Impact of Osteoporosis on Dental Implant Survival, Failure, and Marginal Bone Loss: A Systematic Review and Meta-Analysis
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. PICOS
2.2. Eligibility Criteria
2.3. Information Sources
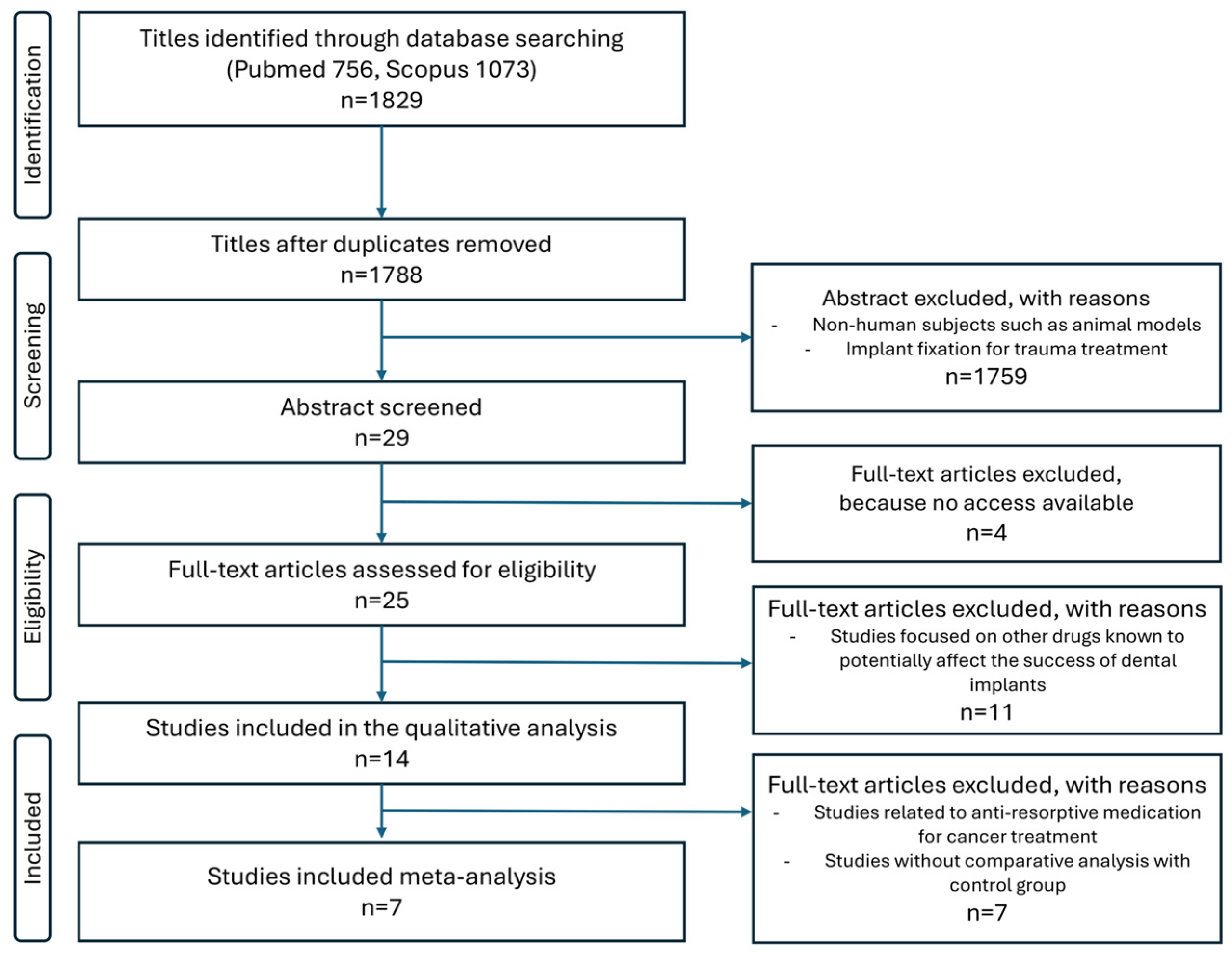
2.4. Search Strategy and Article Selection
- PubMed: (implant OR (dental implant)) AND (osteoporosis OR osteopenia OR (low bone density)) AND ((survive OR (survival rate) OR (success rate)) OR (failure OR fail OR (failure rate))).
- Scopus: (TITLE-ABS-KEY((“implant” OR “dental implant” OR “implant surgery”) AND (“osteoporosis” OR “osteopenia” OR “low bone density”)) AND ((“survive” OR “survival rate” OR “success rate”) OR (“failure” OR “fail” OR “failure rate”))) AND PUBYEAR > 2013 AND PUBYEAR < 2025 AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (SUBJAREA, “MEDI”) OR LIMIT-TO (SUBJAREA, “DENT”)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “cp”)).
2.5. Data Collection Process
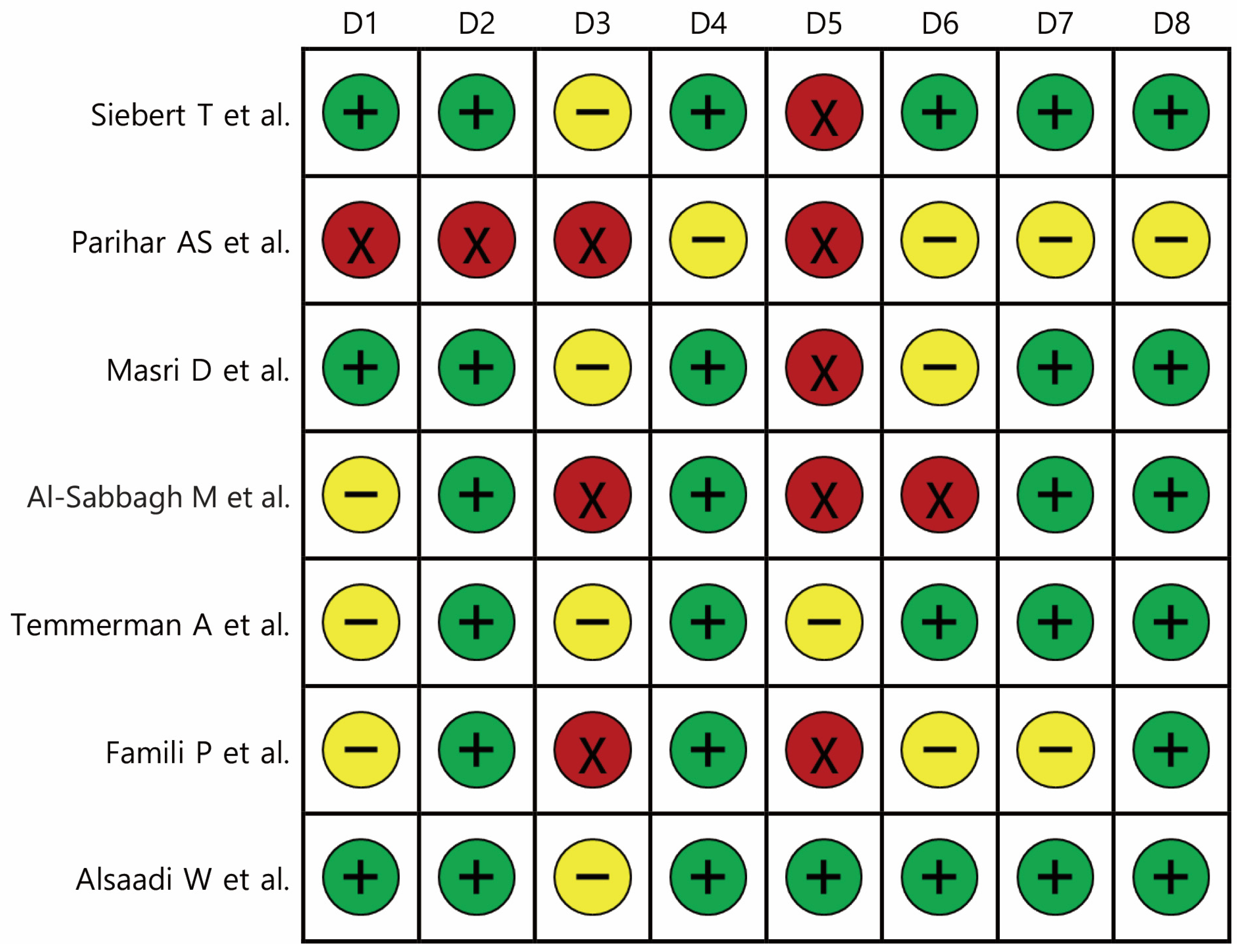
2.6. Evaluation of the Study Risk of Bias
2.7. Statistical Analysis
3. Results
3.1. Study Characteristics
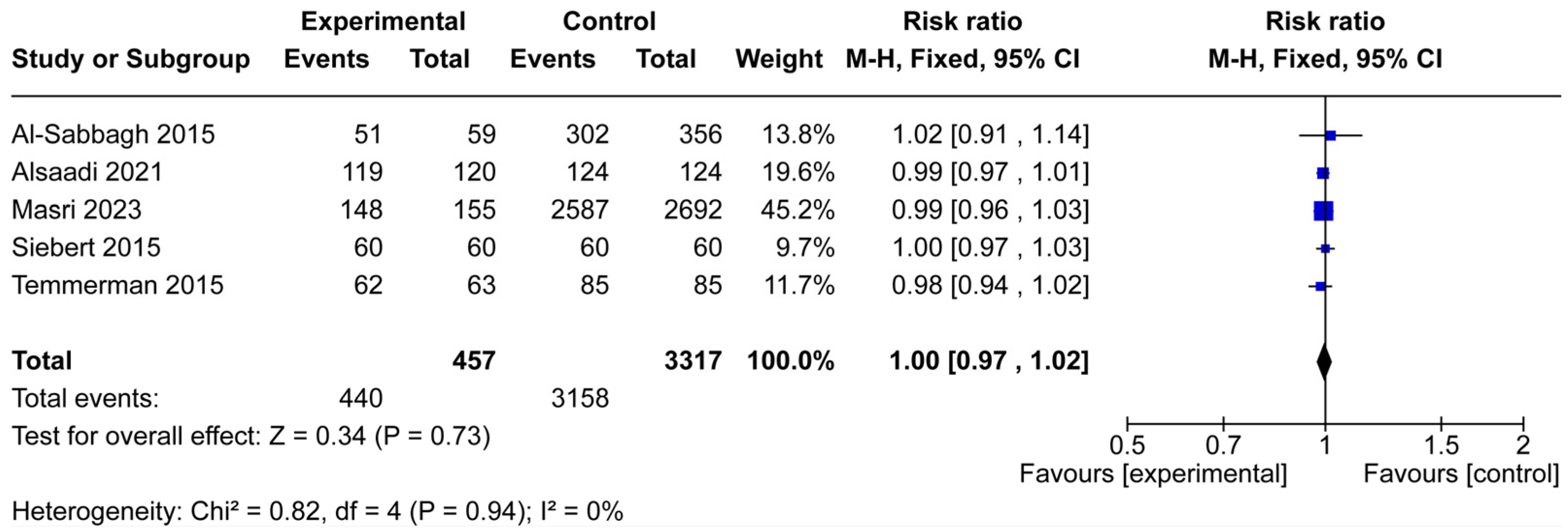
3.2. Implant Survival
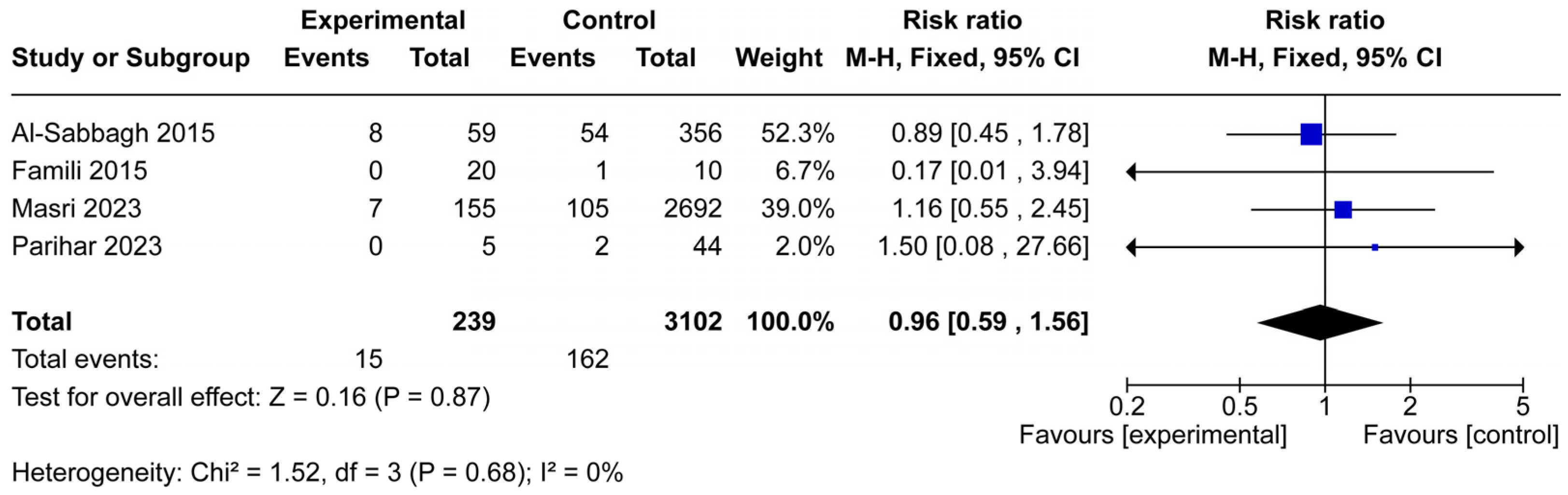
3.3. Implant Failure
3.4. Marginal Bone Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| MRONJ | Medication-related osteonecrosis of the jaws |
| PRISMA-P | Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols |
| RRs | Risk ratios |
| CIs | Confidence intervals |
| MD | Mean difference |
References
- Aung, Y.T.; Eo, M.Y.; Sodnom-Ish, B.; Kim, M.J.; Kim, S.M. Long-term survival rates of tapered self-tapping bone-level implants after immediate placement: A positional effective rationale. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 17. [Google Scholar] [CrossRef] [PubMed]
- Bérczy, K.; Göndöcs, G.; Komlós, G.; Shkolnik, T.; Szabó, G.; Németh, Z. Outcomes of treatment with short dental implants compared with standard-length implants: A retrospective clinical study. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 6. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Hong, N.; Rhee, Y.; Park, W.; Oh, K.C.; Seo, Y.; Lee, H.; Jo, H.G.; Shin, Y.; Kim, J.Y. Clinical outcomes and bone marker changes in postmenopausal women with dental implants: A one-year prospective study. Int. J. Implant Dent. 2025, 11, 41. [Google Scholar] [CrossRef]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Pieralli, S.; Spies, B.C.; Schweppe, F.; Preissner, S.; Nelson, K.; Heiland, M.; Nahles, S. Retrospective long-term clinical evaluation of implant-prosthetic rehabilitations after head and neck cancer therapy. Clin. Oral Implants Res. 2021, 32, 470–486. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, S.-G. Unveiling medication-related osteonecrosis of the jaw: A rapid review of etiology, drug holidays, and treatment strategies. Appl. Sci. 2024, 14, 3314. [Google Scholar] [CrossRef]
- Kim, S.G. Nonessential amino acid is not nonessential in geriatric patients: Implications for maxillofacial wound healing and bone repair. Maxillofac. Plast. Reconstr. Surg. 2025, 47, 12. [Google Scholar] [CrossRef]
- Yip, J.K.; Borrell, L.N.; Cho, S.C.; Francisco, H.; Tarnow, D.P. Association between oral bisphosphonate use and dental implant failure among middle-aged women. J. Clin. Periodontol. 2012, 39, 408–414. [Google Scholar] [CrossRef]
- Niedermaier, R.; Stelzle, F.; Riemann, M.; Bolz, W.; Schuh, P.; Wachtel, H. Implant-supported immediately loaded fixed full-arch dentures: Evaluation of implant survival rates in a case cohort of up to 7 years. Clin. Implant Dent. Relat. Res. 2017, 19, 4–19. [Google Scholar] [CrossRef]
- Tallarico, M.; Canullo, L.; Xhanari, E.; Meloni, S.M. Dental implants treatment outcomes in patient under active therapy with alendronate: 3-year follow-up results of a multicenter prospective observational study. Clin. Oral Implants Res. 2016, 27, 943–949. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Ewers, R.; Morgan, K.; Hirayama, M.; Murcko, L.; Morgan, J.; Bergamo, E.T.P.; Bonfante, E.A. Antiresorptive therapy and dental implant survival: An up to 20-year retrospective cohort study in women. Clin. Oral Investig. 2022, 26, 6569–6582. [Google Scholar] [CrossRef] [PubMed]
- Grisa, A.; Veitz-Keenan, A. Is osteoporosis a risk factor for implant survival or failure? Evid. Based Dent. 2018, 19, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637. [Google Scholar] [CrossRef]
- Siebert, T.; Jurkovic, R.; Statelova, D.; Strecha, J. Immediate implant placement in a patient with osteoporosis undergoing bisphosphonate therapy: 1-year preliminary prospective study. J. Oral Implantol. 2015, 41, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.S.; Sonkar, T.P.; Mahabob, N.; Mohapatra, A.; Feroz, S.M.A.; Shetty, M.; Arasu, T.U. Evaluation for survival of dental implants in medically compromised patients. Int. J. Chem. Biochem. Sci. 2023, 24, 681–684. [Google Scholar]
- Masri, D.; Masri-Iraqi, H.; Nissan, J.; Naishlos, S.; Ben-Zvi, Y.; Rosenfeld, E.; Avishai, G.; Chaushu, L. On the association between dental implants, osteoporosis and bone modulating therapy. Appl. Sci. 2023, 13, 3398. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Thomas, M.V.; Bhavsar, I.; De Leeuw, R. Effect of bisphosphonate and age on implant failure as determined by patient-reported outcomes. J. Oral Implantol. 2015, 41, e287–e291. [Google Scholar] [CrossRef]
- Temmerman, A.; Rasmusson, L.; Kübler, A.; Thor, A.; Quirynen, M. An open, prospective, non-randomized, controlled, multicentre study to evaluate the clinical outcome of implant treatment in women over 60 years of age with osteoporosis/osteopenia: 1-year results. Clin. Oral Implants Res. 2017, 28, 95–102. [Google Scholar] [CrossRef]
- Famili, P.; Zavoral, J.M. Low skeletal bone mineral density does not affect dental implants. J. Oral Implantol. 2015, 41, 550–553. [Google Scholar] [CrossRef]
- Alsaadi, W.; AbouSulaiman, A.; AlSabbagh, M.M. Retrospective study of dental implants survival rate in postmenopausal women with osteoporosis. Int. J. Dent. Oral Sci. 2021, 8, 4259–4266. [Google Scholar] [CrossRef]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47, S11–S20. [Google Scholar] [CrossRef]
- Soares, A.P.; Fischer, H.; Aydin, S.; Steffen, C.; Schmidt-Bleek, K.; Rendenbach, C. Uncovering the unique characteristics of the mandible to improve clinical approaches to mandibular regeneration. Front. Physiol. 2023, 14, 1152301. [Google Scholar] [CrossRef]
- Omi, M.; Mishina, Y. Role of osteoclasts in oral homeostasis and jawbone diseases. Oral Sci. Int. 2020, 18, 14–27. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, F.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, C.; Park, R.W.; Jeon, J.Y. Comparative effectiveness and safety outcomes between denosumab and bisphosphonate in South Korea. J. Bone Miner. Res. 2024, 39, 835–843. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. The efficacy of various bone augmentation procedures for dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int. J. Oral Maxillofac. Implants 2006, 21, 696–710. [Google Scholar] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Diabetes and oral implant failure: A systematic review. J. Dent. Res. 2014, 93, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Herzberg, R.; Dolev, E.; Schwartz-Arad, D. Smoking and complications of onlay bone grafts and sinus lift operations. Int. J. Oral Maxillofac. Implants 2004, 19, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-D.; Jo, H.; Kim, J.-E.; Ohe, J.-Y. A clinical retrospective study of implant as a risk factor for medication-related osteonecrosis of the jaw: Surgery vs. loading. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 31. [Google Scholar] [CrossRef]
- Sulaiman, N.; Hanifah, Y.Z.; Mutalib, N.-S.; Cheung, Y.-B.; Duski, I.; Zainal, H. Bisphosphonates and Dental Implants: A Systematic Review. Materials 2023, 16, 6078. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ha, Y.C.; Kim, T.Y.; Lee, Y.K.; Kim, J.H. Long-Term Efficacy and Safety of Denosumab: Insights beyond 10 Years. Endocrinol. Metab. 2024, 39, 12–25. [Google Scholar]
- Kujanpää, M.; Vuollo, V.; Tiisanoja, A.; Laitala, M.-L.; Sándor, G.K.; Karki, S. Incidence of medication-related osteonecrosis of the jaw and associated antiresorptive drugs in adult Finnish population. Sci. Rep. 2025, 15, 17377. [Google Scholar] [CrossRef] [PubMed]

| Element | Description |
|---|---|
| P (Population) | Patients clinically or radiographically diagnosed with osteopenia or osteoporosis (hereafter collectively referred to as “osteoporotic conditions” unless otherwise specified) |
| I (Intervention/Exposure) | Dental implant placement |
| C (Comparison) | Dental implant placement in systemically healthy individuals without osteopenia or osteoporosis |
| O (Outcomes) | Implant survival, implant failure, marginal bone loss as reported by included studies |
| S (Study design) | Prospective or retrospective cohort studies, non-randomized controlled trials, and case–control studies |
| Focused question | Do patients with osteoporosis experience different implant survival, failure, or marginal bone loss outcomes compared with systemically healthy patients? |
| Author (Year) | Study Design | Experimental Group | Control Group | Age/Sex | Patients | Implants | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | C | T | E | C | T | |||||
| Siebert et al. (2015) [15] | Prospective clinical study | T-score < 2.5 with BP therapy | without osteoporotic disease based on t-scan | ≥54/Females | 12 | 12 | 24 | 60 | 60 | 120 |
| Parihar et al. (2023) [16] | Retrospective study | diagnosed with osteoporosis | Healthy participants | 35–65/(-) * | 2 | 40 | 42 | 5 | 44 | 49 |
| Masri et al. (2023) [17] | Retrospective cohort study | Diagnosis of osteoporosis A: Osteoporosis with BMT B: Osteoporosis without BMT | No osteoporosis | ≤65: 459 implants, 66–79: 265 implants, ≥80: 68 implants/Male: 294 implants, Female: 498 implants | A: 46 B: 26 | 720 | 792 | A: 155 B: 124 | 2692 | 2971 |
| Al-Sabbagh et al. (2015) [18] | Retrospective study | Diagnosis of osteoporosis A: Osteoporosis with BMT B: Osteoporosis without BMT | No osteoporosis | 59.4 ± 13.3/Male: 42% of patients, Female: 58% of patients | A: 39 B: 20 | 356 | 415 | (-) * | (-) * | 963 |
| Temmerman et al. (2016) [19] | Prospective, non-randomized controlled study, multicenter | osteoporosis/osteopenia group subjects had a T-score ≤ −2 | control group had a T-score of ≥−1 | 59–83(mean 69) | 20 | 28 | 48 | 63 | 85 | 148 |
| Famili et al. (2015) [20] | Case–control study | osteoporosis/osteopenia established by report of the treating medical physician | adequate bone density based on DXA measuring | 52–70/Female | 20 | 10 | 30 | 21 | 10 | 31 |
| Alsaadi et al. (2021) [21] | Retrospective study | osteoporosis/osteopenia subjects with a T-score < −1 | subjects with a T-score ≥ −1 | 60.62 ± 5.70/Female | 27 | 26 | 52 | 120 | 124 | 244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Lee, Y.-J.; Kang, Y.-J.; Kim, S.-G.; Rotaru, H. Impact of Osteoporosis on Dental Implant Survival, Failure, and Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6719. https://doi.org/10.3390/jcm14196719
Kim S-Y, Lee Y-J, Kang Y-J, Kim S-G, Rotaru H. Impact of Osteoporosis on Dental Implant Survival, Failure, and Marginal Bone Loss: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(19):6719. https://doi.org/10.3390/jcm14196719
Chicago/Turabian StyleKim, Su-Young, Yoon-Jo Lee, Yei-Jin Kang, Seong-Gon Kim, and Horatiu Rotaru. 2025. "Impact of Osteoporosis on Dental Implant Survival, Failure, and Marginal Bone Loss: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 19: 6719. https://doi.org/10.3390/jcm14196719
APA StyleKim, S.-Y., Lee, Y.-J., Kang, Y.-J., Kim, S.-G., & Rotaru, H. (2025). Impact of Osteoporosis on Dental Implant Survival, Failure, and Marginal Bone Loss: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(19), 6719. https://doi.org/10.3390/jcm14196719







