Point-of-Care Testing and Biomarkers in Biliary Diseases: Current Evidence and Future Directions
Abstract
1. Introduction
2. Epidemiological and Clinical Overview of Biliary Tract Diseases
2.1. Epidemiology and Clinical Burden
2.2. Diagnostic Challenges in Clinical Practice
2.3. Unmet Needs in Biliary Diagnostics
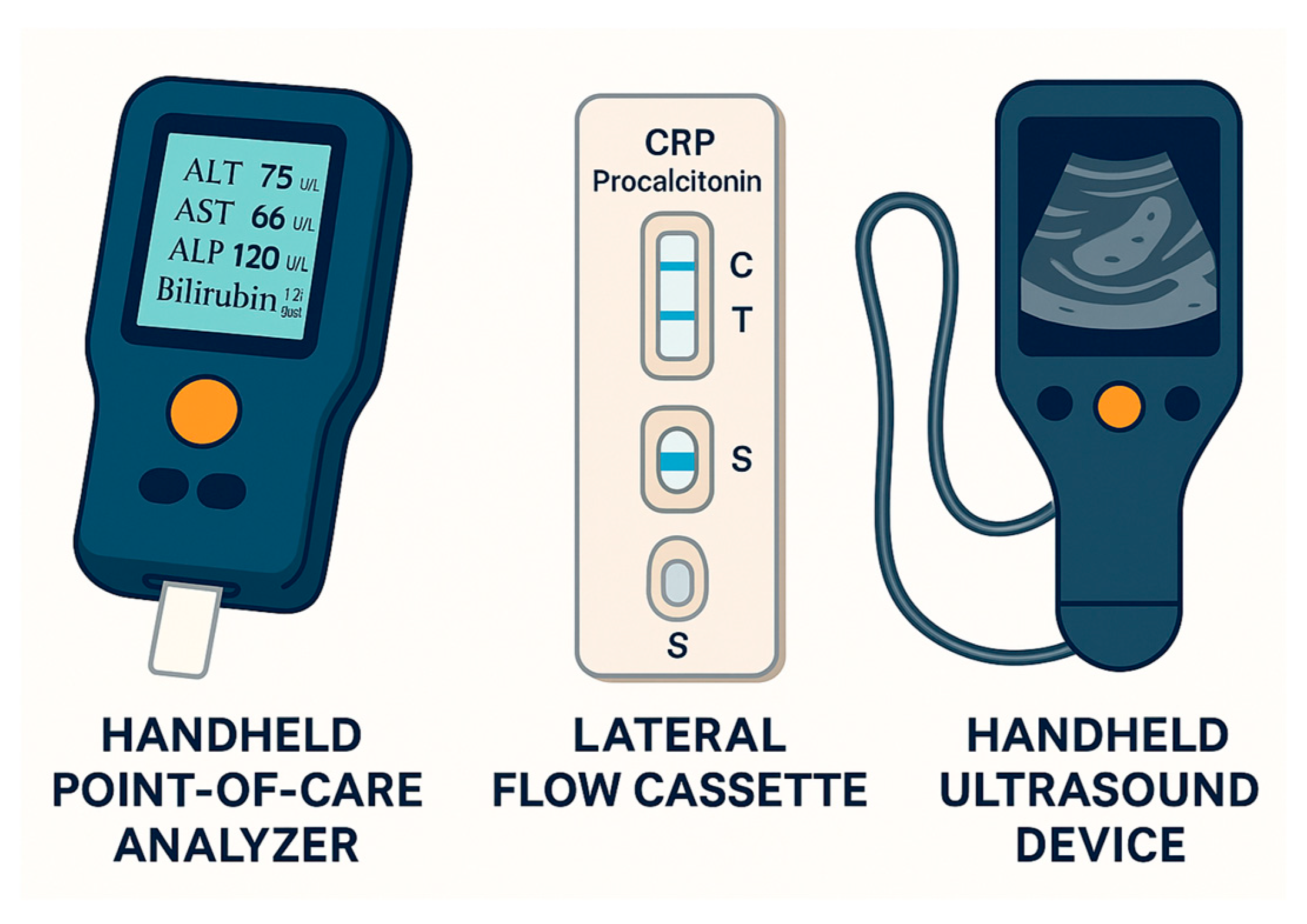
3. Point-of-Care Testing in Biliary Tract Disease
3.1. Principles and Clinical Utility
3.2. Current Point-of-Care Applications
3.2.1. Liver Enzyme and Function Panels
3.2.2. Inflammatory and Infectious Markers
3.2.3. Bedside Ultrasound
3.2.4. Rapid On-Site Evaluation (ROSE)
3.3. Emerging Molecular POC Technologies
3.4. Limitations and Practical Considerations
4. Biomarkers in Biliary Tract Disease
4.1. Clinical Significance and Role of Biomarkers
4.2. Established Serum Biomarkers
4.2.1. Carbohydrate Antigen (CA19-9)
4.2.2. Carcinoembryonic Antigen (CEA)
4.2.3. Cholestatic Enzymes (ALP and GGT)
4.3. Novel and Emerging Biomarkers
4.3.1. Circulating- and Bile-Derived miRNAs
4.3.2. Bile-Derived Protein Biomarkers
4.3.3. Liquid Biopsy: ctDNA in Plasma and Bile
4.3.4. Emerging Serum Protein Biomarkers
4.4. Multimodal and Imaging-Based Biomarker Strategies
4.4.1. Combined Biomarker Panels and Risk Models
4.4.2. AI-Assisted Metabolic Imaging
4.5. Limitations and Future Directions
4.5.1. Clinical Implementation and Cost-Effectiveness
4.5.2. Integrating AI with Multi-Omics
5. Future Directions
5.1. Technological Innovation
5.2. Multimarker and Integrated Models
5.3. Expanding Access and Equity
5.4. Research and Validation Priorities
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| APASL | Asian Pacific Association for the Study of the Liver |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CEACAM6 | Carcinoembryonic antigen-related cell adhesion molecule 6 |
| CRP | C-reactive protein |
| CT | Computed tomography |
| ctDNA | Circulating tumor DNA |
| EASL | European Association for the Study of the Liver |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EUS | Endoscopic ultrasound |
| EUS-FNA | Endoscopic ultrasound-guided fine-needle aspiration |
| FGFR2 | Fibroblast growth factor receptor 2 |
| GGT | Gamma-glutamyl transferase |
| IDH1/2 | Isocitrate dehydrogenase 1/2 |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LFTs | Liver function tests |
| LRG | Leucine-rich alpha-2 glycoprotein |
| miRNA | Microrna |
| MRCP | Magnetic resonance cholangiopancreatography |
| MUC4 | Mucin 4 |
| MUC5AC | Mucin 5AC |
| NAAT | Nucleic acid amplification test |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| PKM2 | Pyruvate kinase M2 |
| POC | Point-of-care |
| POCUS | Point-of-care ultrasound |
| PSC | Primary sclerosing cholangitis |
| ROSE | Rapid on-site evaluation |
| TG18 | Tokyo guidelines 2018 |
| TP53 | Tumor protein p53 |
| WBC | White blood cell count |
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Ma, M.X.; Jayasekeran, V.; Chong, A.K. Benign biliary strictures: Prevalence, impact, and management strategies. Clin. Exp. Gastroenterol. 2019, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Saluja, S.S.; Sharma, R.; Pal, S.; Sahni, P.; Chattopadhyay, T.K. Differentiation between benign and malignant hilar obstructions using laboratory and radiological investigations: A prospective study. HPB 2007, 9, 373–382. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of extrahepatic cholangiocarcinoma. J. Hepatol. 2025, 83, 211–238. [Google Scholar] [CrossRef]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef]
- Luppa, P.B.; Muller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Lahham, S.; Becker, B.A.; Gari, A.; Bunch, S.; Alvarado, M.; Anderson, C.L.; Viquez, E.; Spann, S.C.; Fox, J.C. Utility of common bile duct measurement in ED point of care ultrasound: A prospective study. Am. J. Emerg. Med. 2018, 36, 962–966. [Google Scholar] [CrossRef]
- Wu, X.; Li, K.; Kou, S.; Wu, X.; Zhang, Z. The Accuracy of Point-of-Care Ultrasound in the Detection of Gallbladder Disease: A Meta-analysis. Acad. Radiol. 2024, 31, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Rompianesi, G.; Di Martino, M.; Gordon-Weeks, A.; Montalti, R.; Troisi, R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J. Gastrointest. Oncol. 2021, 13, 332–350. [Google Scholar] [CrossRef]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, R.; Suvarna, K.; Yamada, M.; Kobayashi, K.; Shinkai, N.; Miyake, M.; Takahashi, M.; Jinnai, S.; Shimoyama, R.; Sakai, A.; et al. Application of Artificial Intelligence Technology in Oncology: Towards the Establishment of Precision Medicine. Cancers 2020, 12, 3532. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Hanna, M.G.; Pantanowitz, L.; Dash, R.; Harrison, J.H.; Deebajah, M.; Pantanowitz, J.; Rashidi, H.H. Future of Artificial Intelligence-Machine Learning Trends in Pathology and Medicine. Mod. Pathol. 2025, 38, 100705. [Google Scholar] [CrossRef]
- Dai, F.; Cai, Y.; Yang, S.; Zhang, J.; Dai, Y. Global burden of gallbladder and biliary diseases (1990–2021) with healthcare workforce analysis and projections to 2035. BMC Gastroenterol. 2025, 25, 249. [Google Scholar] [CrossRef] [PubMed]
- Stinton, L.M.; Shaffer, E.A. Epidemiology of gallbladder disease: Cholelithiasis and cancer. Gut Liver 2012, 6, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Kanthan, R.; Senger, J.L.; Ahmed, S.; Kanthan, S.C. Gallbladder Cancer in the 21st Century. J. Oncol. 2015, 2015, 967472. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Catanzaro, E.; Gringeri, E.; Burra, P.; Gambato, M. Primary Sclerosing Cholangitis-Associated Cholangiocarcinoma: From Pathogenesis to Diagnostic and Surveillance Strategies. Cancers 2023, 15, 4947. [Google Scholar] [CrossRef] [PubMed]
- Guerra Ruiz, A.R.; Crespo, J.; Lopez Martinez, R.M.; Iruzubieta, P.; Casals Mercadal, G.; Lalana Garces, M.; Lavin, B.; Morales Ruiz, M. Measurement and clinical usefulness of bilirubin in liver disease. Adv. Lab. Med. 2021, 2, 352–372. [Google Scholar] [CrossRef]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 2021, 13, 1688–1698. [Google Scholar] [CrossRef]
- Nve, E.; Badia, J.M.; Amillo-Zaragueta, M.; Juvany, M.; Mourelo-Farina, M.; Jorba, R. Early Management of Severe Biliary Infection in the Era of the Tokyo Guidelines. J. Clin. Med. 2023, 12, 4711. [Google Scholar] [CrossRef]
- Mishra, S.; Kumari, S.; Husain, N. Liquid biopsy in gallbladder carcinoma: Current evidence and future prospective. J. Liq. Biopsy 2024, 6, 100280. [Google Scholar] [CrossRef]
- Chae, M.S.; Kravchuk, O.A. Point of care ultrasound in rapid diagnosis of acute cholangitis and emphysematous cholecystitis: A case report. Int. J. Emerg. Med. 2025, 18, 29. [Google Scholar] [CrossRef]
- Massaro, K.S.; Costa, S.F.; Leone, C.; Chamone, D.A. Procalcitonin (PCT) and C-reactive protein (CRP) as severe systemic infection markers in febrile neutropenic adults. BMC Infect. Dis. 2007, 7, 137. [Google Scholar] [CrossRef]
- Agnello, L.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Gambino, C.M.; Iacona, A.; Ciaccio, A.M.; Lo Sasso, B.; Ciaccio, M. The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics 2021, 11, 1881. [Google Scholar] [CrossRef]
- Dumbrava, B.A.; Bass, G.A.; Jumean, A.A.; Birido, N.A.; Corbally, M.; Pereira, J.; Biloslavo, A.; Zago, M.; Walsh, T.A. The Accuracy of Point-of-Care Ultrasound (POCUS) in Acute Gallbladder Disease. Diagnostics 2023, 13, 1248. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.-O.; Song, S.A.-O. Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications. Cancers 2024, 16, 1761. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Ma, X.; Efe, C.; Wang, G.; Jeong, S.H.; Abe, K.; Duan, W.; Chen, S.; Kong, Y.; Zhang, D.; et al. APASL clinical practice guidance: The diagnosis and management of patients with primary biliary cholangitis. Hepatol. Int. 2022, 16, 1–23. [Google Scholar] [CrossRef]
- Costache, M.I.; Iordache, S.; Karstensen, J.G.; Saftoiu, A.; Vilmann, P. Endoscopic ultrasound-guided fine needle aspiration: From the past to the future. Endosc. Ultrasound 2013, 2, 77–85. [Google Scholar] [CrossRef]
- Yamao, K.; Sawaki, A.; Mizuno, N.; Shimizu, Y.; Yatabe, Y.; Koshikawa, T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): Past, present, and future. J. Gastroenterol. 2005, 40, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, R.; Duey, M.; Pena, V.; Wanzer, D.; Kirkpatrick, J.; Chau, D.; Sarode, V.R. Role of cytotechnologists in rapid onsite adequacy assessment of cytology materials for diagnostic workup and specimen allocation for ancillary testing using a standardized protocol. J. Am. Soc. Cytopathol. 2020, 9, 67–75. [Google Scholar] [CrossRef]
- Jani, B.S.; Rzouq, F.; Saligram, S.; Lim, D.; Rastogi, A.; Bonino, J.; Olyaee, M. Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Pancreatic Lesions: A Systematic Review of Technical and Procedural Variables. N. Am. J. Med. Sci. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Nehring, P.; Ciszewska, M.; Przybylkowski, A. Indeterminate Biliary Strictures: A Retrospective Study. J. Clin. Med. 2025, 14, 3797. [Google Scholar] [CrossRef]
- Shin, I.S.; Myeong, J.H.; Moon, J.H.; Lee, Y.N.; Park, J.W.; Kim, H.K.; Yang, J.K.; Lee, T.H.; Cho, Y.D.; Park, S.H. Efficacy of Biliary Brush Cytology with Rapid On-Site Cytological Evaluation for the Detection of Malignant Biliary Strictures. J. Gastroenterol. Hepatol. 2025, 40, 750–756. [Google Scholar] [CrossRef]
- Ali, S.; Hawes, R.H.; Kadkhodayan, K.; Rafiq, E.; Navaneethan, U.; Bang, J.Y.; Varadarajulu, S.; Hasan, M.K. Utility of rapid onsite evaluation of touch imprint cytology from endoscopic and cholangioscopic forceps biopsy sampling (with video). Gastrointest. Endosc. 2019, 89, 340–344. [Google Scholar] [CrossRef]
- Mendoza, A.A.-O.; Afify, A.; Howell, L.; Bishop, J.; Lauderdale, A.; Seko, S.; Hermosilla, R.; York, D.; Schaberg, K.B. Evaluation of Optimized Toluidine Blue Stain as an Alternative Stain for Rapid On-Site Evaluation (ROSE). Diagnostics 2025, 15, 1223. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Y.; Zhang, Q.; Zhou, J.; Ding, T.; Feng, J. Advancing point-of-care microbial pathogens detection by material-functionalized microfluidic systems. Trends Food Sci. Technol. 2023, 135, 115–130. [Google Scholar] [CrossRef]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibanez, E.; Cifuentes, A.; Lu, W. Microfluidic biosensors for biomarker detection in body fluids: A key approach for early cancer diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Trung, N.T.; Thau, N.S.; Bang, M.H.; Song, L.H. PCR-based Sepsis@Quick test is superior in comparison with blood culture for identification of sepsis-causative pathogens. Sci. Rep. 2019, 9, 13663. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liang, Z.; Zhang, Y.; Li, T.; Tian, C.; Zhao, J.; Jin, B.; Cao, J.; Lin, Y. Circulating tumor DNA in cholangiocarcinoma: Current clinical applications and future perspectives. Front. Cell Dev. Biol. 2025, 13, 1616064. [Google Scholar] [CrossRef]
- Stenzinger, A.; Vogel, A.; Lehmann, U.; Lamarca, A.; Hofman, P.; Terracciano, L.; Normanno, N. Molecular profiling in cholangiocarcinoma: A practical guide to next-generation sequencing. Cancer Treat. Rev. 2024, 122, 102649. [Google Scholar] [CrossRef]
- Han, G.R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine learning in point-of-care testing: Innovations, challenges, and opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Lucas, B.P.; D’Addio, A.; Block, C.; Manning, H.L.; Remillard, B.; Leiter, J.C. Clinical measurements obtained from point-of-care ultrasound images to assess acquisition skills. Ultrasound J. 2019, 11, 4. [Google Scholar] [CrossRef]
- Marceglia, S.; D’Antrassi, P.; Prenassi, M.; Rossi, L.; Barbieri, S. Point of Care Research: Integrating patient-generated data into electronic health records for clinical trials. AMIA Annu. Symp. Proc. 2017, 2017, 1262–1271. [Google Scholar]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef]
- Qin, X.L.; Wang, Z.R.; Shi, J.S.; Lu, M.; Wang, L.; He, Q.R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 2004, 10, 427–432. [Google Scholar] [CrossRef]
- Xing, H.; Wang, J.; Wang, Y.; Tong, M.; Hu, H.; Huang, C.; Li, D. Diagnostic Value of CA 19-9 and Carcinoembryonic Antigen for Pancreatic Cancer: A Meta-Analysis. Gastroenterol. Res. Pract. 2018, 2018, 8704751. [Google Scholar] [CrossRef]
- Dorrell, R.; Pawa, S.; Zhou, Y.; Lalwani, N.; Pawa, R. The Diagnostic Dilemma of Malignant Biliary Strictures. Diagnostics 2020, 10, 337. [Google Scholar] [CrossRef]
- Shi, T.; Morishita, A.; Kobara, H.; Masaki, T. The Role of microRNAs in Cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 7627. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.N.; Song, D.J.; Tan, J.P.; Cao, Y.; Fan, J.; Wang, Z.; Zhou, J. A High-Accuracy Model Based on Plasma miRNAs Diagnoses Intrahepatic Cholangiocarcinoma: A Single Center with 1001 Samples. Diagnostics 2021, 11, 610. [Google Scholar] [CrossRef]
- Liu, D.S.K.; Puik, J.R.; Veno, M.T.; Mato Prado, M.; Rees, E.; Patel, B.Y.; Merali, N.; Galloway, D.; Chan, G.; Phillips, N.; et al. MicroRNAs as Bile-based biomarkers in pancreaticobiliary cancers (MIRABILE): A cohort study. Int. J. Surg. 2024, 110, 6518–6527. [Google Scholar] [CrossRef]
- Bao, F.; Liu, J.; Chen, H.; Miao, L.; Xu, Z.; Zhang, G. Diagnosis Biomarkers of Cholangiocarcinoma in Human Bile: An Evidence-Based Study. Cancers 2022, 14, 3921. [Google Scholar] [CrossRef]
- O’Neill, R.S.; Stoita, A. Biomarkers in the diagnosis of pancreatic cancer: Are we closer to finding the golden ticket? World J. Gastroenterol. 2021, 27, 4045–4087. [Google Scholar] [CrossRef]
- Arrichiello, G.; Nacca, V.; Paragliola, F.; Giunta, E.F. Liquid biopsy in biliary tract cancer from blood and bile samples: Current knowledge and future perspectives. Explor. Target. Antitumor Ther. 2022, 3, 362–374. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Tavolari, S.; Brandi, G. Circulating Tumor DNA in Biliary Tract Cancer: Current Evidence and Future Perspectives. Cancer Genom. Proteom. 2020, 17, 441–452. [Google Scholar] [CrossRef]
- Awosika, J.A.; Monge, C.; Greten, T.F. Integration of circulating tumor DNA in biliary tract cancer: The emerging landscape. Hepat. Oncol. 2024, 11, 2403334. [Google Scholar] [CrossRef] [PubMed]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Moon, H.C.; Hong, S.J.; Choi, A.; Lee, S.L.; Park, D.H.; Shin, E.; Jo, J.H.; Koh, D.H.; Lee, J.; et al. Enhancing biliary tract cancer diagnosis using AI-driven 3D optical diffraction tomography. Methods 2025, 241, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Beliaev, A.M.; Booth, M.; Rowbotham, D.; Bergin, C. Diagnostic inflammatory markers in acute cholangitis. J. Surg. Res. 2018, 228, 35–41. [Google Scholar] [CrossRef]

| Disease | Classification | Common Symptoms | Risk Factors | Prevalence | Prognosis |
|---|---|---|---|---|---|
| Acute cholangitis | Benign | Fever, jaundice, abdominal pain | Gallstones, biliary strictures | Common | Good with prompt treatment |
| Primary sclerosing cholangitis (PSC) | Benign | Jaundice, pruritus | Autoimmune conditions | Rare | Increased risk of malignancy |
| Gallstone disease | Benign | Abdominal pain | Obesity, advanced age | Very common | Excellent after cholecystectomy |
| Cholangiocarcinoma | Malignant | Jaundice, weight loss | PSC, choledochal cysts | Uncommon | Poor prognosis |
| Gallbladder cancer | Malignant | Abdominal pain, palpable mass | Gallbladder polyps | Rare | Poor prognosis |
| Test Type | Target Marker | Sample Type | Clinical Utility | Time to Result | Limitations |
|---|---|---|---|---|---|
| POC Liver Panel Analyzer | ALT, AST, ALP, GGT, Bilirubin, Albumin | Whole blood/Serum | Initial triage for biliary obstruction or hepatopathy | ~10–15 min | May have lower accuracy than laboratory assays |
| POC Inflammatory Marker | CRP, Procalcitonin | Whole blood | Diagnosis and monitoring of cholangitis | ~15–20 min | False positives in non-infectious inflammation |
| POC Leukocyte Panel | WBC count and differential | Whole blood | Sepsis screening in acute cholangitis | ~10 min | Limited differential accuracy |
| POCUS (bedside ultrasound) | Gallstones, bile duct dilation, sludge | N/A (imaging tool) | Rapid bedside imaging for biliary tract assessment | Immediate | Operator-dependent; limited in obese patients |
| Microfluidic NAAT System | Bacterial DNA, Viral RNA | Bile/Blood/Urine | Pathogen identification in biliary infection | 30–60 min | Not yet validated for routine biliary disease diagnostics |
| ctDNA Panel (lab-on-chip) | KRAS, IDH1/2, FGFR2, TP53 mutations | Plasma/Bile | Detection of biliary tract cancers | 1–2 h | Costly, technically complex; not widely available |
| Biosensor-based miRNA Chip | miR-21, miR-221, miR-200 family | Serum/Bile | Early cancer detection, risk stratification | 30–60 min | Under investigation; standardization and cutoff values lacking |
| Guideline | Primary Diagnostic Approach | Use of POC Tests | Use of Biomarkers | Specific Recommendations | Potential Role for Integration |
|---|---|---|---|---|---|
| AASLD (American Association for the Study of Liver Diseases) [31] | Clinical evaluation, LFTs, ultrasound as first-line; MRCP or EUS for indeterminate cases; ERCP for therapeutic purposes | Not routinely addressed | CA19-9 considered in suspected cholangiocarcinoma (esp. with PSC) | Emphasis on imaging + CA19-9 in select contexts | Incorporation of POC CRP/LFTs in acute cholangitis for rapid triage; biomarker panels for indeterminate strictures |
| EASL (European Association for the Study of the Liver) [4] | Stepwise approach: history/physical, LFTs, ultrasound → advanced imaging (MRCP, CT) → ERCP for diagnosis/therapy | Not specifically addressed | CA19-9, CEA mentioned; emphasize exclusion of benign causes | Support CA19-9 as adjunct but not standalone | Use of POC inflammatory markers to guide early management; miRNA or ctDNA to improve malignant vs. benign differentiation |
| APASL (Asian Pacific Association for the Study of the Liver) [33] | Emphasis on regional epidemiology; ultrasound as first-line; early ERCP for obstructive cholangitis; MRCP/EUS for non-urgent cases | Not specifically addressed | Limited mention; CA19-9 for cholangiocarcinoma suspicion | Prioritizes ERCP in high-incidence regions | POC LFTs/CRP in rural settings; bile-based proteomics for high-risk groups (e.g., hepatolithiasis, choledochal cysts) |
| Tokyo Guidelines 2018 (TG18; for Acute Cholangitis/Cholecystitis) [32] | Diagnostic criteria: systemic inflammation, cholestasis, imaging evidence | Indirectly: early labs and imaging encouraged | No formal biomarker recommendations | Structured severity grading with labs and imaging | Integration of POC CRP/WBC count for faster TG18 severity grading; emerging biomarkers for early sepsis risk prediction |
| Biomarker | Sample Type | Disease Target | Limitations | Clinical Utility |
|---|---|---|---|---|
| CA19-9 | Serum | CCC | False positive: cholestasis, Lewis-neg | Diagnosis, monitoring |
| CEA | Serum | CCC | Low specificity | Adjunctive, monitoring |
| miR-21, -221 | Serum/bile | CCC, GB cancer | Under investigation, no standard cutoff | Early diagnosis |
| MUC5AC | Bile | CCC | Requires ERCP | Malignant vs. benign differentiation |
| ctDNA | Plasma/bile | CCC | Cost, technical complexity | Targeted therapy, prognosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.H.; Lee, K.J.; Park, S.W.; Koh, D.H.; Lee, J. Point-of-Care Testing and Biomarkers in Biliary Diseases: Current Evidence and Future Directions. J. Clin. Med. 2025, 14, 6724. https://doi.org/10.3390/jcm14196724
Jung JH, Lee KJ, Park SW, Koh DH, Lee J. Point-of-Care Testing and Biomarkers in Biliary Diseases: Current Evidence and Future Directions. Journal of Clinical Medicine. 2025; 14(19):6724. https://doi.org/10.3390/jcm14196724
Chicago/Turabian StyleJung, Jang Han, Kyong Joo Lee, Se Woo Park, Dong Hee Koh, and Jin Lee. 2025. "Point-of-Care Testing and Biomarkers in Biliary Diseases: Current Evidence and Future Directions" Journal of Clinical Medicine 14, no. 19: 6724. https://doi.org/10.3390/jcm14196724
APA StyleJung, J. H., Lee, K. J., Park, S. W., Koh, D. H., & Lee, J. (2025). Point-of-Care Testing and Biomarkers in Biliary Diseases: Current Evidence and Future Directions. Journal of Clinical Medicine, 14(19), 6724. https://doi.org/10.3390/jcm14196724







