Sleep Bruxism and Occlusal Function: A Case–Control Study Based on Polysomnography in Young Colombians
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Context
2.3. Participants
- Case group: 20 participants with confirmed sleep bruxism diagnosis via PSG.
- Control group: 20 participants without bruxism, matched by age and sex.
2.4. Sleep Bruxism
- Atypical dental wear facets (anterior or posterior).
- Masseter muscle hypertrophy during voluntary clenching.
- Morning muscle pain, discomfort, or fatigue not attributable to other causes.
2.5. Functional Occlusal Assessment
- Premature contacts.
- Wear facets (attrition, abrasion, erosion).
- Occlusal interferences during work, balancing, and protrusive movements.
2.6. Bias
- Selection bias may have arisen from the inclusion of only students with complete records and valid bruxism diagnostics, potentially limiting generalizability. This was mitigated through probabilistic sampling and strict inclusion criteria.
- Information bias, especially regarding self-reported symptoms, was minimized by employing a hierarchical, three-stage diagnostic protocol (self-report, clinical inspection, PSG), enhancing diagnostic accuracy.
- Observer bias was addressed by ensuring that PSG evaluations and clinical interpretations were conducted by blind professionals, using standardized and validated protocols.
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Comparison of Signs, Symptoms, and Medical History
3.2. Dynamic Occlusal Evaluation
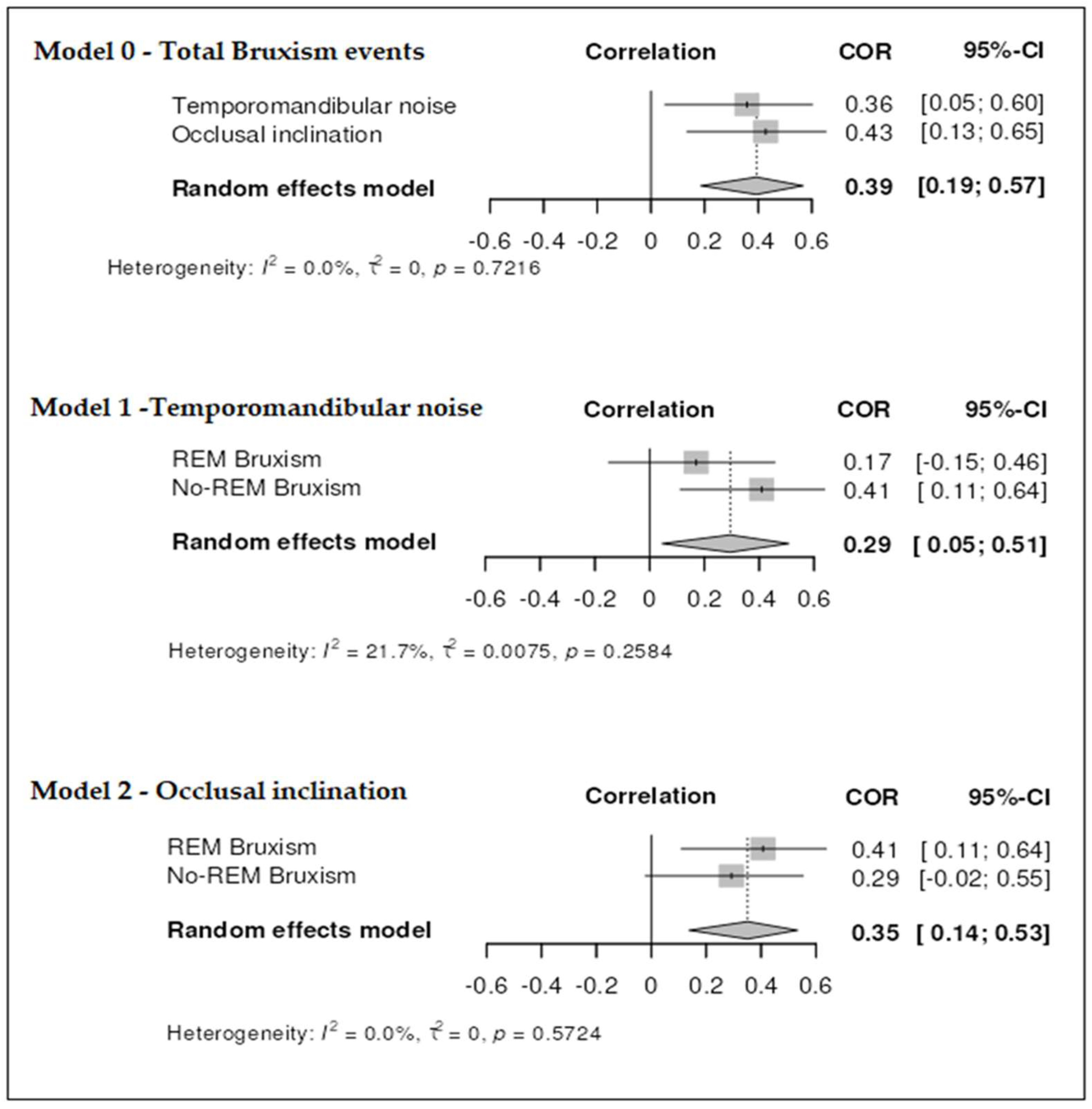
3.3. Correlational Analysis
4. Discussion
5. Clinical and Practical Implications
6. Study Limitations and Strengths
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minakuchi, H.; Fujisawa, M.; Abe, Y.; Iida, T.; Oki, K.; Okura, K.; Tanabe, N.; Nishiyama, A. Managements of sleep bruxism in adult: A systematic review. JPN. Dent. Sci. Rev. 2022, 58, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, M.C.; Lobbezoo, F.; Ahlberg, J.; Bender, S.; Bracci, A.; Colonna, A.; Dal Fabbro, C.; Durham, J.; Glaros, A.G.; Häggman-Henrikson, B.; et al. Updating the Bruxism Definitions: Report of an International Consensus Meeting. J. Oral Rehabil, 2025; early view. [Google Scholar] [CrossRef]
- Bulanda, S.; Ilczuk-Rypuła, D.; Nitecka-Buchta, A.; Nowak, Z.; Baron, S.; Postek-Stefańska, L. Sleep Bruxism in Children: Etiology, Diagnosis, and Treatment-A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 9544. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, N.; Tabatabaei, A.H.; Mohammadi, M.; Rajabi, A. Is bruxism associated with temporomandibular joint disorders? A systematic review and meta-analysis. Evid. Based Dent. 2023, 24, 144. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Pająk, A.; Wójcicki, M.; Litko-Rola, M.; Ginszt, M. Global co-occurrence of bruxism and temporomandibular disorders: A meta-regression analysis. Dent. Med. Probl. 2025, 62, 309–321. [Google Scholar] [CrossRef]
- Song, J.Y. Implant complications in bruxism patients. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 149–150. [Google Scholar] [CrossRef]
- Thomas, D.C.; Manfredini, D.; Patel, J.; George, A.; Chanamolu, B.; Pitchumani, P.K.; Sangalli, L. Sleep bruxism: The past, the present, and the future-evolution of a concept. J. Am. Dent. Assoc. 2024, 155, 329–343. [Google Scholar] [CrossRef]
- Matusz, K.; Maciejewska-Szaniec, Z.; Gredes, T.; Pobudek-Radzikowska, M.; Glapiński, M.; Górna, N.; Przystańska, A. Common therapeutic approaches in sleep and awake bruxism-an overview. Neurol. Neurochir. Pol. 2022, 56, 455–463. [Google Scholar] [CrossRef]
- Casazza, E.; Giraudeau, A.; Payet, A.; Orthlieb, J.D.; Camoin, A. Management of idiopathic sleep bruxism in children and adolescents: A systematic review of the literature. Arch. Pediatr. 2022, 29, 12–20. [Google Scholar] [CrossRef]
- Kuang, B.; Li, D.; Lobbezoo, F.; de Vries, R.; Hilgevoord, A.; de Vries, N.; Huynh, N.; Lavigne, G.; Aarab, G. Associations between sleep bruxism and other sleep-related disorders in adults: A systematic review. Sleep. Med. 2022, 89, 31–47. [Google Scholar] [CrossRef]
- Chung, J.; Lobbezoo, F.; van Selms, M.K.A.; Chattrattrai, T.; Aarab, G.; Mitrirattanakul, S. Physical, psychological and socio-demographic predictors related to patients’ self-belief of their temporomandibular disorders’ aetiology. J. Oral Rehabil. 2021, 48, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Caetano, J.P.; Goettems, M.L.; Nascimento, G.G.; Jansen, K.; da Silva, R.A.; Svensson, P.; Boscato, N. Influence of malocclusion on sleep bruxism and orofacial pain: Data from a study in school children. Clin. Oral Investig. 2024, 28, 142. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Higashiyama, M.; Katagiri, A.; Toyoda, H.; Yamada, M.; Minota, N.; Katsura-Fuchihata, S.; Zhu, Y. Understanding the pathophysiology of sleep bruxism based on human and animal studies: A narrative review. J. Oral Biosci. 2023, 65, 156–162. [Google Scholar] [CrossRef]
- Smardz, J.; Martynowicz, H.; Wojakowska, A.; Michalek-Zrabkowska, M.; Mazur, G.; Wieckiewicz, M. Correlation between Sleep Bruxism, Stress, and Depression-A Polysomnographic Study. J. Clin. Med. 2019, 8, 1344. [Google Scholar] [CrossRef] [PubMed]
- Firmani, M.; Reyes, M.; Becerra, N.; Flores, G.; Weitzman, M.; Espinosa, P. Sleep bruxism in children and adolescents. Rev. Chil. Pediatr. 2015, 86, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Manfredini, D.; Winocur, E. Are bruxism and the bite causally related? J. Oral Rehabil. 2012, 39, 489–501. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA. 2025, 333, 71–74. [Google Scholar] [CrossRef]
- Klasser, G.D.; Rei, N.; Lavigne, G.J. Sleep bruxism etiology: The evolution of a changing paradigm. J. Can. Dent. Assoc. 2015, 81, f2. [Google Scholar]
- Raja, H.Z.; Saleem, M.N.; Mumtaz, M.; Tahir, F.; Iqbal, M.U.; Naeem, A. Diagnosis of Bruxism in Adults: A Systematic Review. J. Coll. Physicians Surg. Pak. 2024, 34, 1221–1228. [Google Scholar] [PubMed]
- Kanclerska, J.; Wieckiewicz, M.; Poreba, R.; Szymanska-Chabowska, A.; Gac, P.; Wojakowska, A.; Frosztega, W.; Michalek-Zrabkowska, M.; Mazur, G.; Martynowicz, H. Polysomnographic Evaluation of Sleep Bruxism Intensity and Sleep Architecture in Nonapneic Hypertensives: A Prospective, Observational Study. J. Clin. Med. 2022, 11, 3113. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Avidan, A.Y.; Foldvary-Schaefer, N.; Malkani, R.G.; During, E.H.; Roland, J.P.; McCarter, S.J.; Zak, R.S.; Carandang, G.; Kazmi, U.; et al. Management of REM sleep behavior disorder: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep. Med. 2023, 19, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.Y.; Kim, M.J.; Lim, Y.J.; Kwon, H.B. Evaluation of eccentric tooth contact on a semi-adjustable articulator by using an occlusal analysis system. J. Prosthet. Dent. 2024, 131, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Zhao, Y.; Wang, Y.; Sun, Y. Design of occlusal wear facets of fixed dental prostheses driven by personalized mandibular movement. J. Prosthet. Dent. 2022, 128, 33–41. [Google Scholar] [CrossRef]
- Pandey, R.; Kamble, R. Comparative evaluation and co-relation in variation of curve of Spee and curve of Wilson in Class II div. 1, Class II div. 2, and Class III as against Class I malocclusion in central India population- an in vitro study. F1000Research 2024, 12, 493. [Google Scholar] [CrossRef]
- Dindaroğlu, F.; Duran, G.S.; Tekeli, A.; Görgülü, S.; Doğan, S. Evaluation of the Relationship between Curve of Spee, WALA-FA Distance and Curve of Wilson in Normal Occlusion. Turk. J. Orthod. 2016, 29, 91–97. [Google Scholar] [CrossRef]
- Aldayel, A.M.; AlGahnem, Z.J.; Alrashidi, I.S.; Nunu, D.Y.; Alzahrani, A.M.; Alburaidi, W.S.; Alanazi, F.; Alamari, A.S.; Alotaibi, R.M. Orthodontics and Temporomandibular Disorders: An Overview. Cureus 2023, 15, e47049. [Google Scholar] [CrossRef]
- Palumbo, S.A.; Robishaw, J.D.; Krasnoff, J.; Hennekens, C.H. Different biases in meta-analyses of case-control and cohort studies: An example from genomics and precision medicine. Ann. Epidemiol. 2021, 58, 38–41. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef] [PubMed]
- Cadar, M.; Almăşan, O. Dental occlusion characteristics in subjects with bruxism. Med. Pharm. Rep. 2024, 97, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Gawda, P. Defining Effect Size Standards in Temporomandibular Joint and Masticatory Muscle Research. Med. Sci. Monit. 2025, 31, e948365. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. The chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, V.; Bornhardt, T.; Velasquez, N. Temporomandibular Joint: Review of Anatomy and Clinical Implications. Dent. Clin. North. Am. 2023, 67, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Xiaojie, X.; Yiling, C.; Honglei, L.; Jiamei, P.; Xiaoyong, W.; Hao, Y.; Hui, C. Comparative analysis of myoelectric activity and mandibular movement in healthy and nonpainful articular temporomandibular disorder subjects. Clin. Oral Investig. 2024, 28, 605. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Bassiur, J.P. Temporomandibular Disorders, Bruxism and Headaches. Neurol. Clin. 2024, 42, 573–584. [Google Scholar] [CrossRef]
- Poluha, R.L.; Canales, G.T.; Bonjardim, L.R.; Conti, P.C.R. Oral behaviors, bruxism, malocclusion and painful temporomandibular joint clicking: Is there an association? Braz. Oral Res. 2021, 35, e090. [Google Scholar] [CrossRef]
- Grossi, M.L.; Castillo, L.O.; Pattussi, M.P.; Pinto, G.M.; Filho, R.T. Validity between signs and symptoms of sleep bruxism against a validated portable electromyographic device. J. Clin. Exp. Dent. 2024, 16, e1354–e1360. [Google Scholar] [CrossRef]
- Manfredini, D.; Lobbezoo, F. Relationship between bruxism and temporomandibular disorders: A systematic review of literature from 1998 to 2008. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 26–50. [Google Scholar] [CrossRef]
- Nagamatsu, S.C.; Minakuchi, H.; Clark, G.T.; Kuboki, T. Relationship between the frequency of sleep bruxism and the prevalence of signs and symptoms of temporomandibular disorders in an adolescent population. Int. J. Prosthodont. 2008, 21, 292–298. [Google Scholar]
- Ita, M.E.; Ghimire, P.; Granquist, E.J.; Winkelstein, B.A. MMPs in tissues retrieved during surgery from patients with TMJ disorders relate to pain more than to radiological damage score. J. Orthop. Res. 2022, 40, 338–347. [Google Scholar] [CrossRef]
- Kluskens, T.J.; Kessler, P.A.; Jansma, B.M.; Kaas, A.; van de Ven, V. Neural Correlates of Tooth Clenching in Patients with Bruxism and Temporomandibular Disorder-Related Pain. J. Oral Facial Pain Headache 2023, 37, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Sun, T.; Shu, J.; Shao, B.; Liu, Z. Effect of Various Degrees of Anterior Disc Displacement on the Biomechanical Response of the Masticatory System. J. Biomech. Eng. 2025, 147, 041006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Chen, J.; Liu, Y.; Pan, L.L.; Guo, Y.X.; Zhang, Z.M.; Li, Q.; Chen, Y.J. Regulation of CeA-Vme projection in masseter hyperactivity caused by restraint stress. Front. Cell Neurosci. 2024, 18, 1509020. [Google Scholar] [CrossRef]
- Bornhardt, T.; Iturriaga, V. Sleep Bruxism: An Integrated Clinical View. Sleep. Med. Clin. 2021, 16, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Tago, C.; Aoki, S.; Sato, S. Status of occlusal contact during sleep bruxism in patients who visited dental clinics-A study using a Bruxchecke. Cranio 2017, 24, 1–7. [Google Scholar]
- Fuentes, A.D.; Sforza, C.; Miralles, R.; Ferreira, C.L.; Mapelli, A.; Lodetti, G.; Martin, C. Assessment of electromyographic activity in patients with temporomandibular disorders and natural mediotrusive occlusal contact during chewing and tooth grinding. Cranio 2017, 35, 152–161. [Google Scholar] [CrossRef]
- Mendoza-Martiarena, Y.; Paredes-Coz, G.; Alvarado-Menacho, S.; Watanabe-Velásquez, R. Relationship between Mediotrusive Occlusal Contacts and Temporomandibular Disorders in Young Adults without Psychosocial Disorders: A Case-Control Study. J. Int. Soc. Prev. Community Dent. 2025, 15, 91–99. [Google Scholar] [CrossRef]
- Klitynska, O.V.; Martyts, Y.M.; Gurando, V.R.; Layoch, N.V. Effectiveness of bruxism treatment in young adults. Wiad. Lek. 2024, 77, 417–423. [Google Scholar] [CrossRef]
- Nascimento, B.L.; Vieira, A.R.; Bezamat, M.; Ignácio, S.A.; Souza, E.M. Occlusal problems, mental health issues and non-carious cervical lesions. Odontology 2022, 110, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Oppitz, L.R.; Arantes, A.C.M.; Garanhani, R.R.; Costa, C.A.; Araujo, C.M.; Tanaka, O.M.; Andreis, P.K.D.S.; Schappo, C.; Ignácio, S.A.; Johann, A.C.B.R.; et al. Efficiency of mixed and rigid occlusal stabilization splints: Randomized clinical trial. Braz. Oral Res. 2024, 38, e017. [Google Scholar] [CrossRef] [PubMed]
- Albagieh, H.; Alomran, I.; Binakresh, A.; Alhatarisha, N.; Almeteb, M.; Khalaf, Y.; Alqublan, A.; Alqahatany, M. Occlusal splints-types and effectiveness in temporomandibular disorder management. Saudi Dent. J. 2023, 35, 70–79. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamashita, S. A Study for Determining the Inclination of the Occlusal Plane from the Mandibular Functional Trajectory. Int. J. Dent. 2022, 2022, 6713881. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romanillos, E.; Feijóo, G.; Martín-Vacas, A.; Mourelle-Martínez, M.R.; Gallardo-López, N.E.; Caleya, A.M. Analysis of the Relationship Between Unilateral Posterior Crossbite and Alterations in the Eruptive Trajectory of Maxillary Canines, the Occlusal Plane, and the Inclination of the Labial Commissure. Children 2025, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, E.; Heikinheimo, K.; Suominen, A.; Evälahti, M.; Michelotti, A.; Svedström-Oristo, A.L.; Rice, D.P. Longitudinal trends in temporomandibular joint disorder symptoms, the impact of malocclusion and orthodontic treatment: A 20-year prospective study. J. Oral Rehabil. 2023, 50, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Chen, C.; Wong, H.C.; Yow, M.; Tan, E. Temporomandibular disorders in prospective orthodontic patients. Angle Orthod. 2021, 91, 377–383. [Google Scholar] [CrossRef]
- Kapagiannidou, D.; Koutris, M.; Wetselaar, P.; Visscher, C.M.; van der Zaag, J.; Lobbezoo, F. Association between polysomnographic parameters of sleep bruxism and attrition-type tooth wear. J. Oral Rehabil. 2021, 48, 687–691. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Lobbezoo, F. Bruxism definition: Past, present, and future-What should a prosthodontist know? J. Prosthet. Dent. 2022, 128, 905–912. [Google Scholar] [CrossRef]
- Goldstein, G.; DeSantis, L.; Goodacre, C. Bruxism: Best Evidence Consensus Statement. J. Prosthodont. 2021, 30, 91–101. [Google Scholar] [CrossRef]
| Sociodemographic Variables | Sleep Bruxism Group (n = 20) | Control Group (n = 20) | p-Value | Effect Size Classification | |
|---|---|---|---|---|---|
| Age (years) | 22.85 ± 3.67 | 20.35 ± 1.57 | 0.009 | 0.88 (d) | Large |
| Male | 5 (25%) | 4 (20%) | 0.705 | 0.06 (w) | Small |
| Female | 15 (75%) | 16 (80%) | |||
| Daily smoker | 0 (0%) | 1 (5%) | 0.310 | 0.95 (w) | Large |
| Coffee >4 times/week | 0 (0%) | 2 (10%) | 0.146 | 0.90 (w) | Large |
| Self-reported stress | 8 (40%) | 6 (30%) | 0.507 | 0.10 (w) | Moderate |
| Anxiety medication | 1 (5%) | 3 (15%) | 0.292 | 0.17 (w) | Moderate |
| Sleep medication | 1 (5%) | 2 (10%) | 0.548 | 0.09 (w) | Small |
| Signs, Clinical Symptoms, and History | Presents Condition | Sleep Bruxism Group | Control Group | p-Value | df | w | Effect Size Classification |
|---|---|---|---|---|---|---|---|
| Fatigue or sore jaw | Yes | 16 (80%) | 15 (75%) | 0.708 | 1 | 0.059 | Small |
| No | 4 (20%) | 5 (25%) | |||||
| Sore teeth or gums | Yes | 9 (45%) | 7 (46.15%) | 0.516 | 1 | 0.102 | Moderate |
| No | 11 (55%) | 13 (53.85%) | |||||
| Headache in the temporal region | Yes | 11 (55%) | 12 (60%) | 0.751 | 1 | 0.050 | Small |
| No | 9 (45%) | 8 (40%) | |||||
| Daytime grinding | Yes | 7 (35%) | 6 (30%) | 0.740 | 1 | 0.052 | Small |
| No | 13 (65%) | 14 (70%) | |||||
| Nighttime grinding | Yes | 8 (40%) | 5 (25%) | 0.310 | 1 | 0.160 | Moderate |
| No | 12 (60%) | 15 (75%) | |||||
| Nighttime clenching | Yes | 9 (45%) | 5 (25%) | 0.184 | 1 | 0.209 | Moderate |
| No | 11 (55%) | 15 (75%) | |||||
| Wear facets | Yes | 14 (70%) | 15 (75%) | 0.718 | 1 | 0.057 | Small |
| No | 6 (30%) | 5 (25%) | |||||
| Masseter voluntary hypertrophy contraction | Yes | 8 (40%) | 10 (50%) | 0.527 | 1 | 0.101 | Moderate |
| No | 12 (60%) | 10 (50%) | |||||
| Discomfort in masticatory muscles | Yes | 12 (60%) | 12 (60%) | 1 | 1 | 0 | Null |
| No | 8 (40%) | 8 (40%) | |||||
| Dental hypersensitivity | Yes | 9 (45%) | 6 (30%) | 0.327 | 1 | 0.154 | Moderate |
| No | 11 (55%) | 14 (70%) | |||||
| Temporomandibular joint locking | Yes | 0 (0%) | 3 (15%) | 0.071 | 1 | 0.284 | Moderate |
| No | 20 (100%) | 17 (85%) | |||||
| Occlusal impressions on buccal mucosa | Yes | 11 (55%) | 9 (45%) | 0.527 | 1 | 0.100 | Moderate |
| No | 9 (45%) | 11 (55%) | |||||
| Dental impressions on lingual edges | Yes | 4 (20%) | 7 (35%) | 0.287 | 1 | 0.168 | Moderate |
| No | 16 (80%) | 13 (65%) | |||||
| Temporomandibular pain on palpation | Yes | 2 (10%) | 4 (20%) | 0.377 | 1 | 0.139 | Moderate |
| No | 18 (90%) | 16 (80%) | |||||
| Temporomandibular pain when opening or closing the mouth | Yes | 4 (20%) | 2 (10%) | 0.377 | 1 | 0.139 | Moderate |
| No | 16 (80%) | 18 (90%) | |||||
| Presence of joint noise | Yes | 7 (35%) | 1 (5%) | 0.017 | 1 | 0.375 | Large |
| No | 13 (65%) | 19 (95%) | |||||
| Presence of functional anterior guidance | Yes | 16 (80%) | 16 (80%) | 1 | 1 | 0 | Null |
| No | 4 (20%) | 4 (20%) | |||||
| Presence of premature contacts | Yes | 19 (95%) | 17 (85%) | 0.292 | 1 | 0.166 | Moderate |
| No | 1 (5%) | 3 (15%) |
| Dynamic Variables | Groups | Statisticians | |||||
|---|---|---|---|---|---|---|---|
| SD | SE | 95% IC | p-Value | d | Effect Size Classification | ||
| Right lateral interferences | Bruxism | 0.74 | 0.16 | 0.65 (0.30 a 0.99) | 0.53 | 0.128 | Small |
| Control | 0.82 | 0.18 | 0.55 (0.16 a 0.93) | ||||
| Left lateral interferences | Bruxism | 0.75 | 0.16 | 0.45 (0.09 a 0.80) | 0.22 | 0.374 | Moderate |
| Control | 0.85 | 0.19 | 0.75 (0.35 a 1.14) | ||||
| Right balancing interferences | Bruxism | 0.58 | 0.13 | 0.31 (0.03 a 0.59) | 0.07 | 0.556 | Moderate |
| Control | 0.91 | 0.20 | 0.75 (0.32 a 1.17) | ||||
| Left balancing interferences | Bruxism | 0.75 | 0.16 | 0.45 (0.09 a 0.80) | 0.04 | 0.723 | Large |
| Control | 0.22 | 0.05 | 0.05 (−0.05 a 0.16) | ||||
| Protrusion interferences | Bruxism | 0.97 | 0.21 | 0.70 (0.24 a 1.16) | 0.98 | 0.085 | Null |
| Control | 1.13 | 0.26 | 0.79 (0.24 a 1.34) | ||||
| Horizontal overbite (mm) | Bruxism | 0.68 | 0.15 | 1.50 (1.17 a 1.82) | 0.67 | 0.092 | Null |
| Control | 0.83 | 0.19 | 1.57 (1.17 a 1.98) | ||||
| Vertical overbite (mm) | Bruxism | 0.67 | 0.15 | 1.65 (1.33 a 1.96) | 0.21 | 0.829 | Large |
| Control | 0.84 | 0.19 | 1.04 (1.53 a 2.35) | ||||
| Premature contacts | Bruxism | 0.32 | 0.07 | 1.00 (0.84 a 1.15) | 0.48 | 2.154 | Large |
| Control | 0.52 | 0.12 | 0.94 (0.69 a 1.20) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aristizabal-Hoyos, J.A.; López-Soto, O.; Fuentes-Barría, H.; Aguilera-Eguía, R.; Angarita-Davila, L.; Rojas-Gómez, D. Sleep Bruxism and Occlusal Function: A Case–Control Study Based on Polysomnography in Young Colombians. J. Clin. Med. 2025, 14, 6733. https://doi.org/10.3390/jcm14196733
Aristizabal-Hoyos JA, López-Soto O, Fuentes-Barría H, Aguilera-Eguía R, Angarita-Davila L, Rojas-Gómez D. Sleep Bruxism and Occlusal Function: A Case–Control Study Based on Polysomnography in Young Colombians. Journal of Clinical Medicine. 2025; 14(19):6733. https://doi.org/10.3390/jcm14196733
Chicago/Turabian StyleAristizabal-Hoyos, Juan Alberto, Olga López-Soto, Héctor Fuentes-Barría, Raúl Aguilera-Eguía, Lissé Angarita-Davila, and Diana Rojas-Gómez. 2025. "Sleep Bruxism and Occlusal Function: A Case–Control Study Based on Polysomnography in Young Colombians" Journal of Clinical Medicine 14, no. 19: 6733. https://doi.org/10.3390/jcm14196733
APA StyleAristizabal-Hoyos, J. A., López-Soto, O., Fuentes-Barría, H., Aguilera-Eguía, R., Angarita-Davila, L., & Rojas-Gómez, D. (2025). Sleep Bruxism and Occlusal Function: A Case–Control Study Based on Polysomnography in Young Colombians. Journal of Clinical Medicine, 14(19), 6733. https://doi.org/10.3390/jcm14196733







