High Salivary 3-Nitrotyrosine Levels in Periodontitis
Abstract
1. Introduction
2. Methods
2.1. Design and Subjects
2.2. Definitions
2.3. Variables Recorded
2.4. Salivary Samples
2.5. Salivary 3-NT Concentrations Analysis
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Sánchez-Medrano, A.G.; Martinez-Martinez, R.E.; Soria-Guerra, R.; Portales-Perez, D.; Bach, H.; Martinez-Gutierrez, F. A systematic review of the protein composition of whole saliva in subjects with healthy periodontium compared with chronic periodontitis. PLoS ONE 2023, 18, e0286079. [Google Scholar] [CrossRef]
- Gomes, P.R.; Rocha, M.D.; Lira, J.A.; Coelho, F.A.; Alves, E.H.; Nascimento, H.M.; Oliveira, S.M.; Carmo, R.R.; Araújo, H.T.; Silva, F.R.; et al. Salivary biomarkers present in patients with periodontitis without clinical distinction: Findings from a meta-analysis. Med. Oral Patol. Oral Cir. Bucal. 2023, 28, e457–e466. [Google Scholar] [CrossRef]
- Arroyo, E.; Oliveira-Alves, M.G.; Chamorro-Petronacci, C.M.; Marichalar-Mendia, X.; Bravo-López, S.B.; Blanco-Carrión, J.; Pérez-Sayáns, M. Protein-based salivary biomarkers for the diagnosis of periodontal diseases: Systematic review and meta-analysis. J. Taibah Univ. Med. Sci. 2022, 18, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, G.; Santonocito, S.; Lupi, S.M.; Amato, M.; Spagnuolo, G.; Pesce, P.; Isola, G. Impact of local drug delivery and natural agents as new target strategies against periodontitis: New challenges for personalized therapeutic approach. Ther. Adv. Chronic. Dis. 2023, 14, 20406223231191043. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Fu, Y.; Yao, S.; Huang, L. Programmed cell death of periodontal ligament cells. J. Cell. Physiol. 2023, 238, 1768–1787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, C.; Zhang, Y.; Hu, T.; Cheng, R. PANoptosis is a compound death in periodontitis: A systematic review of ex vivo and in vivo studies. Oral Dis. 2024, 30, 1828–1842. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Chu, P.M.; Tuan, V.P.; Te, J.S.; Lee, I.T. The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights. Antioxidants 2020, 9, 1211. [Google Scholar] [CrossRef]
- Tsikas, D. What we—authors, reviewers and editors of scientific work—can learn from the analytical history of biological 3-nitrotyrosine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1058, 68–72. [Google Scholar] [CrossRef]
- Bandookwala, M.; Thakkar, D.; Sengupta, P. Advancements in the Analytical Quantification of Nitroxidative Stress Biomarker 3-Nitrotyrosine in Biological Matrices. Crit. Rev. Anal. Chem. 2020, 50, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide-derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [PubMed]
- Campolo, N.; Issoglio, F.M.; Estrin, D.A.; Bartesaghi, S.; Radi, R. 3-Nitrotyrosine and related derivatives in proteins: Precursors, radical intermediates and impact in function. Essays Biochem. 2020, 64, 111–133. [Google Scholar] [CrossRef]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A multifaceted oxidizing and nitrating metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofac. Res. 2016, 6, 66–75. [Google Scholar] [CrossRef]
- Solomon, S.M.; Matei, M.N.; Badescu, A.C.; Jelihovschi, I.; Martu-Stefanache, A.; Teusan, A.; Martu, S.; Iancu, L.S. Evaluation of DNA Extraction Methods from Saliva as a Source of PCR—Amplifiable Genomic DNA. Rev. Chim. 2015, 66, 2101–2103. [Google Scholar]
- Huang, Z.; Yang, X.; Huang, Y.; Tang, Z.; Chen, Y.; Liu, H.; Huang, M.; Qing, L.; Li, L.; Wang, Q.; et al. Saliva—A new opportunity for fluid biopsy. Clin. Chem. Lab. Med. 2022, 61, 4–32. [Google Scholar] [CrossRef]
- Kumari, S.; Samara, M.; Ampadi Ramachandran, R.; Gosh, S.; George, H.; Wang, R.; Pesavento, R.P.; Mathew, M.T. A Review on Saliva-Based Health Diagnostics: Biomarker Selection and Future Directions. Biomed. Mater. Devices 2023, 2, 121–138. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z. Saliva biomarkers in oral disease. Clin. Chim. Acta 2023, 548, 117503. [Google Scholar] [CrossRef] [PubMed]
- Malathi, M.; Rajesh, E.; Babu, N.A.; Jimson, S. Saliva as A Diagnostic Tool. Biomed. Pharmacol. J. 2016, 9, 867–870. [Google Scholar] [CrossRef]
- Čižmárová, B.; Tomečková, V.; Hubková, B.; Hurajtová, A.; Ohlasová, J.; Birková, A. Salivary Redox Homeostasis in Human Health and Disease. Int. J. Mol. Sci. 2022, 23, 10076. [Google Scholar] [CrossRef]
- Tóthová, L.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Zhang, X.; Mao, Y.; Ji, Y.; Shi, L.; Cai, W.; Wang, P.; Wu, G.; Gan, X.; et al. Enhanced Oxidative Damage and Nrf2 Downregulation Contribute to the Aggravation of Periodontitis by Diabetes Mellitus. Oxidative Med. Cell. Longev. 2018, 2018, 9421019. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Naruse, K.; Kobayashi, Y.; Miyajima, S.; Mizutani, M.; Kikuchi, T.; Soboku, K.; Nakamura, N.; Sokabe, A.; Tosaki, T.; et al. Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J. Clin. Periodontol. 2012, 39, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Lohinai, Z.; Stachlewitz, R.; Virág, L.; Székely, A.D.; Haskó, G.; Szabó, C. Evidence for reactive nitrogen species formation in the gingivomucosal tissue. J. Dent. Res. 2001, 80, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Yuan, H.; Zhang, X.; Febbraio, M.; Pan, Y.; Huang, S.; Liu, Z. Mitochondrial biogenesis disorder and oxidative damage promote refractory apical periodontitis in rat and human. Int. Endod. J. 2024, 57, 1326–1342. [Google Scholar] [CrossRef]
- Pârvu, A.E.; Alb, S.F.; Crăciun, A.; Taulescu, M.A. Efficacy of subantimicrobial-dose doxycycline against nitrosative stress in chronic periodontitis. Acta. Pharmacol. Sin. 2013, 34, 247–254. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S149–S161. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef]
- Lorente, L.; Hernández Marrero, E.; Abreu González, P.; Lorente Martín, A.D.; González-Rivero, A.F.; Marrero González, M.J.; Hernández Marrero, C.; Hernández Marrero, O.; Jiménez, A.; Hernández Padilla, C.M. Observational prospective study to determine the association and diagnostic utility of salivary nitrites levels in periodontitis. Quintessence Int. 2025, 56, 100–107. [Google Scholar]
- Lorente, L.; Hernández Marrero, E.; Abreu González, P.; Lorente Martín, A.D.; González-Rivero, A.F.; Marrero González, M.J.; Hernández Marrero, C.; Hernández Marrero, O.; Jiménez, A.; Hernández Padilla, C.M. Low salivary uric acid levels are independently associated with periodontitis. World J. Clin. Cases 2025, 13, 105911. [Google Scholar] [CrossRef]
- Lorente, L.; Hernández Marrero, E.; Abreu González, P.; Lorente Martín, A.D.; González-Rivero, A.F.; Marrero González, M.J.; Hernández Marrero, C.; Hernández Marrero, O.; Jiménez, A.; Hernández Padilla, C.M. High Salivary Malondialdehyde Levels Are Associated with Periodontitis Independently of Other Risk Factors. J. Clin. Med. 2025, 14, 2993. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Nanakaly, H.; Nouri Ahmed, S.; Warya Azeez, H. Effect of periodontal therapy on serum and salivary Interleukin-1 beta (IL-1β) and malondialdehyde levels in chronic periodontitis. Cell. Mol. Biol. 2024, 70, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.R.; Lee, A.J.L.; Zhao, J.J.; Chan, Y.H.; Fu, J.H.; Ma, M.; Tay, S.H. Higher odds of periodontitis in systemic lupus erythematosus compared to controls and rheumatoid arthritis: A systematic review, meta-analysis and network meta-analysis. Front. Immunol. 2024, 15, 1356714. [Google Scholar] [CrossRef]
- Sánchez-Alonso, F.; Sánchez-Piedra, C.; González-Dávila, E.; Díaz-González, F. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: A case-control study. Arthritis Res. Ther. 2019, 21, 27. [Google Scholar]
- Shiau, H.J.; Reynolds, M.A. Sex differences in destructive periodontal disease: A systematic review. J. Periodontol. 2010, 81, 1379–1389. [Google Scholar] [CrossRef]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Enteghad, S.; Shirban, F.; Nikbakht, M.H.; Bagherniya, M.; Sahebkar, A. Relationship Between Diabetes Mellitus and Perio-dontal/Peri-Implant Disease: A Contemporaneous Review. Int. Dent. J. 2024, 74, 426–445. [Google Scholar] [CrossRef]
- Tada, A.; Tano, R.; Miura, H. The relationship between tooth loss and hypertension: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 13311. [Google Scholar] [CrossRef]
- Adam, M. Obesity as a risk factor for periodontitis—Does it really matter? Evid.-Based Dent. 2023, 24, 48–49. [Google Scholar] [CrossRef]
- Salvi, G.E.; Roccuzzo, A.; Imber, J.C.; Stähli, A.; Klinge, B.; Lang, N.P. Clinical periodontal diagnosis. Periodontology 2000, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Tamaki, N.; Orihuela-Campos, R.C.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Briguglio, F.; Paterniti, I.; Mazzon, E.; Oteri, G.; Militi, D.; Cordasco, G.; Cuzzocrea, S. Emerging role of PPAR-β/δ in inflammatory process associated to experimental periodontitis. Mediat. Inflamm. 2011, 2011, 787159. [Google Scholar] [CrossRef] [PubMed]
- Elmazoglu, Z.; Bek, Z.A.; Sarıbaş, G.S.; Özoğul, C.; Goker, B.; Bitik, B.; Aktekin, C.N.; Karasu, Ç. TLR4, RAGE, and p-JNK/JNK mediated inflammatory aggression in osteoathritic human chondrocytes are counteracted by redox-sensitive phenolic olive compounds: Comparison with ibuprofen. J. Tissue Eng. Regen. Med. 2020, 14, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 126) | 3-Nitrotyrosine (ng/mL) Median (p 25–75) | |
|---|---|---|
| Without Periodontitis—n (%) | 66 (52.4) | 4.20 (2.53–8.95) |
| Periodontitis stage I—n (%) | 17 (13.5) | 5.68 (2.53–10.43) |
| Periodontitis stage II—n (%) | 24 (19.0) | 5.88 (3.79–9.48) |
| Periodontitis stage III—n (%) | 13 (10.3) | 5.85 (3.32–10.90) |
| Periodontitis stage IV—n (%) | 6 (4.8) | 7.27 (4.38–21.35) |
| Subjects Without Periodontitis (n = 66) | Subjects with Periodontitis (n = 60) | p-Value | |
|---|---|---|---|
| Gender female—n (%) | 47 (71.2) | 37 (61.7) | 0.26 |
| Age (years)—median (p 25–75) | 40 (30–48) | 60 (51–68) | <0.001 |
| Arterial hypertension—n (%) | 4 (6.1) | 18 (30.0) | 0.001 |
| Cardiovascular disease—n (%) | 0 | 4 (6.7) | 0.049 |
| Hyperocholesterolemia—n (%) | 2 (3.0) | 2 (3.3) | 0.99 |
| Diabetes mellitus—n (%) | 0 | 5 (8.3) | 0.02 |
| Rheumatoid arthritis—n (%) | 1 (1.5) | 4 (6.7) | 0.19 |
| Metrotexate for rheumatoid arthritis—n (%) | 1 (1.5) | 0 | 0.99 |
| Immunosupressive therapy—n (%) | 1 (1.5) | 2 (3.3) | 0.61 |
| Radiotherapy—n (%) | 0 | 2 (3.3) | 0.23 |
| Body mass index (kg/m2)—median (p 25–75) | 24.5 (22.4–26.6) | 24.8 (22.6–28.2) | 0.37 |
| Obesity—n (%) | 7 (10.6) | 9 (15.0) | 0.59 |
| Never smoker—n (%) | 54 (81.8) | 25 (41.7) | <0.001 |
| Coffee—n (%) | 55 (83.3) | 53 (88.3) | 0.46 |
| Tea—n (%) | 9 (13.6) | 3 (5.0) | 0.13 |
| Alcohol—n (%) | 25 (37.9) | 31 (51.7) | 0.15 |
| Salivary 3-NT levels (ng/mL)—median (p 25–75) | 4.20 (2.53–8.95) | 5.78 (3.62–9.69) | 0.02 |
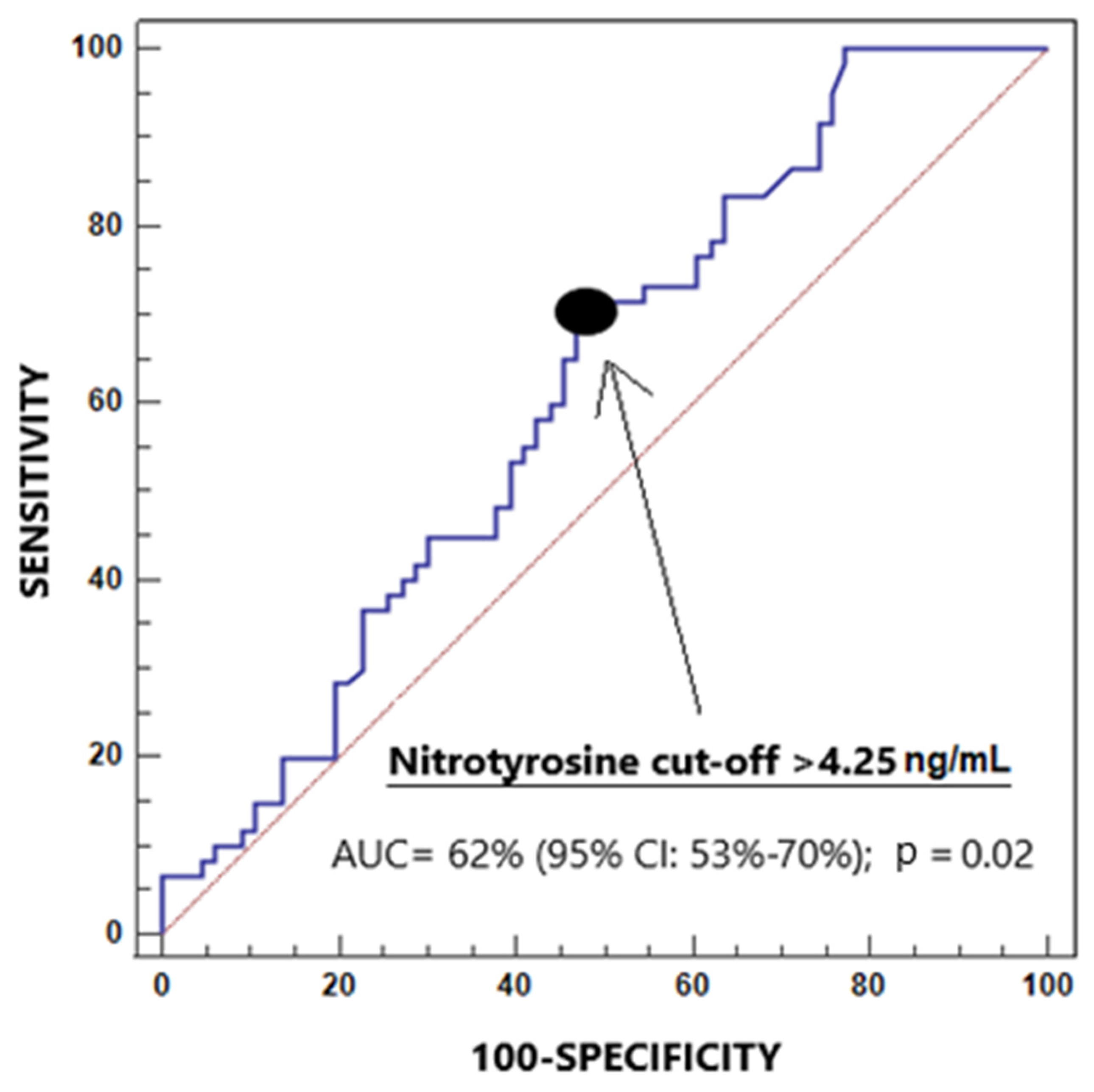
| Salivary 3-NT levels > 4.25 ng/mL—n (%) | 32 (48.5) | 43 (71.7) | 0.01 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Age (years) | 1.12 | 1.064–1.168 | <0.001 |
| Salivary 3-nitrotyrosine levels > 4.25 ng/mL | 3.22 | 1.180–8.789 | 0.02 |
| Never smoker (yes vs. non) | 0.36 | 0.129–0.989 | 0.048 |
| Arterial hypertension (yes vs. non) | 1.13 | 0.260–4.865 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, L.; Hernández Marrero, E.; Abreu González, P.; Lorente Martín, A.D.; González-Rivero, A.F.; Marrero González, M.J.; Hernández Marrero, C.; Hernández Marrero, O.; Jiménez, A.; Hernández Padilla, C.M. High Salivary 3-Nitrotyrosine Levels in Periodontitis. J. Clin. Med. 2025, 14, 6785. https://doi.org/10.3390/jcm14196785
Lorente L, Hernández Marrero E, Abreu González P, Lorente Martín AD, González-Rivero AF, Marrero González MJ, Hernández Marrero C, Hernández Marrero O, Jiménez A, Hernández Padilla CM. High Salivary 3-Nitrotyrosine Levels in Periodontitis. Journal of Clinical Medicine. 2025; 14(19):6785. https://doi.org/10.3390/jcm14196785
Chicago/Turabian StyleLorente, Leonardo, Esther Hernández Marrero, Pedro Abreu González, Angel Daniel Lorente Martín, Agustín F. González-Rivero, María José Marrero González, Carmen Hernández Marrero, Olga Hernández Marrero, Alejandro Jiménez, and Cándido Manuel Hernández Padilla. 2025. "High Salivary 3-Nitrotyrosine Levels in Periodontitis" Journal of Clinical Medicine 14, no. 19: 6785. https://doi.org/10.3390/jcm14196785
APA StyleLorente, L., Hernández Marrero, E., Abreu González, P., Lorente Martín, A. D., González-Rivero, A. F., Marrero González, M. J., Hernández Marrero, C., Hernández Marrero, O., Jiménez, A., & Hernández Padilla, C. M. (2025). High Salivary 3-Nitrotyrosine Levels in Periodontitis. Journal of Clinical Medicine, 14(19), 6785. https://doi.org/10.3390/jcm14196785






