Subchorionic Placental Cyst in a Woman with Fetal Growth Restriction: A Case Report and Review of the Literature
Abstract
1. Introduction
2. Case Report
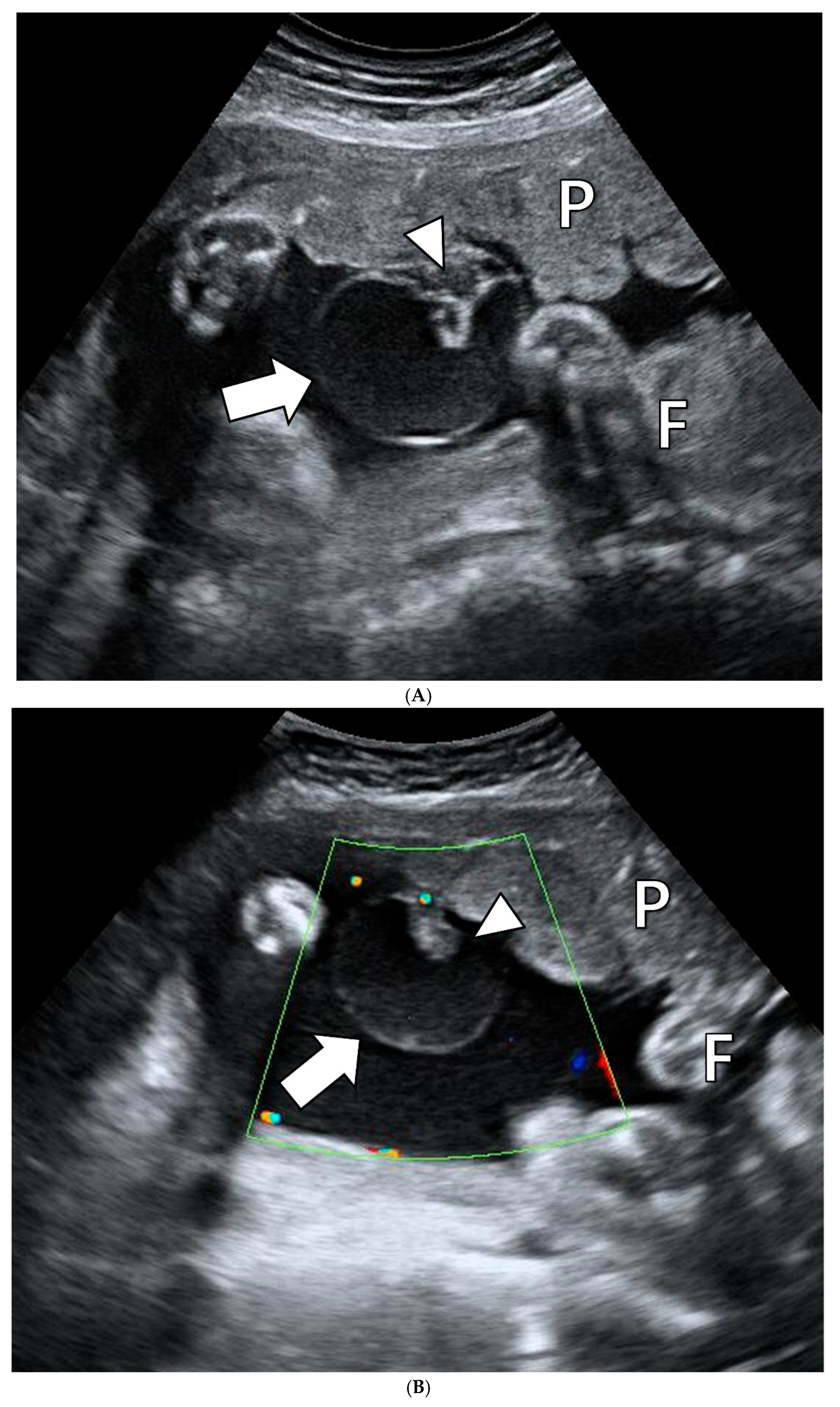
2.1. Clinical Presentation
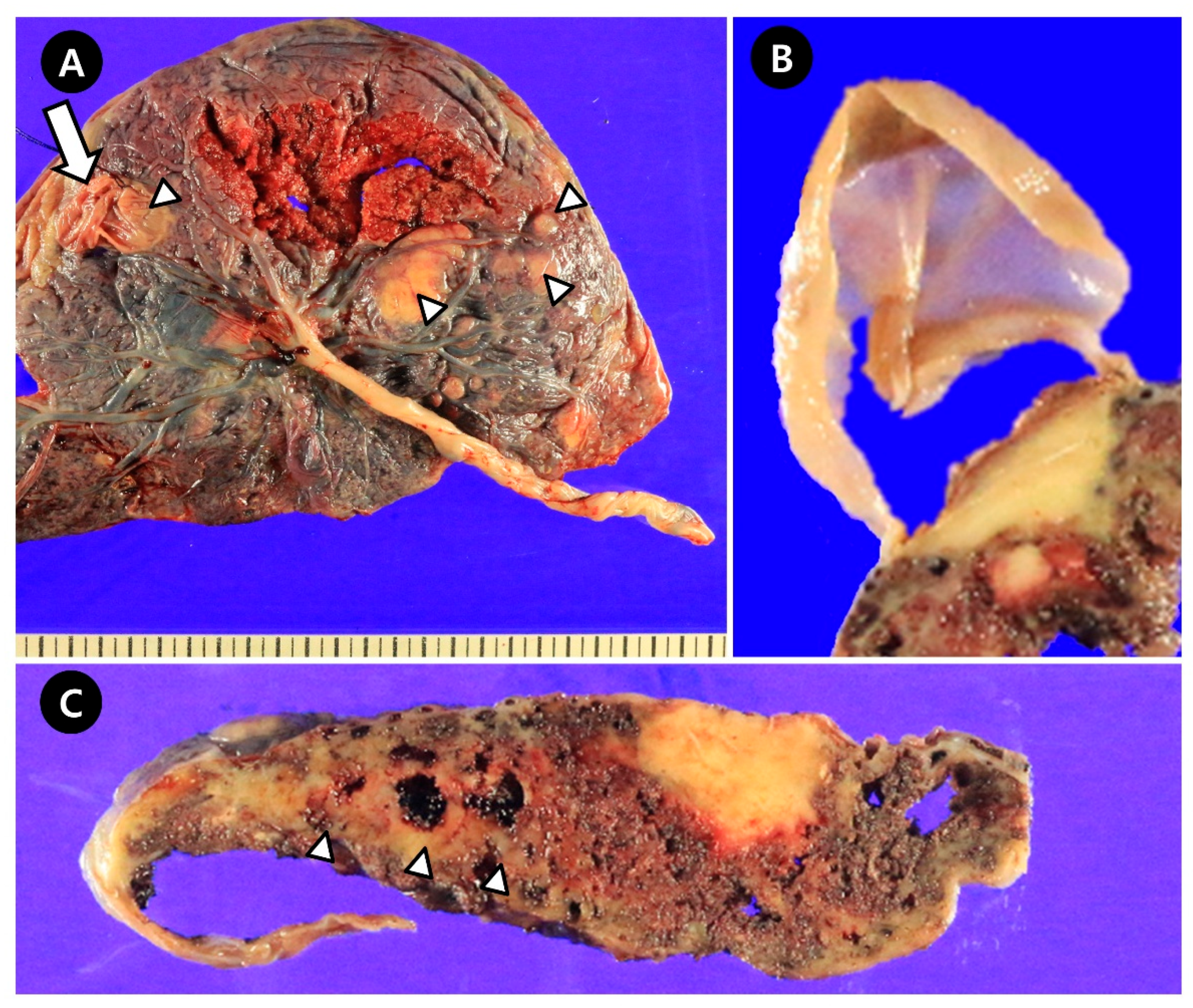
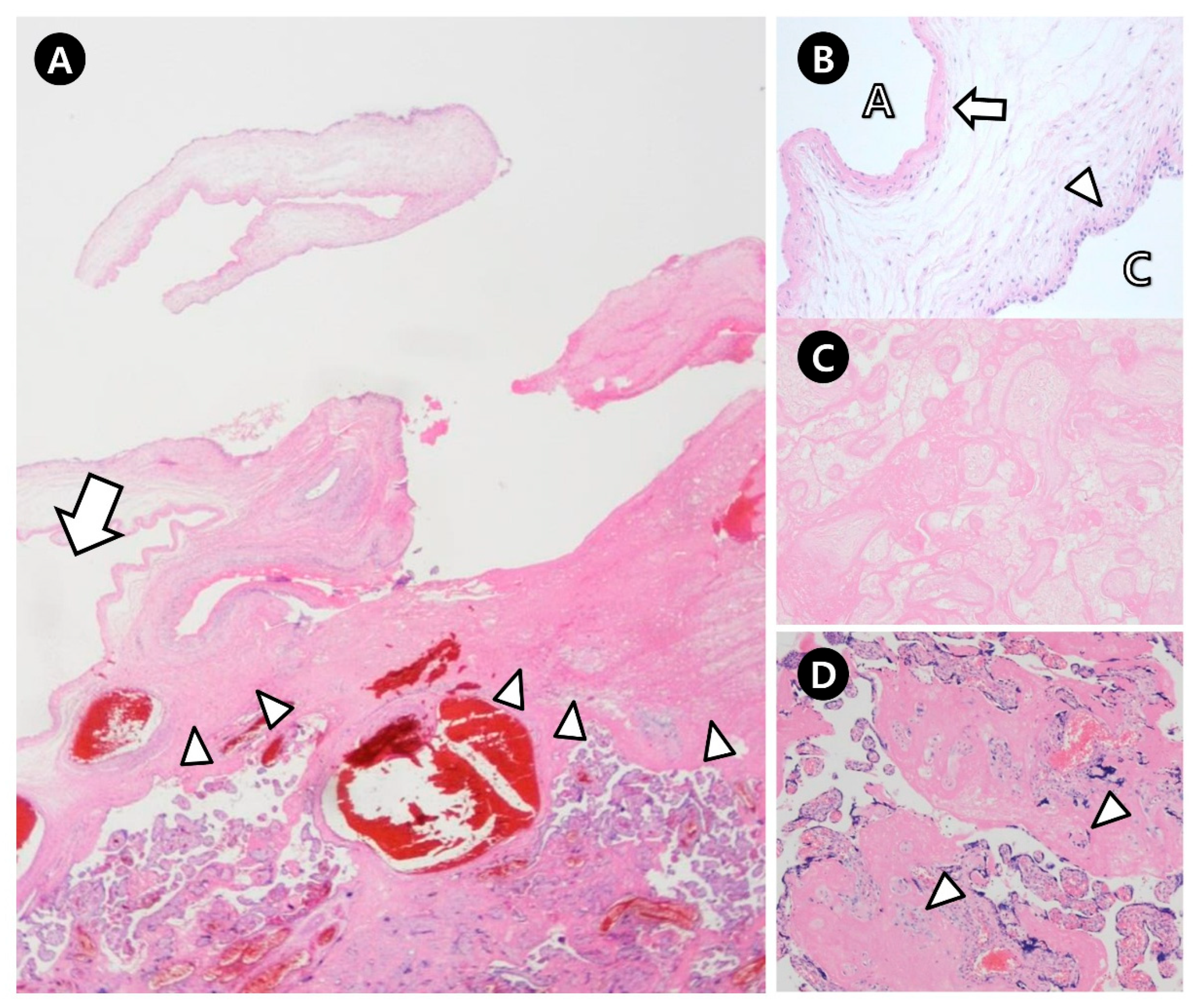
2.2. Pathologic Findings
3. Discussion
3.1. Patient Characteristics and Ultrasonographic Findings of SPCs
3.2. Differential Diagnosis of SPCs
3.3. Etiology of SPCs
3.4. Placental Hypoxia and Infarction in Cases with SPCs
3.5. Relationship Between the Size, Number, and Location of SPCs and Adverse Pregnancy Outcomes
3.6. Placental Weight in Cases with SPCs
3.7. Adverse Pregnancy Outcomes in Cases with SPCs
3.8. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulis, T.S.; Rochelson, B.L.; Williamson, A.K. Massive Subchorionic Placental Cyst and Poor Fetal Growth: A Case Report. J. Reprod. Med. 2015, 60, 458–460. [Google Scholar]
- Witters, I.; Sieprath, P.; Van Holsbeke, C.; Theyskens, C.; Deraedt, K. Prenatal diagnosis of multiple large subchorionic placental cysts with intracystic hemorraghe. Facts Views Vis. Obgyn. 2018, 9, 223–225. [Google Scholar]
- Lin, C.J.; Su, C.F.; Tsai, H.J.; Lee, C.K. A large subchorionic placental cyst with thalassemia minor without fetal growth restriction. Taiwan J. Obstet. Gynecol. 2018, 57, 161–162. [Google Scholar] [CrossRef]
- Brown, D.L.; DiSalvo, D.N.; Frates, M.C.; Davidson, K.M.; Genest, D.R. Placental surface cysts detected on sonography: Histologic and clinical correlation. J. Ultrasound Med. 2002, 21, 641–646; quiz 647–648. [Google Scholar] [CrossRef]
- Vernof, K.K.; Benirschke, K.; Kephart, G.M.; Wasmoen, T.L.; Gleich, G.J. Maternal floor infarction: Relationship to X cells, major basic protein, and adverse perinatal outcome. Am. J. Obstet. Gynecol. 1992, 167, 1355–1363. [Google Scholar] [CrossRef]
- Stolla, M.; Refaai, M.A.; Conley, G.; Spinelli, S.; Casey, A.; Katerji, H.; McRae, H.L.; Blumberg, N.; Phipps, R.; Metlay, L.A.; et al. Placental Chorionic Cyst Fluid Has Prothrombotic Properties and Differs from Amniotic Fluid. Pediatr. Dev. Pathol. 2019, 22, 304–314. [Google Scholar] [CrossRef]
- Kirkinen, P.; Jouppila, P. Intrauterine membranous cyst: A report of antenatal diagnosis and obstetric aspects in two cases. Obstet. Gynecol. 1986, 67, 26s–30s. [Google Scholar] [CrossRef]
- Raga, F.; Ballester, M.J.; Osborne, N.G.; Bonilla-Musoles, F. Subchorionic placental cyst: A cause of fetal growth retardation--ultrasound and color-flow Doppler diagnosis and follow-up. J. Natl. Med. Assoc. 1996, 88, 285–288. [Google Scholar] [PubMed]
- Ferrara, N.; Menditto, C.; Di Marino, M.P.; Ciccarelli, A.; Gerosolima, G.; Menditto, V. Subchorionic placental cyst: Histopathological and clinical aspects in two cases. Pathologica 1996, 88, 439–443. [Google Scholar] [PubMed]
- Tam, W.H.; Fung, H.Y.; Fung, T.Y.; Lau, T.K.; To, K.F. Intra-uterine growth retardation and transverse lie due to massive subchorionic thrombohematoma and overlying large subchorionic cyst. Acta Obstet. Gynecol. Scand. 1997, 76, 381–383. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Luis, J.; Oneson, R.H.; Santolaya-Forgas, J. Placental surface cyst with contents less echogenic than amniotic fluid on a second-trimester ultrasonographic evaluation. Ultrasound Obstet. Gynecol. 2004, 23, 627–628. [Google Scholar] [CrossRef]
- Hong, S.C.; Yoo, S.W.; Kim, T.; Yeom, B.W.; Kim, Y.T.; Lee, K.W.; Kim, S.H. Prenatal diagnosis of a large subchorionic placental cyst with intracystic hematomas: A case report. Fetal Diagn. Ther. 2007, 22, 259–263. [Google Scholar] [CrossRef]
- Shimada, T.; Inoue, T.; Abe, S.; Hiraki, K.; Miura, K.; Hayashi, T.; Masuzaki, H. Placental multiple chorionic cysts in maternal scleroderma. J. Med. Ultrason. 2010, 37, 209–212. [Google Scholar] [CrossRef]
- Soyama, H.; Miyamoto, M.; Sasa, H.; Yoshida, M.; Takano, M.; Furuya, K. Multiple placental surface cysts with intracystic hemorrhaging: A case report and review of the literature. J. Obstet. Gynaecol. Res. 2017, 43, 1346–1349. [Google Scholar] [CrossRef]
- Chansriniyom, N.; Romero, R.; Warintaksa, P.; Meyyazhagan, A.; Promsonthi, P.; Singhsnaeh, A.; Chaemsaithong, P. Prenatal diagnosis of subamniotic placental cysts. Am. J. Obstet. Gynecol. 2025, 233, 135.e1–135.e7. [Google Scholar] [CrossRef] [PubMed]
- Almog, B.; Shehata, F.; Aljabri, S.; Levin, I.; Shalom-Paz, E.; Shrim, A. Placenta weight percentile curves for singleton and twins deliveries. Placenta 2011, 32, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.A.; Kouba, I.; Stefanov, D.G.; Jackson, F.I.; Mansoor, S.; Aloysius, S.P.; O’Sullivan-Bakshi, T.; Mccalla, B.; Bracero, L.A.; Blitz, M.J. Presence and Size of Placental Lakes on 20-Week Fetal Anatomy Ultrasound and Obstetrical Outcomes. J. Obstet. Gynaecol. Can. 2024, 46, 102458. [Google Scholar] [CrossRef]
- Na, E.D.; Lee, K.J.; Kim, M.J.; Yi, H.J.; Kim, J.Y. Placental mesenchymal dysplasia associated with severe preeclampsia: A case report Korean. J. Obstet. Gynecol. 2011, 54, 459–463. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Bloom, S.L.; Dashe, J.S.; Spong, C.Y.; Hoffman, B.L.; Casey, B.M. Williams Obstetrics, 25th ed.; McGraw Hill: São Paulo, Brazil, 2018. [Google Scholar]
- Kim, E.N.; Lee, J.Y.; Shim, J.Y.; Hwang, D.; Kim, K.C.; Kim, S.R.; Kim, C.J. Clinicopathological characteristics of miscarriages featuring placental massive perivillous fibrin deposition. Placenta 2019, 86, 45–51. [Google Scholar] [CrossRef]
- Parks, W.T. Placental hypoxia: The lesions of maternal malperfusion. Semin. Perinatol. 2015, 39, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Facchinetti, F.; Yin, H.; Saade, G.R.; Longo, M. Single-nucleotide polymorphisms in genes involved in placental function and unexplained stillbirth. Am. J. Obstet. Gynecol. 2012, 207, 316.e1–316.e7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.P.; Arcot, M.; Munisamaiah, M.; Balakrishna, S. Novel association of SNP rs479200 in EGLN1 gene with predisposition to preeclampsia. Gene 2019, 705, 1–4. [Google Scholar] [CrossRef] [PubMed]
| Case No. | Age (Years) | GA at Diagnosis (Weeks) | Cyst Size (cm) 1 | Number of Cysts 2 | Weight of the Placenta (g) 3 |
|---|---|---|---|---|---|
| 1 [7] | 39 | 30 | 5 | 1 | 530 |
| 2 [7] | 38 | 32 | 8 | 1 | 370 4 |
| 3 [5] | 29 | Postpartum | 4 | Multiple (>5) | 385 4 |
| 4 [8] | 28 | 12 | 7 | 1 | 480 4 |
| 5 [9] | 31 | 36 | 6 | 1 | 435 4 |
| 6 [9] | 22 | 16 | 12 | 1 | 450 4 |
| 7 [10] | 35 | 16 | 8 | 1 | 390 4 |
| 8 [11] | 31 | 26 | 4.6 | 1 | 619 |
| 9 [12] | 35 | <31 | 7.2 | 1 | 450 4 |
| 10 [13] | 36 | 24 | 3 | Multiple (>3) | 320 |
| 11 [1] | 29 | 72/7 | 20 | 1 | 383 4 |
| 12 [14] | 29 | 26 | 7.7 | 5 | 592 |
| 13 [2] | 37 | 26 | 6 | 2 | 513 |
| 14 [3] | 33 | 28 | 9 | 1 | 800 |
| 15 [15] | NA | 22 | 5.8 | Multiple (>5) | 330 |
| 16 (present case) | 40 | 353/7 | 4.1 | 1 | 421 4 |
| Case No. | GA at Delivery (Weeks) | FGR 1 | Delivery Mode | Birth Weight (g, Percentile) 2 | Adverse Obstetrical Findings |
|---|---|---|---|---|---|
| 1 [7] | 376/7 | Yes | VD | 2540 (9–14) | Abnormal Doppler ultrasound: Absent end diastolic velocity in UA and in the fetal descending aorta, and reduced velocity in the fetal umbilical vein, Preterm labor |
| 2 [7] | 39 | No | VD | 3250 (40) | PIH |
| 3 [5] | 346/7 | Yes | CS | 1325 (<1) | Previous cesarean section due to fetal distress with FGR and placenta previa, Non-reassuring fetal status |
| 4 [8] | 37 | Yes | CS | 2100 (4) | Non-reassuring fetal status, Abnormal Doppler ultrasound: Low impedance in MCA (RI: 0.61), higher impedance in UA (RI: 0.78), and decreased umbilical/cerebral index (0.88) |
| 5 [9] | 395/7 | No | VD | 3250 (29) | None |
| 6 [9] | 39 | No | CS | 2950 (27) | None |
| 7 [10] | 34 | Yes | CS | 1290 (1) | Oligohydramnios, Amniocentesis: 45XY, t(13q,14q) |
| 8 [11] | >37 | No | VD | 3660 (95) | None |
| 9 [12] | 37 | No | CS | 3330 (85) | Non-reassuring fetal status |
| 10 [13] | 29 | No | CS | 1092 (41) | Non-reassuring fetal status, Short cervix (2 cm) |
| 11 [1] | 36 | No | CS | 2585 (48) | None |
| 12 [14] | 32 | Yes | CS | 1389 (21) | Previous one spontaneous abortion, and one CS due to IUFD caused by placental abruption, Preterm labor, Placental abruption |
| 13 [2] | 34 | Yes | CS | 1850 (25) | None |
| 14 [3] | 37 | No | CS | 2950 (60) | None |
| 15 [15] | 294/7 | Yes | CS | 730 (5%) | Non-reassuring fetal status, Abnormal Doppler ultrasound: A reversed A-wave in the ductus venosus, an elevated peak systolic velocity of MCA (1.58 multiples of the median), and persistent reversed end-diastolic velocities in UA, Single umbilical artery, Neonatal anemia |
| 16 (present case) | 366/7 | Yes | CS | 1240 (<1) | 6 previous spontaneous abortions, Non-reassuring fetal status, Abnormal Doppler ultrasound: Increased PI in UA (PI: 2.01), RI in UA (0.99), Decreased S/D ratio (117.09), Preterm labor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Jang, M.-H.; Koo, Y.-J.; Lee, D.-H. Subchorionic Placental Cyst in a Woman with Fetal Growth Restriction: A Case Report and Review of the Literature. J. Clin. Med. 2025, 14, 6698. https://doi.org/10.3390/jcm14196698
Kim H-S, Jang M-H, Koo Y-J, Lee D-H. Subchorionic Placental Cyst in a Woman with Fetal Growth Restriction: A Case Report and Review of the Literature. Journal of Clinical Medicine. 2025; 14(19):6698. https://doi.org/10.3390/jcm14196698
Chicago/Turabian StyleKim, Hyo-Shin, Min-Hye Jang, Yu-Jin Koo, and Dae-Hyung Lee. 2025. "Subchorionic Placental Cyst in a Woman with Fetal Growth Restriction: A Case Report and Review of the Literature" Journal of Clinical Medicine 14, no. 19: 6698. https://doi.org/10.3390/jcm14196698
APA StyleKim, H.-S., Jang, M.-H., Koo, Y.-J., & Lee, D.-H. (2025). Subchorionic Placental Cyst in a Woman with Fetal Growth Restriction: A Case Report and Review of the Literature. Journal of Clinical Medicine, 14(19), 6698. https://doi.org/10.3390/jcm14196698






