Pro-Inflammatory Cytokines as Early Predictors of Chronic Rheumatologic Disease Following Chikungunya Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
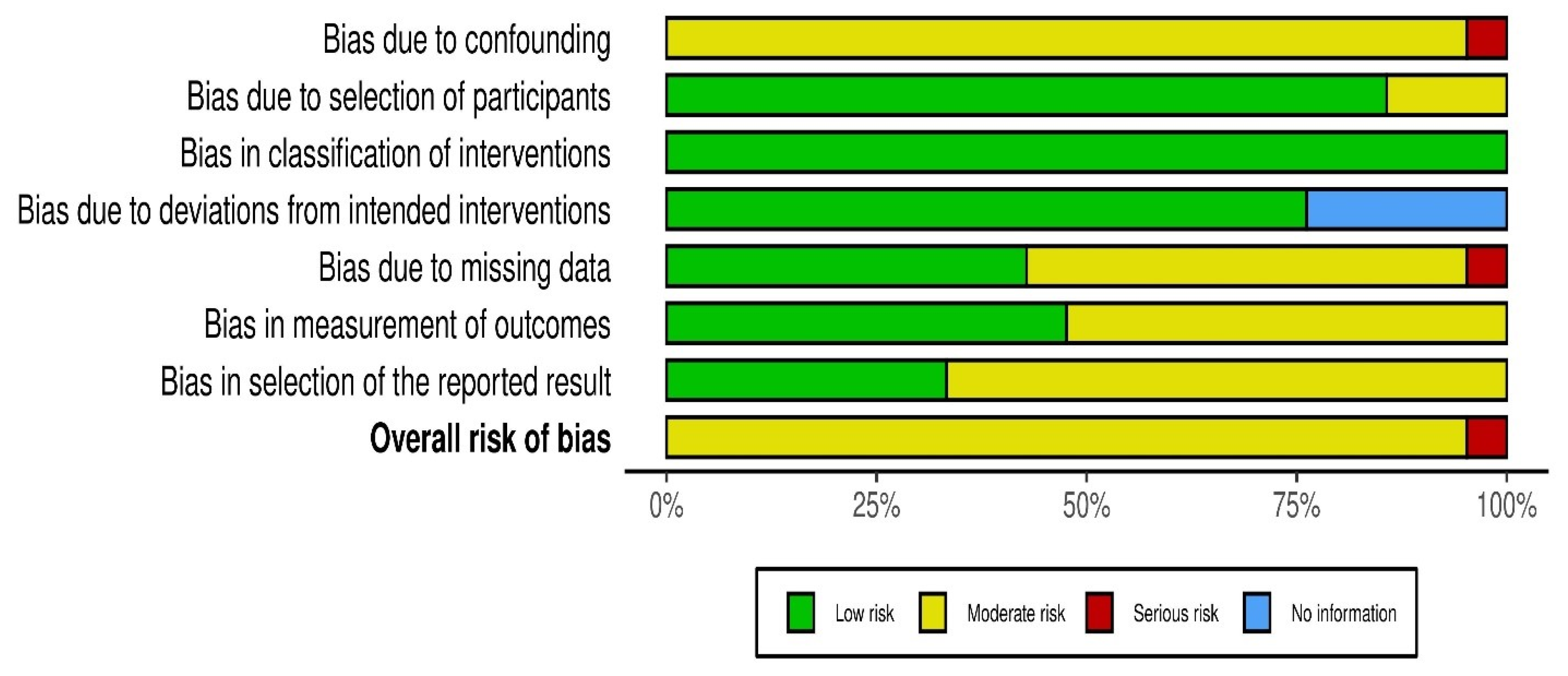
2.5. Quality Assessment and Risk of Bias
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Schilte, C.; Staikovsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A.; Singh, S.K. Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Neglected Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Sissoko, D.; Malvy, D.; Ezzedine, K.; Renault, P.; Moscetti, F.; Ledrans, M.; Pierre, V. Post-epidemic Chikungunya disease on Reunion Island: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Neglected Trop. Dis. 2009, 3, e389. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.-J.; Bandjee, M.-C.J.; Trotot, P.K.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Chow, A.; Her, Z.; Ong, E.K.S.; Chen, J.-M.; Dimatatac, F.; Kwek, D.J.C.; Barkham, T.; Yang, H.; Rénia, L.; Leo, Y.-S.; et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 2011, 203, 149–157. [Google Scholar] [CrossRef]
- Cavalcanti, N.G.; MeloVilar, K.; Duarte, A.L.B.P.; Rêgo, M.J.B.d.M.; Pereira, M.C.; Pitta, I.d.R.; Marques, C.D.L.; Pitta, M.G.D.R. IL-27 in patients with Chikungunya fever: A possible chronicity biomarker? Acta. Trop. 2019, 196, 48–51. [Google Scholar] [CrossRef]
- Teng, T.-S.; Kam, Y.-W.; Lee, B.; Hapuarachchi, H.C.; Wimal, A.; Ng, L.-C.; Ng, L.F.P. A Systematic Meta-analysis of Immune Signatures in Patients With Acute Chikungunya Virus Infection. J. Infect. Dis. 2015, 211, 1925–1935. [Google Scholar] [CrossRef]
- Tanabe, I.S.B.; Tanabe, E.L.L.; Santos, E.C.; Martins, W.V.; Araújo, I.M.T.C.; Cavalcante, M.C.A.; Lima, A.R.V.; Câmara, N.O.S.; Anderson, L.; Yunusov, D.; et al. Cellular and Molecular Immune Response to Chikungunya Virus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 345. [Google Scholar] [CrossRef]
- Amaral, J.K.; Bilsborrow, J.B.; Schoen, R.T. Chronic Chikungunya Arthritis and Rheumatoid Arthritis: What They Have in Common. Am. J. Med. 2020, 133, e91–e97. [Google Scholar] [CrossRef]
- Silveira-Freitas, J.E.P.; Campagnolo, M.L.; Dos Santos Cortez, M.; de Melo, F.F.; Zarpelon-Schutz, A.C.; Teixeira, K.N. Long chikungunya? An overview to immunopathology of persistent arthralgia. World J. Virol. 2024, 13, 89985. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Poo, Y.-S.; Alves, J.C.; Almeida, R.P.; Mostafavi, H.; Tang, P.C.H.; Bucala, R.; Teixeira, M.M.; Taylor, A.; Zaid, A.; et al. Interleukin-17 Contributes to Chikungunya Virus-Induced Disease. mBio 2022, 13, e00289-22. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.F.P.; Chow, A.; Sun, Y.-J.; Kwek, D.J.C.; Lim, P.-L.; Dimatatac, F.; Ng, L.-C.; Ooi, E.-E.; Choo, K.-H.; Her, Z.; et al. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE 2009, 4, e4261. [Google Scholar] [CrossRef]
- Kelvin, A.A.; Banner, D.; Silvi, G.; Moro, M.L.; Spataro, N.; Gaibani, P.; Cavrini, F.; Pierro, A.; Rossini, G.; Cameron, M.J.; et al. Inflammatory Cytokine Expression Is Associated with Chikungunya Virus Resolution and Symptom Severity. PLoS Neglected Trop. Dis. 2011, 5, e1279. [Google Scholar] [CrossRef]
- Chopra, A.; Venugopalan, A. Persistent rheumatic musculoskeletal pain and disorders at one year post-chikungunya epidemic in south Maharashtra—A rural community based observational study with special focus on naïve persistent rheumatic musculoskeletal cases and selected cytokine expression. Indian J. Rheumatol. 2011, 6 (Suppl. S1), 5–11. [Google Scholar] [CrossRef]
- Lohachanakul, J.; Phuklia, W.; Thannagith, M.; Thonsakulprasert, T.; Ubol, S. High concentrations of circulating interleukin-6 and monocyte chemotactic protein-1 with low concentrations of interleukin-8 were associated with severe chikungunya fever during the 2009–2010 outbreak in Thailand. Microbiol. Immunol. 2012, 56, 134–138. [Google Scholar] [CrossRef]
- Venugopalan, A.; Ghorpade, R.P.; Chopra, A. Cytokines in acute chikungunya. PLoS ONE 2014, 9, e111305. [Google Scholar] [CrossRef]
- Reddy, V.; Mani, R.S.; Desai, A.; Ravi, V. Correlation of plasma viral loads and presence of Chikungunya IgM antibodies with cytokine/chemokine levels during acute Chikungunya virus infection. J. Med. Virol. 2014, 86, 1393–1401. [Google Scholar] [CrossRef]
- Raad, J.J.; Rosero, A.S.; Martínez, J.V.; Parody, A.; Raad, R.J.; Tovar, D.C.; López, P.C.; Ramírez, M.G.; Magdaniel, J.B.; Celedón, L.A. Immunological response of a population from the Caribbean region of Colombia infected with the chikungunya virus. Rev. Colomb. Reumatol. 2016, 23, 85–91. (In English) [Google Scholar] [CrossRef]
- Sepúlveda-Delgado, J.; Vera-Lastra, O.L.; Trujillo-Murillo, K.; Canseco-Ávila, L.; Sánchez-González, R.; Gómez-Cruz, O.; Lugo-Trampe, A.; Fernández-Salas, I.; Danis-Lozano, R.; Contreras-Contreras, A.; et al. Inflammatory biomarkers, disease activity index, and self-reported disability may be predictors of chronic arthritis after chikungunya infection: Brief report. Clin. Rheumatol. 2017, 36, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Tritsch, S.; Reid, S.P.; Martins, K.; Encinales, L.; Pacheco, N.; Amdur, R.L.; Porras-Ramirez, A.; Rico-Mendoza, A.; Li, G.; et al. The Cytokine Profile in Acute Chikungunya Infection is Predictive of Chronic Arthritis 20 Months Post Infection. Diseases 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Mukhopadhyay, S. Oxidative damage markers and inflammatory cytokines are altered in patients suffering with post-chikungunya persisting polyarthralgia. Free. Radic. Res. 2018, 52, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Abella, J.; Rojas, Á.; Rojas, C.; Rondón, F.; Medina, Y.; Peña, M.; Campo, A.; Rico, Á.; Mercado, M. Clinical and immunological features of post-chikungunya virus chronic arthritis and its effect on functional ability and quality of life in a cohort of Colombian patients. Rev. Colomb. Reumatol. 2019, 26, 253–259. (In English) [Google Scholar] [CrossRef]
- Ninla-Aesong, P.; Mitarnun, W.; Noipha, K. Proinflammatory Cytokines and Chemokines as Biomarkers of Persistent Arthralgia and Severe Disease After Chikungunya Virus Infection: A 5-Year Follow-Up Study in Southern Thailand. Viral Immunol. 2019, 32, 442–452. [Google Scholar] [CrossRef]
- Jacob-Nascimento, L.C.; Carvalho, C.X.; Silva, M.M.O.; Kikuti, M.; Anjos, R.O.; Fradico, J.R.B.; Campi-Azevedo, A.C.; Tauro, L.B.; Campos, G.S.; Moreira, P.S.D.S.; et al. Acute-Phase Levels of CXCL8 as Risk Factor for Chronic Arthralgia Following Chikungunya Virus Infection. Front. Immunol. 2021, 12, 744183. [Google Scholar] [CrossRef]
- Chang, A.Y.-H.; Hernández, A.S.; Forero-Mejía, J.; Tritsch, S.R.; Mendoza-Torres, E.; Encinales, L.; Bonfanti, A.C.; Proctor, A.M.; Simon, G.L.; Simmens, S.J.; et al. The Natural History of Post-Chikungunya Viral Arthritis Disease Activity and T-cell Immunology: A Cohort Study. J. Cell Immunol. 2024, 6, 64–75. [Google Scholar] [CrossRef]
- Lozano-Parra, A.; Herrera, V.; Villar, L.Á.; Urcuqui-Inchima, S.; Valdés-López, J.F.; Garrido, E.M.R. Acute Immunological Biomarkers for Predicting Chronic Rheumatologic Disease After Chikungunya Virus Infection. Trop. Med. Infect. Dis. 2025, 10, 195. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chaaithanya, I.K.; Muruganandam, N.; Sundaram, S.G.; Kawalekar, O.; Sugunan, A.P.; Manimunda, S.P.; Ghosal, S.R.; Muthumani, K.; Vijayachari, P. Role of Proinflammatory Cytokines and Chemokines in Chronic Arthropathy in CHIKV Infection. Viral Immunol. 2011, 24, 265–271. [Google Scholar] [CrossRef]
- Chopra, A.; Anuradha, V.; Ghorpade, R.; Saluja, M. Acute Chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: A 2-year prospective rural community study. Epidemiol. Infect. 2012, 140, 842–850. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Reynoso, G.V.; Winkler, E.S.; Mack, M.; Diamond, M.S.; Hickman, H.D.; Morrison, T.E. MyD88-dependent influx of monocytes and neutrophils impairs lymph node B cell responses to chikungunya virus infection via Irf5, Nos2 and Nox2. PLoS Pathog. 2020, 16, e1008292. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- DiVietro, J.A.; Smith, M.J.; Smith, B.R.; Petruzzelli, L.; Larson, R.S.; Lawrence, M.B. Immobilized IL-8 triggers progressive activation of neutrophils rolling in vitro on P-selectin and intercellular adhesion molecule-1. J. Immunol. 2001, 167, 4017–4025. [Google Scholar] [CrossRef]
- Wauquier, N.; Becquart, P.; Nkoghe, D.; Padilla, C.; Ndjoyi-Mbiguino, A.; Leroy, E.M. The Acute Phase of Chikungunya Virus Infection in Humans Is Associated With Strong Innate Immunity and T CD8 Cell Activation. J. Infect. Dis. 2010, 204, 115–123. [Google Scholar] [CrossRef]
- Zaid, A.; Gérardin, P.; Taylor, A.; Mostafavi, H.; Malvy, D.; Mahalingam, S. Review: Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis Rheumatol. 2018, 70, 484–495. [Google Scholar] [CrossRef]
- Ng, W.H.; Amaral, K.; Javelle, E.; Mahalingam, S. Chronic chikungunya disease (CCD): Clinical insights, immunopathogenesis and therapeutic perspectives. QJM Int. J. Med. 2024, 117, 489–494. [Google Scholar] [CrossRef]

| Author (Year) | Study Design | Sample Size | Follow-Up Duration | Mean Age (SD or Range) | No. of Male/Female |
|---|---|---|---|---|---|
| Ng et al., 2009 [14] | Retrospective | 10 | 2 weeks post-illness | 22–65 years (median 35) | 10 M/0 F |
| Hoarau et al., 2010 [4] | Cohort | 15 | Up to Month 18 post-infection | Mean: Recovered = 50.3 year (SD = 13.7); Chronic = 70.7 year (SD = 15.5) | Recovered: 1 M/5 F; Chronic: 1 M/8 F |
| Kelvin et al., 2011 [15] | Cohort | 60 (50 with CHIKV + 10 healthy controls) | Acute, 6 months, 12 months | N/A | N/A |
| Chow et al., 2011 [6] | Case–control | 30 | Acute (median 4 days), early convalescent (median 10 days), late convalescent (4–6 weeks), chronic (2–3 months) | Mean 39 years (range 23–67 years; median 36.5) | 26 M/4 F |
| Chopra et al., 2011 [16] | Cross-sectional | CHIKV (Blood: n = 141); Pre-CHIKV MSK (Blood: n = 20); Post-CHIKV MSK (Blood: n = 121) | 1-year post-epidemic | Majority > 45 years | 54 M/74 F |
| Chaaithanya et al., 2011 [30] | Cohort | 22 patients (6 acute, 6 recovered, 10 chronic) + 6 healthy controls | 10 months | Mean 34.5 years (30.5 controls, 34.5 recovered, 41.5 chronic) | N/A |
| Chopra A. et al., 2012 [31] | Prospective | 509 acute CHIKV cases | Up to 24 months | N/A | Male–female ratio = 1:3 |
| Lohachanakul et al., 2012 [17] | Cross-sectional | 62 enrolled (35 confirmed CHIKV: 15 severe, 20 mild; 27 non-CHIKF) | 30 days (blood sampling at day 1 and day 30) | Severe CHIKF: 34.9 ± 14.3; Mild CHIKF: 38.0 ± 16.5; non-CHIKF: 37.0 ± 17.5 | 23 M/39 F |
| Schilte et al., 2013 [2] | Cohort | 147 | 36 months | Stratified by age groups (≤35 y: 30, 36–50 y: 28, 51–60: 24, 61–70: 20, >70: 45) | 73 M/74 F |
| Venugopalan et al., 2014 [18] | Prospective | 132 cases (110 symptomatic, 22 recovered); plus 49 survey controls and 80 healthy controls | 6–22 months | Median: 36 years (cases) and 41 years (healthy controls) | Symptomatic: 1:1.5; Recovered: 1:3; Healthy controls: 1:1 |
| Reddy et al., 2014 [19] | Cohort | 48 patients with CHIKV infection (and 37 healthy controls) | Up to 12 weeks in 4 patients with persistent arthralgia; otherwise, acute phase (2–10 days post-onset) | Mean 40.6 years (range 21–80); persistent arthralgia group mean 52.5 years (42–57) | 31 M/17 F |
| Raad et al., 2016 [20] | Prospective | 109 | 9 months | Range 22–82 years; majority (56%) between 41 and 60 years | 11% M/89% F |
| Delgado et al., 2017 [21] | Prospective | 10 | 12 months | 48 ± 15.04 years | 4 M/6 F |
| Chang et al., 2018 [22] | Case–control | 242 (121 with chronic arthritis, 121 without joint pain) | Median 20 months post-infection | With arthritis: 49 ± 17 years; Without arthritis: 48 ± 17 years | Female: 89% in both groups (108 F and 13 M per group) |
| Banerjee et al., 2018 [23] | Case–control | 65 (25 with persisting polyarthralgia, 15 without, 25 healthy controls) | Patients assessed post 10–30 days of infection; persisting symptoms evaluated after 10 days | Median ages: Controls 47 (23–75), Without polyarthralgia 39 (20–64), With polyarthralgia 55 (35–76) | Controls 12 M/13 F, Without polyarthralgia 6 M/9 F, With polyarthralgia 10 M/15 F |
| Abella et al., 2019 [24] | Cross-sectional | 94 | Symptoms >3 months; mean 278 ± 87.8 days since onset | 57 ± 14.99 years | 22 M/72 F |
| Cavalcanti et al., 2019 [7] | Case–control | 45 patients with CHIKV + 49 healthy controls | Subacute (3–12 weeks) and chronic (>12 weeks) phases | 55.2 ± 13.8 years (patients); 51 ± 8.95 years (controls) | 9 M/36 F (patients); 12 M/37 F (controls) |
| Ninla-aesong et al., 2019 [25] | Cross-sectional | 123 (63 persistent arthralgia, 30 fully recovered, 30 healthy controls) | 5 years | N/A | N/A |
| Jacob-Nascimento et al., 2021 [26] | Cohort | 253 CHIKV-positive patients + (81 OAFD controls and 15 healthy controls) | 2.5 years post-infection | Median 34 years (IQR 22–44) for CHIKV patients | 125 M/128 F |
| Yu-hen Chang et al., 2024 [27] | Cohort | 40 | 2 years | 52.6 ±14.5 years | 6 M/34 F |
| Lozano-Parra et al., 2025 [28] | Case–control | 46 febrile patients (11 cases + 35 controls Piedecuesta; 14 cases + 20 controls Capitanejo) | Capitanejo: 2.2 years; Piedecuesta: 7.7 years | Median: 33.5 years (IQR 19) | Piedecuesta cases 81.2% female vs. 40% controls; Capitanejo cases 85.7% female vs. 65% controls |
| Author (Year) | Acute Symptoms | Clinical Severity of Acute Infection |
|---|---|---|
| Ng et al., 2009 [14] | Fever (100%), arthralgia (90%), rash (50%), conjunctivitis (40%), GI symptoms (30%), headache (30%), eye pain (20%), back pain (20%), myalgia (10%), arthritis (10%) | 5 severe, 5 non-severe |
| Hoarau et al., 2010 [4] | Fever (38.1 ± 1 °C), rash, arthralgia | High viral load associated with severe and chronic outcomes |
| Kelvin et al., 2011 [15] | Fever, rash, headache, nausea, vomiting, myalgia, arthralgia/joint pain (severe joint pain = defining feature) | Defined as severe, mild, or non-symptomatic based on questionnaire at 6 and 12 months |
| Chow et al., 2011 [6] | Arthralgia (60%), fever (46.7%), myalgia (46.7%), rash (43.3%); joint swelling in 4 patients | Severe in 53.3% (defined by fever > 38.5 °C, pulse > 100/min, or platelet count < 100 × 109/L) |
| Chopra et al., 2011 [16] | Fever, joint pains, body aches | N/A |
| Chaaithanya et al., 2011 [30] | Fever, joint pain; some had rash, myalgia, headache | Mild to severe febrile illness with arthralgia |
| Chopra A. et al., 2012 [31] | Fever (95.9%), chills (75.6%), polyarthralgia (93.1%), myalgia (81.3%), skin rash (33.6%), headache (18.9%), fatigue (43.2%) | Mostly self-limiting but severe joint/musculoskeletal pain; elderly affected more severely |
| Autoantibodies (Anti-CCP, RF)Lohachanakul et al., 2012 [17] | Fever, myalgia, arthralgia/arthritis, rash | Mild CHIKF (fever < 38.5 °C, PR < 100, platelets > 100 × 109/L, recovery ≤ 30 days); severe CHIKF (fever > 38.5 °C, PR > 100, platelets < 100 × 109/L, some with prolonged arthralgia > 30 days) |
| Schilte et al., 2013 [2] | Febrile arthralgia | Febrile arthralgia |
| Venugopalan et al., 2014 [18] | High fever, severe musculoskeletal pain, polyarthralgia, headaches, fatigue, rash | Typical cases: severe febrile polyarthralgia; probable cases: milder illness |
| Reddy et al., 2014 [19] | Fever (100%), arthralgia (83%), myalgia (63%), headache (42%), rash (29%), GI symptoms (29%), conjunctival redness (6%) | Categorized by viral load (high vs. low) |
| Raad et al., 2016 [20] | Fever (89%), rash (73%), headache (69%), arthralgia (76%), myalgia (72%), back pain (61%), periarticular edema (55%), nausea (31%), vomiting (19%), mucosal bleeding (5%), asthenia (82%), cutaneous manifestations (80%), meningoencephalitis (57%) | All had persistent arthralgia; 10% polyarthritis |
| Delgado et al., 2017 [21] | Fever, arthralgia, myalgia, rash, arthritis, nausea, vomiting, back pain, headache, purpura, mucosal bleeding | 6/10 severe (DAS-28 > 5.1); 4/10 moderate/mild |
| Chang et al., 2018 [22] | Fever >38 °C, severe joint pain/arthritis, acute onset erythema multiforme | Severe joint pain/arthritis |
| Banerjee et al., 2018 [23] | Fever, joint pain | N/A |
| Abella et al., 2019 [24] | Fever (96.8%), rash (98.9%), myalgia (97.9%), headache (98.9%), fatigue (98.4%), abdominal pain (96.8%), nausea/emesis (97.9%), low back pain (63.8%), conjunctivitis (31.9%), adenopathy (41.5%) | 11.7% hospitalized at onset |
| Cavalcanti et al., 2019 [7] | All patients reported persistent joint pain | N/A |
| Ninla-aesong et al., 2019 [25] | High fever, severe polyarthralgia, myalgia, fatigue; typical rash and headache | N/A |
| Jacob-Nascimento et al., 2021 [26] | Fever, rash, headache, myalgia (92%), polyarthralgia (81%), symmetric arthralgia (82%), swollen joints (41%) | Mostly mild-to-moderate febrile disease |
| Yu-hen Chang et al., 2024 [27] | N/A | N/A |
| Lozano-Parra et al., 2025 [28] | Fever, joint pain, acute febrile syndrome | N/A |
| Author (Year) | Timing of Cytokine Measurement | Patient Cytokine Levels (pg/mL) | Control Cytokine Levels (pg/mL) | Notes/Findings |
|---|---|---|---|---|
| Ng et al., 2009 [14] | Acute, day 2–19 (median 4.5) | IL-6: 100–150; IL-1β: 20–30; RANTES: 2000; IL-2R, IL-5, IL-7, IL-8, IL-10, IL-15, IFN-α: 50–400; IP-10 and MIG: >10,000; Eotaxin: 50–100; HGF, FGF-basic, VEGF: 200–500; EGF: <5 | IL-6: <5; IL-1β: <5; RANTES: 10,000; Eotaxin: >200; EGF: 50–100 | Strong pro-inflammatory cytokine elevation; suppression of RANTES and Eotaxin |
| Hoarau et al., 2010 [4] | Acute (day 0–5), day 15, 6 w, 3–18 m | Chronic: TNF-α: 41 vs. Recovered: 1.5; IL-8: 37 vs. 11.3; IL-6: 11.2 vs. 9.8; IFN-γ: 757.5 vs. 1037.5; IL-12: 782.6 vs. 381 (up to 1650 at M12); IL-4: 14.5 vs. 89.5; IL-13: 14.5 vs. 49.8 | No control | Chronic disease linked to persistent TNF-α, IL-8, IL-12 elevation |
| Kelvin et al., 2011 [15] | Acute, 6 m, 12 m | IP-10: 7000 → <1000 after seroconversion; MIG: acute high → ↓1000–10,000 fold by 6–12 m; IL-10: ↓3-fold; IL-6, CXCL9, CXCL10 higher in high IgG group | No control | Strong IP-10 and MIG elevation in acute, decline over time |
| Chow et al., 2011 [6] | Acute (day 4), early (day 10), late (4–6 w), chronic (2–3 m) | IL-6: 128–256 vs. recovered 32–64; GM-CSF: 128 vs. 32; IL-17: 64 (only chronic); Eotaxin: 128 vs. 512; HGF: 256 vs. 512–1024 | No control | Chronic arthralgia group had higher IL-6, GM-CSF, IL-17 |
| Chopra et al., 2011 [16] | 1-year post-CHIKV | IFN-γ 8.23, CXCL-10 2.67, TNF-α 40.65, IL-13 280.7 | Healthy: IFN-γ 3.1, CXCL-10 3.2, TNF-α 32.3, IL-13 181.4 | IFN-γ, TNF-α, IL-13 elevated compared to controls |
| Chaaithanya et al., 2011 [30] | Acute (5–7 days), Chronic/Recovered (10 m) | Acute: IL-6 11.8, IL-8 754.1, MCP-1 354.9; Recovered: IL-6 0.01, IL-8 1911.6; Chronic: IL-6 63.5, IL-8 12,543.6, MCP-1 2539.8 | IL-6: 0.06; IL-8: 573.7; MCP-1: 190; MIP-1α: 60.4; MIP-1β: 78.5 | Chronic patients showed massive IL-6 and IL-8 rise |
| Chopra et al., 2012 [31] | Acute, 6–24 m follow-up | IL-6 baseline 262 (7.8 times higher); remained elevated up to 24 m | Controls baseline IL-6 low | IL-6 persistence linked to chronic disease |
| Lohachanakul et al., 2012 [17] | Day 1 and 30 | Severe: IL-6 43, MCP-1 1561, IL-8 71.8; Mild: IL-6 27.9, MCP-1 1019, IL-8 122 | Non-CHIKF: IL-6 15.7, MCP-1 413.4, IL-8 148.1 | Higher MCP-1 in severe CHIKF |
| Schilte et al., 2013 [2] | 36 m post-infection | Similar to controls | Arthralgia vs non-arthralgia: IFN-γ 0.9 vs. 0.9; IL-1α 1.4 vs. 1.4; IL-1β 2.4 vs. 2.4; IL-6 undetectable vs. 5.8; IL-17 0.9 vs. 0.9; TNF-α 2.2 vs. 2.2; MCP-1 150 vs. 170; IP-10 207 vs. 214 | Minimal long-term cytokine differences |
| Venugopalan et al., 2014 [18] | Within 4 w (acute, subacute, extended) | IFN-α 4.7; IFN-β 38.1; IFN-γ 26.8; IP-10 66.1; IL-1β 8.2; TNF-α 107.5; MCP-1 1869; IL-4 170.5; IL-6 251; IL-10 73; IL-13 576 | No control | Broad pro-inflammatory and Th2 cytokine elevation |
| Reddy et al., 2014 [19] | Days 2–10, follow-up 1–12 w | Peak w2: IL-1β 12.4; IL-6 34.1; IL-8 1078; MIG 739; MCP-1 687.3 | IL-1β 2.7; IL-6 2.6; IL-8 4.9; MIG 31.5; MCP-1 34.9 | Strong acute spike, returned near baseline |
| Raad et al., 2016 [20] | Mean 49 days | IL-1β, IL-2, IL-6, IL-8, IL-17, TNF-α, IFN-γ ↑ in 95% | No control | Generalized cytokine elevation |
| Delgado et al., 2017 [21] | Diagnosis, day10, 3 m, 12 m | IL-6: 4.5 at M12; CRP 0.35; ESR 15; RF 20 | No control | Persistent IL-6 at 1 year |
| Chang et al., 2018 [22] | Acute | TNF-α 0.65; IL-2 0.57; IL-4 0.50; IL-13 0.80 (NS) | No control | Mild elevations only |
| Banerjee et al., 2018 [23] | Follow-up (10–30 days) | IL-6, IFN-γ, CXCL-9 ↑; IL-10 ↓; TGF-β1 ↑ | No control | Linked to persistent polyarthralgia |
| Abella et al., 2019 [24] | Chronic (>3 m) | IL-6 mean 4.5; IL-17 mean 1.5 | No control | IL-6 detectable in about 65% |
| Cavalcanti et al., 2019 [7] | Subacute (3–12 w), Chronic (>12 w) | Patients: IL-27 210, IL-17A 22, IL-29 62.5, TGF-β 40.4 | Controls lower values (e.g., IL-27 62.5, IL-17A 3.9) | IL-27 and IL-17A persistently high |
| Ninla-aesong et al., 2019 [25] | 5 years post-infection | TNF-α 1.8; IL-1β 0; IL-6 6.8; IL-8 117.5; IL-12 13.1; IFN-γ 0; MCP-1 274.5 | No control | Low-grade inflammation persists long term |
| Jacob-Nascimento et al., 2021 [26] | Acute (≤7 days), Convalescent (15–40 days) | Acute: IL-8 90, IL-6 12, MCP-1 250, RANTES 1200, IP-10 2000, IL-1β 12, TNF 20 | No control | IL-6 and IL-8 higher in acute |
| Yu-hen Chang et al., 2024 [27] | 4 y post-infection | IL-6: 1648 (2019) → 1382 (2021); IL-10: 245 → 385; IL-12p70: 116 → 214; TNF-α: 474 → 458; IFN-γ: 441 → 366; IL-17α: 343 → 290 | No control | Persistent elevation of IL-6, TNF-α, IL-17α |
| Lozano-Parra et al., 2025 [28] | Acute (≤7 days) and subacute (≥14 days) | Acute: IL-6 7.7; IL-8 19.1; CXCL9 7230; CXCL10 6333; Subacute: IL-6 2.6; IL-8 67.4; CXCL9 4033; CXCL10 523 | Controls: IL-6 4.9; IL-8 27.3; CXCL9 7926; CXCL10 24,223 | Acute cases show lower CXCL10 vs. controls |
| Author (Year) | Development of Chronic Rheumatologic Disease | Type of Rheumatologic Manifestations | Duration of Chronic Symptoms |
|---|---|---|---|
| Ng et al., 2009 [14] | No (follow-up limited to 2 weeks only) | 2 patients had persistent arthralgia (>2 weeks); 1 had arthritis (knee effusion) | Persistent arthralgia reported >2 weeks |
| Hoarau et al., 2010 [4] | Yes | Chronic relapsing arthralgia, RA-like arthritis | ≥12 months, some up to 18 months |
| Kelvin et al., 2011 [15] | Yes | Persistent joint pain, arthralgia, arthritis (mono-/oligo-/polyarthritis, tenosynovitis, fibromyalgia-like symptoms) | Up to 12 months post-infection |
| Chow et al., 2011 [6] | Yes | Persistent arthralgia (joint pain), no persistent swelling | 2–3 months after infection onset |
| Chopra et al., 2011 [16] | Yes | Osteoarthritis (48%), Non-specific arthralgia (27%), Undifferentiated inflammatory arthritis (15%), Others (2%) | ≥1 year |
| Chaaithanya et al., 2011 [30] | Yes, (only in 10/22 patients) | Chronic joint pain/arthritis resembling RA | Up to 10 months |
| Chopra A. et al., 2012 [31] | Yes | Predominantly non-specific arthralgias (NSA); undifferentiated inflammatory arthritis rare (0.3% at 1 year, 0.07% at 2 years) | 1 year: 4.1% prevalence; 2 years: 1.6% prevalence (chronic musculoskeletal pain) |
| Lohachanakul et al., 2012 [17] | Yes—6/15 severe CHIKF patients had prolonged arthralgia (>30 days) | Prolonged arthralgia/arthritis; 1 case chronic arthritis | >30 days (for prolonged cases) |
| Schilte et al., 2013 [2] | Yes (60% had long-term arthralgia at 36 months) | Predominantly symmetrical polyarthralgia (70%) | Up to 36 months post-infection |
| Venugopalan et al., 2014 [18] | Yes—some cases had persistent MSK symptoms beyond 1 month | Persistent musculoskeletal pain, arthritis, polyarthralgia | Extended symptomatic phase: 15–30 days |
| Reddy et al., 2014 [19] | Yes, in 4 patients with persistent arthralgia | Arthralgia (persistent) | Up to 12 weeks |
| Raad et al., 2016 [20] | Yes (72% had persistent arthralgia and periarticular edema after 9 months) | Symmetrical polyarthritis, persistent arthralgia, periarticular edema | ≥9 months follow-up, symptoms persisted |
| Delgado et al., 2017 [21] | Yes—2 patients developed chronic arthritis | Chronic polyarthralgia, polyarthritis, myalgias (joints: fingers, wrists, knees, ankles, toes) | >12 months (persisted at 1-year follow-up) |
| Chang et al., 2018 [22] | Yes (chronic arthritis/joint pain at 20 months) | Chronic arthritis (joint pain/swelling) | Up to 20 months post-infection |
| Banerjee et al., 2018 [23] | Yes | Chronic joint pain and inflammation (polyarthralgia) | Persisted beyond 10 days, up to months |
| Abella et al., 2019 [24] | Yes—all included patients had chronic rheumatologic manifestations (>3 months) | Symmetrical arthritis, synovitis (29.8%), tender/swollen joints, persistent arthralgia | Mean 278 ± 87.8 days (range: 91–365 days) |
| Cavalcanti et al., 2019 [7] | Yes—21 patients (46.67%) were in chronic phase (>12 weeks) | Arthritis, persistent arthralgia, swollen/painful joints | Median disease duration 12 weeks (range: 8.5–20 weeks); chronic defined as >12 weeks |
| Ninla-aesong et al., 2019 [25] | Yes—63/123 had persistent arthralgia (80.9% severe pain, 19.05% non-severe) | Persistent arthralgia, severe vs. non-severe joint pain, stiffness, swelling | 5 years post-infection |
| Jacob-Nascimento et al., 2021 [26] | Yes—chronic arthralgia occurred in 42.5% (62/146) | Chronic arthralgia (polyarthralgia, symmetric arthralgia, swollen joints) | >3 months after acute infection |
| Yu-hen Chang et al., 2024 [27] | Yes (persistent arthritis 4–6 years post-CHIKV infection) | Persistent arthritis, mainly affecting small joints (MCP, IFP, wrists); pain, stiffness, disability | 4–6 years post-infection (study follow-up adds 2 more years) |
| Lozano-Parra et al., 2025 [28] | Yes | Rheumatoid arthritis, spondylarthritis, SLE, post-viral arthritis, post-viral arthralgia, tenosynovitis, bursitis, fasciitis, fibromyalgia | ≥3 months; up to 7.7 years follow-up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conforti, A.; Lavalle, G.; Varini, F.; Lucchetti, L.; Cataldi, G.; Faticoni, A.; Ruggiero, M.; Gentile, M.; Gimignani, G.; Bassetti, M. Pro-Inflammatory Cytokines as Early Predictors of Chronic Rheumatologic Disease Following Chikungunya Virus Infection. J. Clin. Med. 2025, 14, 6720. https://doi.org/10.3390/jcm14196720
Conforti A, Lavalle G, Varini F, Lucchetti L, Cataldi G, Faticoni A, Ruggiero M, Gentile M, Gimignani G, Bassetti M. Pro-Inflammatory Cytokines as Early Predictors of Chronic Rheumatologic Disease Following Chikungunya Virus Infection. Journal of Clinical Medicine. 2025; 14(19):6720. https://doi.org/10.3390/jcm14196720
Chicago/Turabian StyleConforti, Alessandro, Gabriella Lavalle, Farbizio Varini, Linda Lucchetti, Giulia Cataldi, Augusto Faticoni, Marco Ruggiero, Martina Gentile, Giancarlo Gimignani, and Matteo Bassetti. 2025. "Pro-Inflammatory Cytokines as Early Predictors of Chronic Rheumatologic Disease Following Chikungunya Virus Infection" Journal of Clinical Medicine 14, no. 19: 6720. https://doi.org/10.3390/jcm14196720
APA StyleConforti, A., Lavalle, G., Varini, F., Lucchetti, L., Cataldi, G., Faticoni, A., Ruggiero, M., Gentile, M., Gimignani, G., & Bassetti, M. (2025). Pro-Inflammatory Cytokines as Early Predictors of Chronic Rheumatologic Disease Following Chikungunya Virus Infection. Journal of Clinical Medicine, 14(19), 6720. https://doi.org/10.3390/jcm14196720









