B.; Frascello, K.; Bondue, A. Effectiveness and Utility of Genetic Testing in Establishing a Diagnosis of Hereditary Transthyretin Amyloidosis. J. Clin. Med. 2025, 14, 6821. https://doi.org/10.3390/jcm14196821
Singh A, Wyatt J, Théaudin M, Karam C, Kasper D, Streubel B, Frascello K, Bondue A. Effectiveness and Utility of Genetic Testing in Establishing a Diagnosis of Hereditary Transthyretin Amyloidosis. Journal of Clinical Medicine. 2025; 14(19):6821. https://doi.org/10.3390/jcm14196821
Chicago/Turabian StyleSingh, Akash, John Wyatt, Marie Théaudin, Chafic Karam, David Kasper, Berthold Streubel, Karen Frascello, and Antoine Bondue. 2025. "Effectiveness and Utility of Genetic Testing in Establishing a Diagnosis of Hereditary Transthyretin Amyloidosis" Journal of Clinical Medicine 14, no. 19: 6821. https://doi.org/10.3390/jcm14196821
APA StyleSingh, A., Wyatt, J., Théaudin, M., Karam, C., Kasper, D., Streubel, B., Frascello, K., & Bondue, A. (2025). Effectiveness and Utility of Genetic Testing in Establishing a Diagnosis of Hereditary Transthyretin Amyloidosis. Journal of Clinical Medicine, 14(19), 6821. https://doi.org/10.3390/jcm14196821






