The Impact of Respiratory Function on Functionality and Mortality in ALS Patients
Abstract
1. Introduction
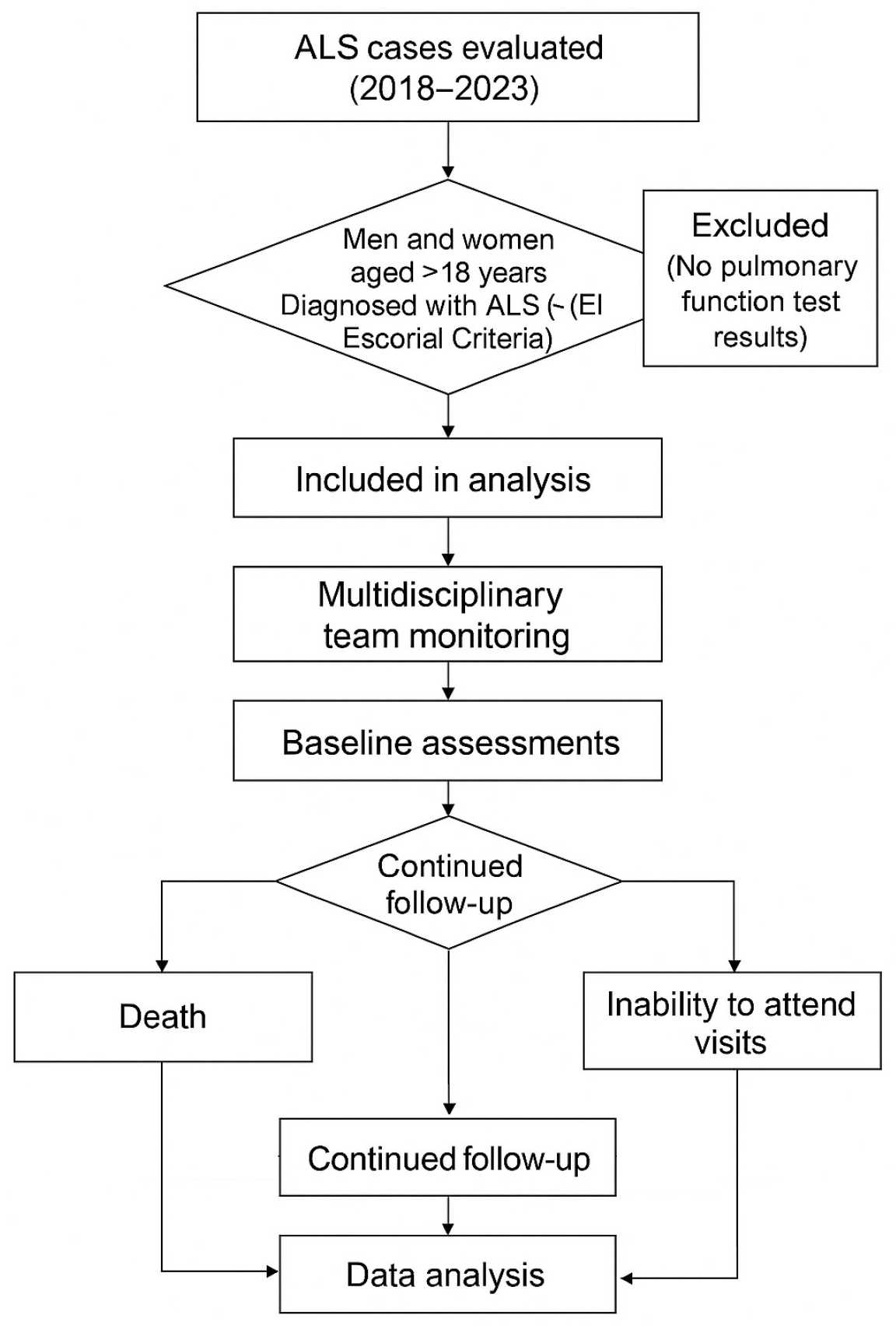
2. Materials and Methods
2.1. Study Design
2.2. Procedures and Data Collection
2.3. Spirometry
2.4. Respiratory Muscle Strength
2.5. Functionality and Stage of the Disease
2.6. Statistical Analysis
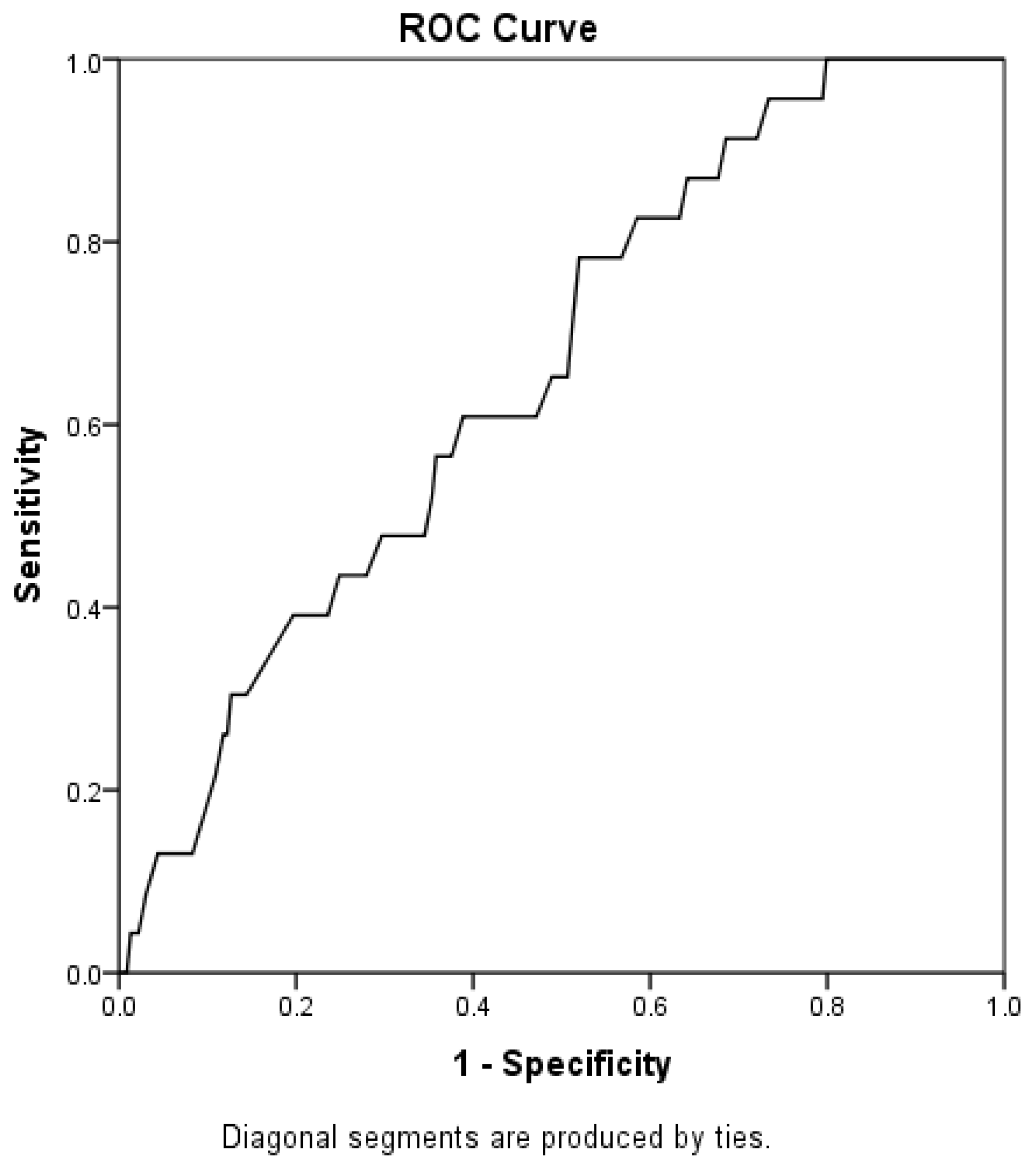
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| PEF | Peak Expiratory Flow |
| FVC | Forced Vital Capacity |
| SNIP | Sniff Nasal Inspiratory Pressure |
| MIP | Maximal Inspiratory Pressure |
| MEP | Maximal Expiratory Pressure |
| ALSFRS-R | Amyotrophic Lateral Sclerosis Functional Rating Scale |
| SVC | Slow Vital Capacity |
| VC | Vital Capacity |
| PCF | Peak Cough Flow |
| PFT | Pulmonary Function Test |
| HUOL | Hospital Universitário Onofre Lopes |
| REC | Research Ethics Committee |
| UFRN | Universidade Federal Do Rio Grande Do Norte |
| ATS | American Toracic Society |
| ERS | European Respiratory Society |
| FRC | Functional Residual Capacity |
| BCa | Bias-Corrected And Accelerated |
| GLM | Generalized Linear Model |
| AUC | Area Under The Curve |
| BMI | Body Mass Index |
| FEV1 | Forced Expiratory Volume In The First Second |
| MVV | Maximal Voluntary Ventilation |
| MEF75% | Maximum Expiratory Flow At 75% |
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 1707. [Google Scholar] [CrossRef]
- Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C. Skeletal Muscle in ALS: An Unappreciated Therapeutic Opportunity? Cells 2021, 10, 525. [Google Scholar] [CrossRef]
- Gordon, P.H. Amyotrophic Lateral Sclerosis: An update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013, 4, 295–310. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Costa, J.; Swash, M.; de Carvalho, M. Awaji Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis: A systematic review. Arch. Neurol. 2012, 69, 1410–1416. [Google Scholar] [CrossRef]
- Geevasinga, N.; Loy, C.T.; Menon, P.; de Carvalho, M.; Swash, M.; Schrooten, M.; Van Damme, P.; Gawel, M.; Sonoo, M.; Higashihara, M.; et al. Awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis: A systematic review using individual patient data. Clin. Neurophysiol. 2016, 127, 2684–2691. [Google Scholar] [CrossRef]
- Sancho, J.; Martínez, D.; Bures, E.; Díaz, J.L.; Ponz, A.; Servera, E. Bulbar impairment score and survival of stable amyotrophic lateral sclerosis patients after noninvasive ventilation initiation. ERJ Open Res. 2018, 4, 00159-2017. [Google Scholar] [CrossRef]
- Garcia, L.N.; Silva, A.V.; Carrete, H.; Favero, F.M.; Fontes, S.V.; Moneiro, M.T.; Oliveira, A.S.B. Relação entre degeneração do trato córtico-espinhal através de ressonância magnética e escala funcional (ALSFRS) em pacientes com esclerose lateral amiotrófica. Arq. Neuro-Psiquiatr. 2007, 65, 869–874. [Google Scholar] [CrossRef]
- Polkey, M.I.; Lyall, R.A.; Yang, K.; Johnson, E.; Leigh, P.N.; Moxham, J. Respiratory Muscle Strength as a Predictive Biomarker for Survival in Amyotrophic Lateral Sclerosis. Am. J. Respir. Crit. Care Med. 2017, 195, 86–95. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Mollar, E.; Melissari, L.; Buscema, M.; Bagnoli, E.; Cabona, C.; Gemelli, C.; Vignolo, M.; Maranzana, C.; Marogna, M.; et al. Longitudinal respiratory trajectories in motor neuron disease phenotypes: Multiparametric characterization and clinical management. Respir. Med. 2025, 239, 108003. [Google Scholar] [CrossRef]
- Aslan, G.K.; Gurses, H.N.; Issever, H.; Kiyan, E. Effects of respiratory muscle training on pulmonary functions in patients with slowly progressive neuromuscular disease: A randomized controlled trial. Clin. Rehabil. 2014, 28, 573–581. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- ENCALS. Standard Operations Manual for Outcome Measures, Version 2.0; ENCALS: Utrecht, The Netherlands, 2018.
- Shoesmith, C.; Abrahao, A.; Benstead, T.; Chum, M.; Dupre, N.; Izenberg, A.; Johnston, W.; Kalra, S.; Leddin, D.; O’Connell, C.; et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. CMAJ 2020, 192, E1453–E1468. [Google Scholar] [CrossRef]
- Shefner, J.M.; Watson, M.L.; Meng, L.; Wolff, A.A.; Neals/Cytokinetics STUDY Team. A study to evaluate safety and tolerability of repeated doses of tirasemtiv in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 574–581. [Google Scholar] [CrossRef]
- Hermann, W.; Langner, S.; Freigang, M.; Fischer, S.; Storch, A.; Günther, R.; Hermann, A. Affection of Respiratory Muscles in ALS and SMA. J. Clin. Med. 2022, 11, 1163. [Google Scholar] [CrossRef]
- Sales de Campos, P.; Olsen, W.L.; Wymer, J.P.; Smith, B.K. Respiratory therapies for Amyotrophic Lateral Sclerosis: A state of the art review. Chron. Respir. Dis. 2023, 20, 14799731231175915. [Google Scholar] [CrossRef]
- Carvalho, M.; Swash, M.; Pinto, S. Diaphragmatic Neurophysiology and Respiratory Markers in ALS. Front. Neurol. 2019, 10, 143. [Google Scholar] [CrossRef]
- Murray, D.; Rooney, J.; Al-Chalabi, A.; Bunte, T.; Chiwera, T.; Choudhury, M.; Chio, A.; Fenton, L.; Fortune, J.; Maidment, L.; et al. Correlations between measures of ALS respiratory function: Is there an alternative to FVC? Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 495–504. [Google Scholar] [CrossRef]
- Rooney, J.; Murray, D.; Meldrum, D.; Al-Chalabi, A.; Bunte, T.; Chiwera, T.; Choudhury, M.; Chio, A.; Fenton, L.; Fortune, J.; et al. REVEALS-a longitudinal cohort study of multifaceted respiratory assessment in ALS. Amyotroph. Lateral Scler. Front. Degener. 2024, 25, 661–671. [Google Scholar] [CrossRef]
- Baumann, F.; Henderson, R.D.; Morrison, S.C.; Brown, M.; Hutchinson, N.; Douglas, J.A.; Robinson, P.J.; McCombe, P.A. Use of respiratory function tests to predict survival in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 194–202. [Google Scholar] [CrossRef]
- Lyall, R.A.; Donaldson, N.; Polkey, M.I.; Leigh, P.N.; Moxham, J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001, 124 Pt 10, 2000–2013. [Google Scholar] [CrossRef]
- Daghlas, S.A.; Govindarajan, R.; Pooled Resource Open-Access ALS Clinical Trials Consortium. Relative effects of forced vital capacity and ALSFRS-R on survival in ALS. Muscle Nerve 2021, 64, 346–351. [Google Scholar] [CrossRef]
- Kjældgaard, A.L.; Pilely, K.; Olsen, K.S.; Jessen, A.H.; Lauritsen, A.Ø.; Pedersen, S.W.; Svenstrup, K.; Karlsborg, M.; Thagesen, H.; Blaabjerg, M.; et al. Prediction of survival in amyotrophic lateral sclerosis: A nationwide, Danish cohort study. BMC Neurol. 2021, 21, 164. [Google Scholar] [CrossRef]
- Pirola, A.; De Mattia, E.; Lizio, A.; Sannicolò, G.; Carraro, E.; Rao, F.; Sansone, V.; Lunetta, C. The prognostic value of spirometric tests in Amyotrophic Lateral Sclerosis patients. Clin. Neurol. Neurosurg. 2019, 184, 105456. [Google Scholar] [CrossRef]
- Andrews, J.A.; Meng, L.; Kulke, S.F.; Rudnicki, S.A.; Wolff, A.A.; Bozik, M.E.; Malik, F.I.; Shefner, J.M. Association Between Decline in Slow Vital Capacity and Respiratory Insufficiency, Use of Assisted Ventilation, Tracheostomy, or Death in Patients With Amyotrophic Lateral Sclerosis. JAMA Neurol. 2018, 75, 58–64. [Google Scholar] [CrossRef]
- Pereira, C.A.; Sato, T.; Rodrigues, S.C. New reference values for forced spirometry in white adults in Brazil. J. Bras. Pneumol. 2007, 33, 397–406. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef]
- de Lima, J.C.C.; Resqueti, V.R.; Marcelino, A.A.; da Fonsêca, J.D.M.; Paz, A.L.; Dias, F.A.L.; Otto-Yañez, M.; Fregonezi, G.A.F. Reliability of maximal respiratory nasal pressure tests in healthy young adults. PLoS ONE 2023, 18, e0287188. [Google Scholar] [CrossRef]
- Araújo, P.R.S.; Fonseca, J.D.M.D.; Marcelino, A.A.; Moreno, M.A.; Dornelas de Andrade, A.F.; Yañez, M.O.; Torres-Castro, R.; Resqueti, V.R.; Fregonezi, G.A.F. Reference values for respiratory muscle strength and maximal voluntary ventilation in the Brazilian adult population: A multicentric study. PLoS ONE 2024, 19, e0313209. [Google Scholar] [CrossRef]
- Araújo, P.R.; Resqueti, V.R.; Nascimento Junior, J.; Carvalho Lde, A.; Cavalcanti, A.G.; Silva, V.C.; Silva, E.; Moreno, M.A.; Andrade Ade, F.; Fregonezi, G.A. Reference values for sniff nasal inspiratory pressure in healthy subjects in Brazil: A multicenter study. J. Bras. Pneumol. 2012, 38, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Guedes, K.; Pereira, C.; Pavan, K.; Valério, B.C. Cross-cultural adaptation and validation of ALS Functional Rating Scale-Revised in Portuguese language. Arq. Neuro-Psiquiatr. 2010, 68, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.C.; Rojas-Garcia, R.; Scott, K.M.; Scotton, W.; Ellis, C.E.; Burman, R.; Wijesekera, L.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012, 135 Pt 3, 847–852. [Google Scholar] [CrossRef]
- LaFleur, B.J.; Greevy, R.A. Introduction to permutation and resampling-based hypothesis tests. J. Clin. Child Adolesc. Psychol. 2007, 38, 286–294. [Google Scholar] [CrossRef]
- Raymond, J.; Oskarsson, B.; Mehta, P.; Horton, K. Clinical characteristics of a large cohort of US participants enrolled in the National Amyotrophic Lateral Sclerosis (ALS) Registry, 2010–2015. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 413–420. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Vasconcelos, K.; Oliveira, A.S.B.; Fuchs, L.F.P.; Simões, R.S.; Girão, M.J.B.C.; Soares Júnior, J.M.; Baracat, E.C. Estrogens: Possible protection against Amyotrophic Lateral Sclerosis? Rev. Assoc. Medica Bras. 2019, 65, 576–577. [Google Scholar] [CrossRef]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef]
- Pinto, S.; Turkman, A.; Pinto, A.; Swash, M.; de Carvalho, M. Predicting respiratory insufficiency in amyotrophic lateral sclerosis: The role of phrenic nerve studies. Clin. Neurophysiol. 2009, 120, 941–946. [Google Scholar] [CrossRef]
- Morgan, R.K.; McNally, S.; Alexander, M.; Conroy, R.; Hardiman, O.; Costello, R.W. Use of Sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med. 2005, 171, 269–274. [Google Scholar] [CrossRef]
- Polla, B.; D’Antona, G.; Bottinelli, R.; Reggiani, C. Respiratory muscle fibres: Specialisation and plasticity. Thorax 2004, 59, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Suárez, A.A.; Pessolano, F.A.; Monteiro, S.G.; Ferreyra, G.; Capria, M.E.; Mesa, L.; Dubrovsky, A.; De Vito, E.L. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am. J. Phys. Med. Rehabil. 2002, 81, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Huang, J.C.; Chen, J.; Hu, W.; Xu, L.Q.; Guo, Q.F. Peak expiratory flow is a reliably household pulmonary function parameter correlates with disease severity and survival of patients with amyotrophic lateral sclerosis. BMC Neurol. 2022, 22, 105. [Google Scholar] [CrossRef] [PubMed]
| Mean (Minimum–Maximum) | |
|---|---|
| Age (years) | 55.7 (18–82) |
| Sex Male/Female (%) | 49 (66.2)/25 (33.8) |
| Weight (kg) | 65.6 (39–92) |
| Height (cm) | 164.80 (148–185) |
| BMI (kg/m2) | 24.10 (16.23–33.32) |
| Bulbar type | 16 (21.6) |
| Spinal type | 38 (51.3) |
| Spinal bulbar type | 3 (4.1) |
| N° information of type | 17 (23) |
| FVC (L) | 2.99 (0.83–5.23) |
| FVC%pred | 0.76 (0.18–1.14) |
| FEV1 (L) | 2.26 (0.64–4.22) |
| FEV1%pred | 0.70 (0.21–1.08) |
| FEV1/FVC% | 0.74 (0.32–0.96) |
| FEV1/FVC%pred | 0.94 (0.49–1.16) |
| FEF25–75 | 2.74 (0.41–10.04) |
| FEF25–75%pred | 0.64 (0.13–1.37) |
| PEF (L/s) | 3.29 (0.25–8.34) |
| PEF%pred | 0.46 (0.09–1.28) |
| ALSFRS-R | 37.2 (18.00–47) |
| MIP (cmH2O) | 63.4 (8.79–139) |
| MEP (cmH2O) | 74.1 (9–163) |
| SNIP (cmH2O) | 58 (13–113) |
| Mortality | ALSFRS-R | |||
|---|---|---|---|---|
| Variables | rpb (95% CI) | p Valor | r (95% CI) | p Valor |
| PEF | −0.10 (−0.20–0.01) | 0.110 | 0.27 (0.15–0.37) | <0.001 |
| PEF%pred | −0.16 (−0.23–0.07) | 0.013 | 0.32 (0.21–0.42) | <0.001 |
| SNIP | −0.24 (−0.34–0.12) | 0.001 | 0.62 (0.52–0.69) | <0.001 |
| MIP | −0.20 (−0.32–0.07) | 0.009 | 0.51 (0.38–0.62) | <0.001 |
| MEP | −0.21 (−0.31–0.08) | 0.007 | 0.51 (0.40–0.60) | <0.001 |
| FVC | −0.25 (−0.34–0.12) | <0.001 | 0.53 (0.43–0.62) | <0.001 |
| FVC%pred | −0.26 (−0.39–0.11) | <0.001 | 0.62 (0.52–0.71) | <0.001 |
| Dependent Variable | Independent Variable | Coefficient | p Valor | OR (CI 95%) |
|---|---|---|---|---|
| Mortality | PEF%pred | 1.09 | <0.001 | 2.99 (2.05–4.34) |
| MIP | 0.02 | <0.001 | 1.02 (1.01–1.03) | |
| SNIP | 0.01 | 0.001 | 1.01 (1.00–1.01) | |
| Constant | 0.42 | 0.42 | - | |
| ALSFRS-R | PEF%pred | −3.11 | 0.299 | 0.04 (0.00–15.64) |
| SNIP | 0.19 | <0.001 | 1.21 (1.11–1.31) | |
| MIP | 0.07 | 0.038 | 1.07 (1.00–1.15) | |
| Constant | 20.22 | <0.001 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciel, A.C.d.M.G.; Resqueti, V.R.; Costa, L.M.d.; Silva, A.A.M.d.; Fonseca, J.D.M.d.; Vieira, R.G.d.S.; Pondofe, K.d.M.; Otto-Yáñez, M.; Vilaró, J.; Torres-Castro, R.; et al. The Impact of Respiratory Function on Functionality and Mortality in ALS Patients. J. Clin. Med. 2025, 14, 6702. https://doi.org/10.3390/jcm14196702
Maciel ACdMG, Resqueti VR, Costa LMd, Silva AAMd, Fonseca JDMd, Vieira RGdS, Pondofe KdM, Otto-Yáñez M, Vilaró J, Torres-Castro R, et al. The Impact of Respiratory Function on Functionality and Mortality in ALS Patients. Journal of Clinical Medicine. 2025; 14(19):6702. https://doi.org/10.3390/jcm14196702
Chicago/Turabian StyleMaciel, Ana Cristina de Medeiros Garcia, Vanessa Regiane Resqueti, Lariza Maria da Costa, Ana Aline Marcelino da Silva, Jéssica Danielle Medeiros da Fonseca, Rayane Grayce da Silva Vieira, Karen de Medeiros Pondofe, Matías Otto-Yáñez, Jordi Vilaró, Rodrigo Torres-Castro, and et al. 2025. "The Impact of Respiratory Function on Functionality and Mortality in ALS Patients" Journal of Clinical Medicine 14, no. 19: 6702. https://doi.org/10.3390/jcm14196702
APA StyleMaciel, A. C. d. M. G., Resqueti, V. R., Costa, L. M. d., Silva, A. A. M. d., Fonseca, J. D. M. d., Vieira, R. G. d. S., Pondofe, K. d. M., Otto-Yáñez, M., Vilaró, J., Torres-Castro, R., Vera-Uribe, R., Ribeiro-Samora, G. A., Nagem, D., Valentim, R. A., Dourado Júnior, M. E. T., & Fregonezi, G. (2025). The Impact of Respiratory Function on Functionality and Mortality in ALS Patients. Journal of Clinical Medicine, 14(19), 6702. https://doi.org/10.3390/jcm14196702









