Combined Liquid-Based Cytology and Conventional Smear Provides Better Sensitivity and Adequacy Rates After Endoscopic Ultrasound-Guided Tissue Acquisition of Abdominal Masses: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process
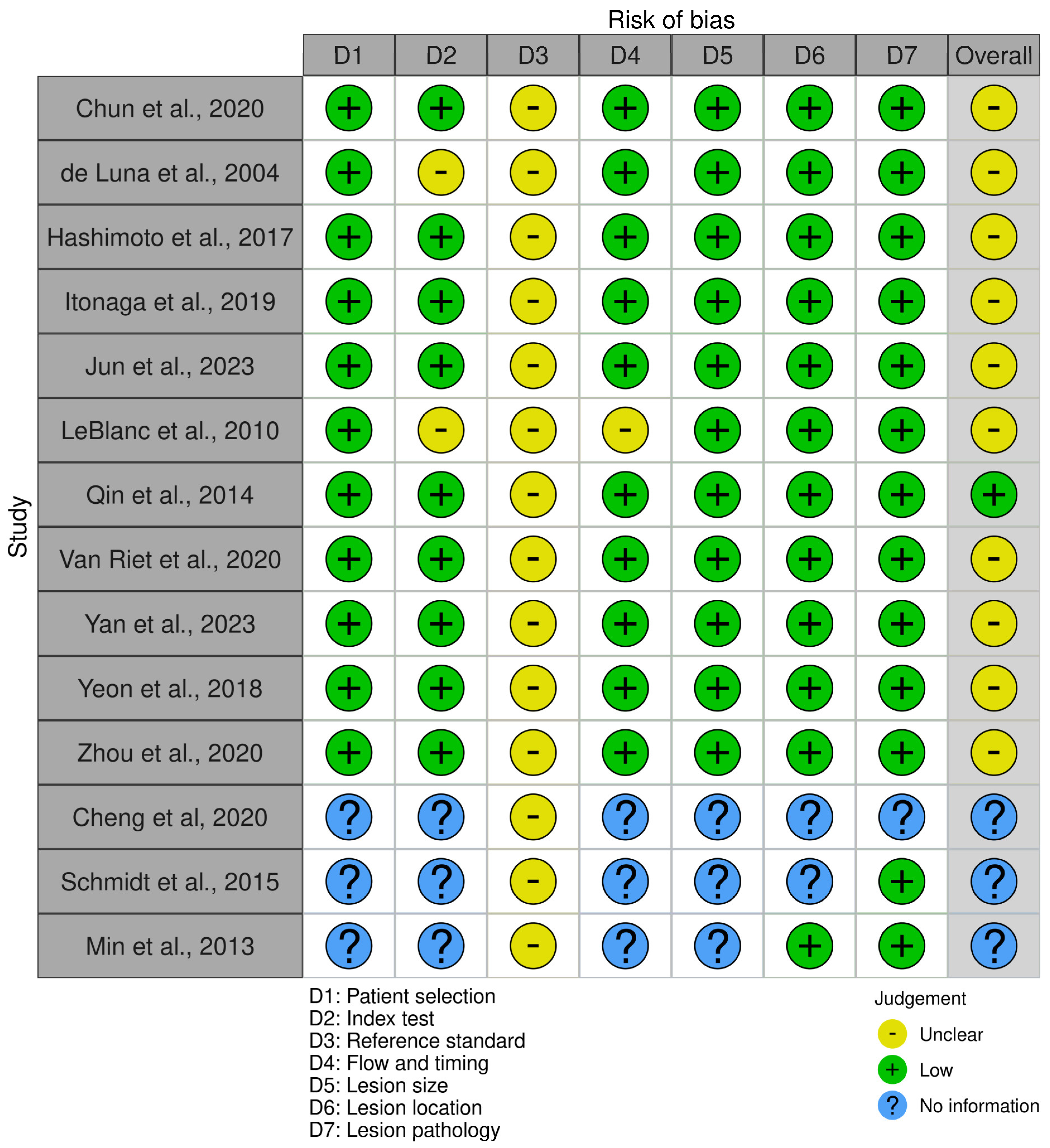
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
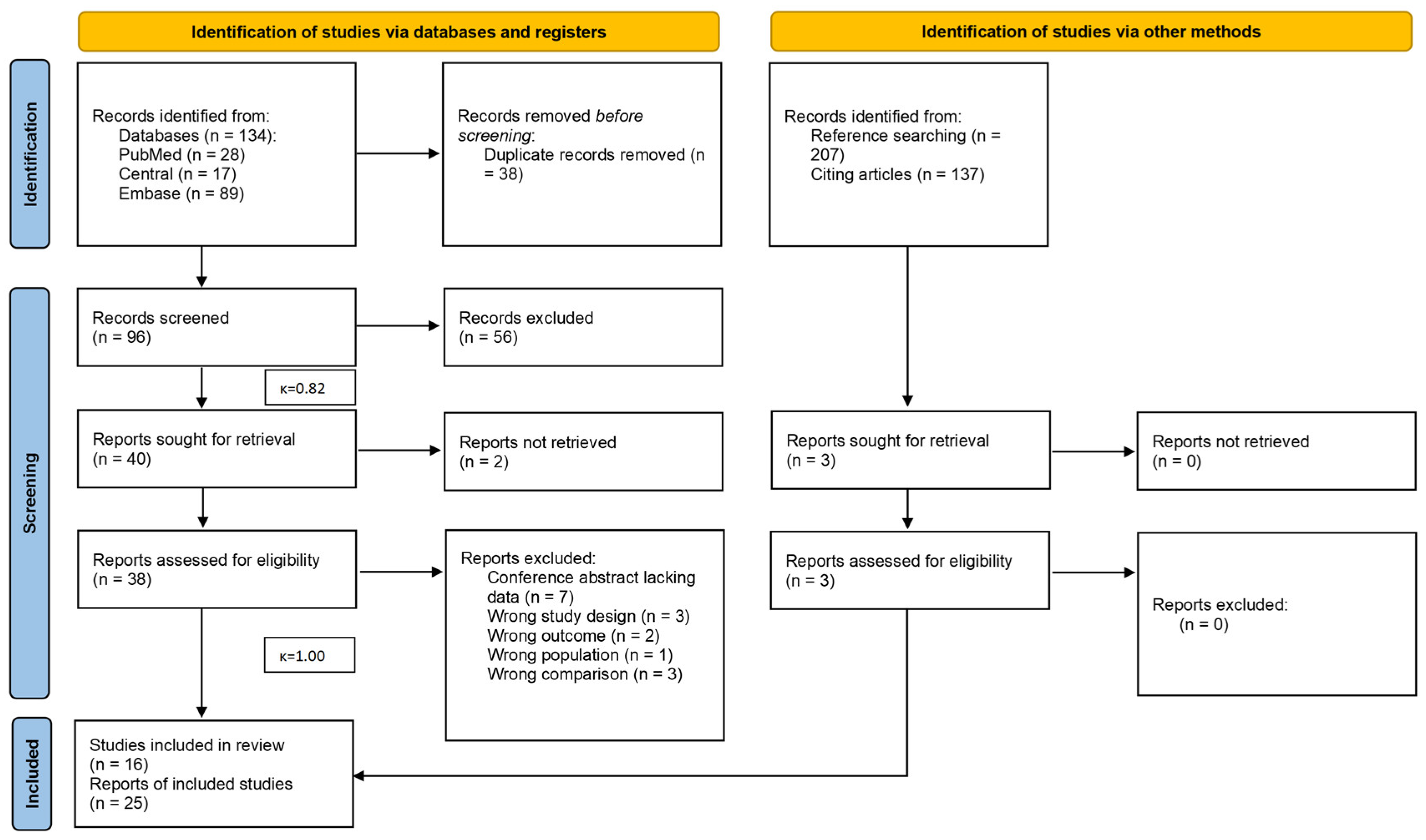
3.1. Search and Selection
3.2. Basic Characteristics of Included Studies
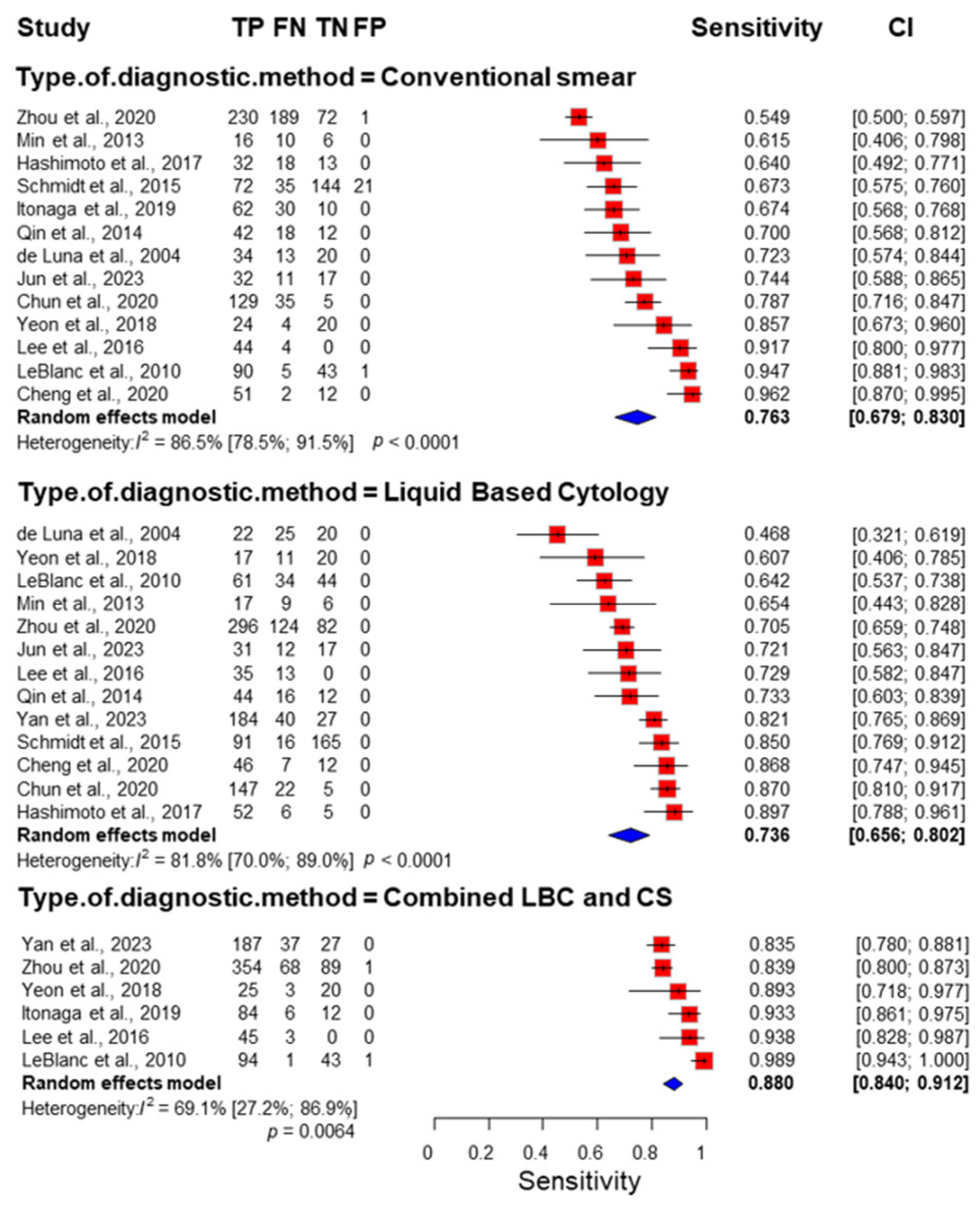
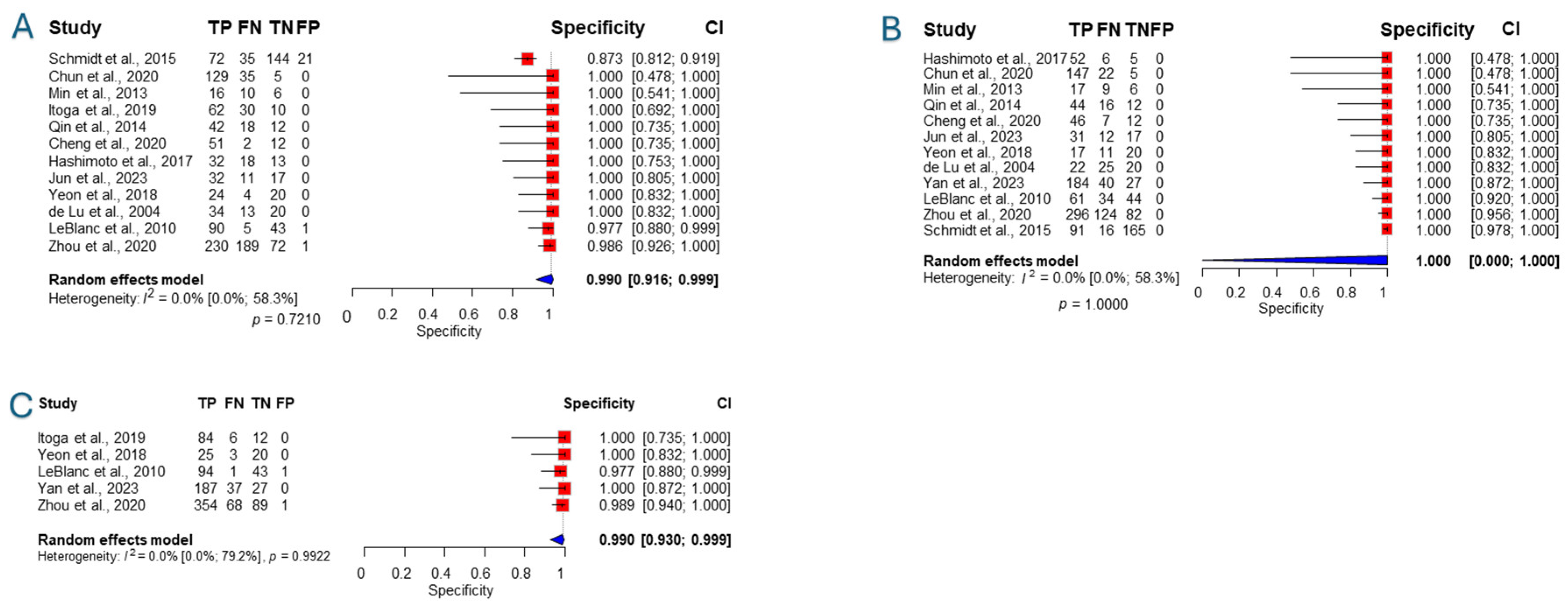
3.3. Sensitivity and Specificity
3.4. Accuracy
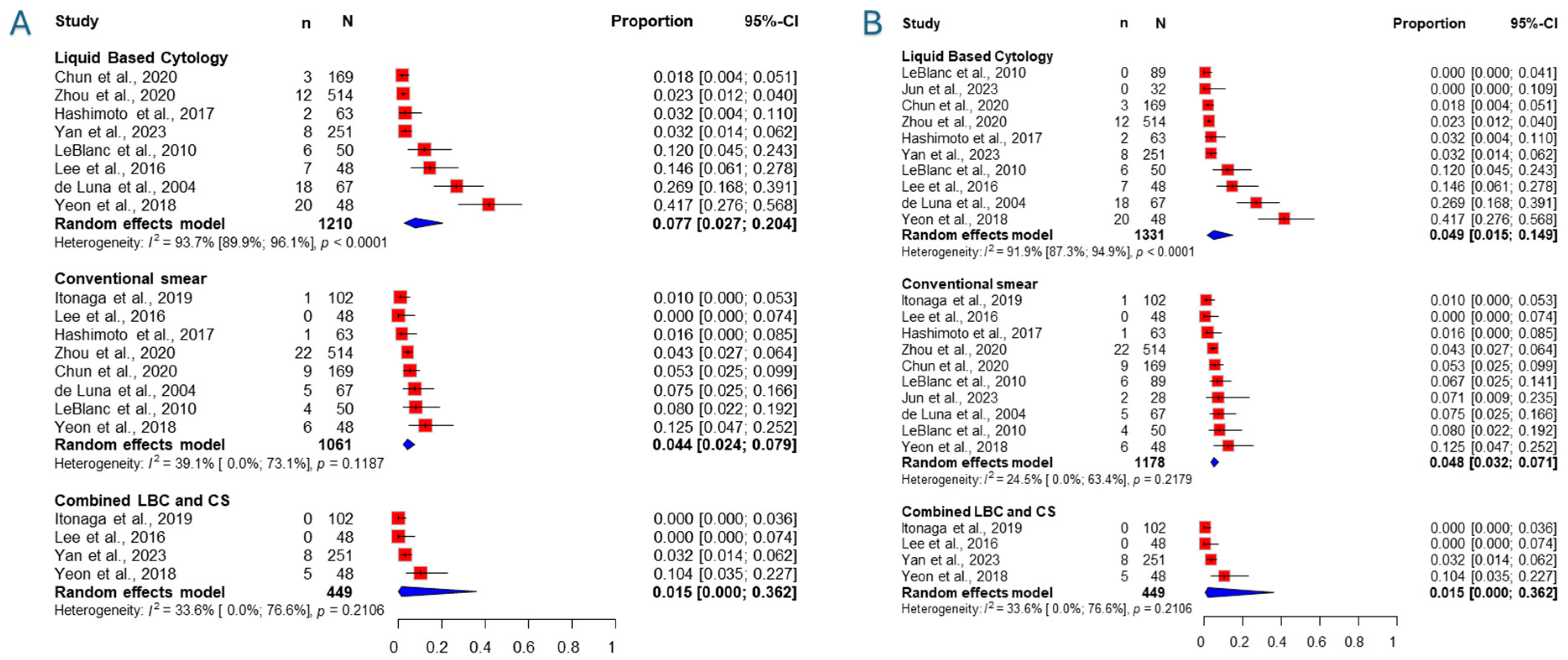
3.5. Inadequacy Rate
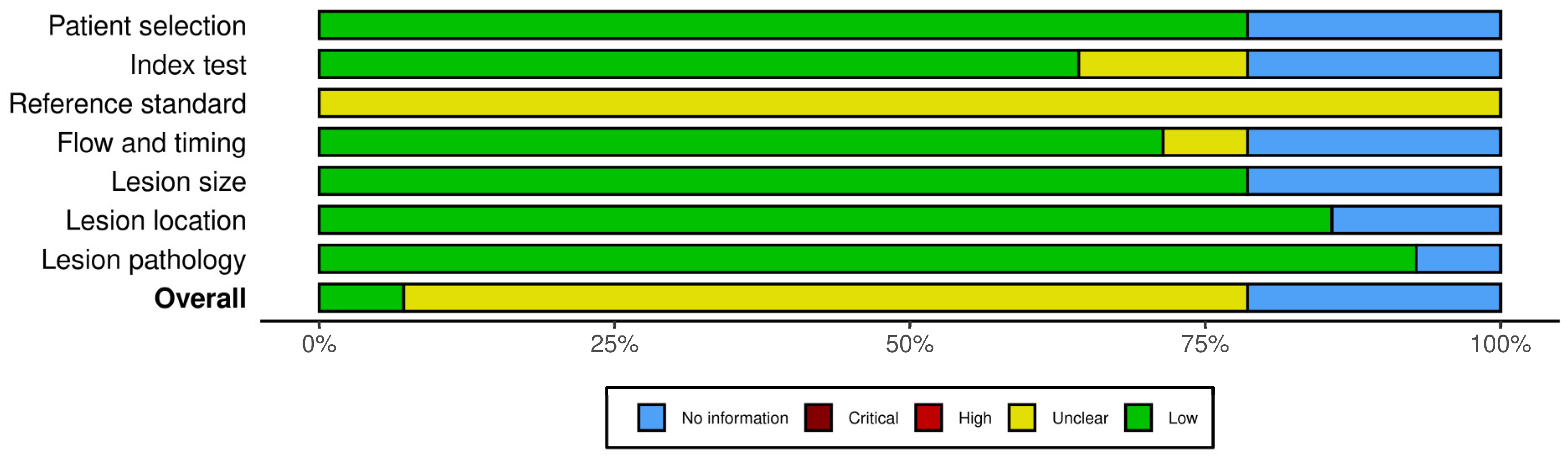
3.6. Risk of Bias Assessment
3.7. Publication Bias and Heterogeneity
4. Discussion
4.1. Strengths and Limitations
4.2. Implication for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O'Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Pan, C.Y.; Wang, S.M.; Cai, D.H.; Ma, J.Y.; Li, S.Y.; Guo, Y.; Jing, S.; Zhendong, J.; Wang, K. Adverse events of 20–22G second-generation endoscopic ultrasound-guided fine-needle biopsy needles for solid lesions in the upper gastrointestinal tract and adjacent organs: Systematic review and meta-analysis. Dig. Endosc. 2025, 37, 490–500. [Google Scholar] [CrossRef]
- Engh, M.A.; Teutsch, B.; Schulze Wenning, A.; Hadani, Y.; Almog, O.; Veres, D.S.; Hegyi, P.; Erőss, B. Contrast-enhanced endoscopic ultrasound likely does not improve diagnostic adequacy during endoscopic ultrasound guided tissue acquisition: A systematic review and meta-analysis. Pancreatology 2024, 24, 649–660. [Google Scholar] [CrossRef]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848.e5. [Google Scholar] [CrossRef]
- Chandan, S.; Mohan, B.P.; Khan, S.R.; Ofosu, A.; Dhaliwal, A.S.; Shah, A.R.; Bhogal, N.; Mashiana, H.S.; Mashiana, S.S.; Kassab, L.L.; et al. Comparison of EUS-guided conventional smear and liquid-based cytology in pancreatic lesions: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E1611–E1622. [Google Scholar] [CrossRef]
- Pan, H.H.; Zhou, X.X.; Zhao, F.; Chen, H.Y.; Zhang, Y. Diagnostic value of liquid-based cytology and smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration: A meta-analysis. World J. Clin. Cases 2020, 8, 3006–3020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Ma, S.Y.; Liu, N.; Wei, Z.C.; Gao, X.; Hao, Y.J.; Liu, Y.X.; Cai, Y.Q.; Wang, J.H. Comparison of smear cytology with liquid-based cytology in pancreatic lesions: A systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 3308–3319. [Google Scholar] [CrossRef]
- Pouw, R.E.; Barret, M.; Biermann, K.; Bisschops, R.; Czakó, L.; Gecse, K.B.; de Hertogh, G.; Hucl, T.; Iacucci, M.; Jansen, M.; et al. Endoscopic tissue sampling—Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Park, S.W.; Kim, S.H.; Cho, C.M.; Choi, J.H.; Choi, E.K.; Lee, T.H.; Cho, E.; Lee, J.K.; Song, T.J.; et al. Clinical and Technical Guideline for Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition of Pancreatic Solid Tumor: Korean Society of Gastrointestinal Endoscopy (KSGE). Gut Liver 2021, 15, 354–374. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef]
- Pitman, M.B.; Centeno, B.A.; Ali, S.Z.; Genevay, M.; Stelow, E.; Mino-Kenudson, M.; Castillo, C.F.; Schmidt, C.M.; Brugge, W.R.; Layfield, L.J. Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology guidelines. Diagn. Cytopathol. 2014, 42, 338–350. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.C.; Kerby, C.R.; Patel, A.; Cooper, N.J.; Quinn, T.; Sutton, A.J. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med. Res. Methodol. 2019, 19, 81. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Cole, S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006, 59, 1331–1332; author reply 1332–1333. [Google Scholar] [CrossRef]
- Pustejovsky, J.E.; Tipton, E. Meta-analysis with Robust Variance Estimation: Expanding the Range of Working Models. Prev. Sci. 2022, 23, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Stijnen, T.; Hamza, T.H.; Ozdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010, 29, 3046–3067. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef]
- Cheng, G.; Liang, Y.; Hu, D. Diagnostic efficacy of different pathological methods of tissue samples obtained by EUS-FNA: A prospective study. Digestion 2020, 102, 99. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, E.B.; Kim, K.; Oh, Y.; Yoon, S.M.; Chae, H.B.; Park, S.M.; Youn, S.J.; Han, J.H. Comparison of liquid-based cytology (CellPrepPlus®) and conventional smears in pancreaticobiliary disease. J. Gastroenterol. Hepatol. 2016, 31, 247. [Google Scholar] [CrossRef]
- Chun, J.W.; Lee, K.; Lee, S.H.; Kim, H.; You, M.S.; Hwang, Y.J.; Paik, W.H.; Ryu, J.K.; Kim, Y.T. Comparison of liquid-based cytology with conventional smear cytology for EUS-guided FNA of solid pancreatic masses: A prospective randomized noninferiority study. Gastrointest. Endosc. 2020, 91, 837–846.e1. [Google Scholar] [CrossRef]
- Chun, J.W.; Lee, K.; You, M.S.; Hwang, Y.J.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T.; Kim, H.; Lee, S.H. Comparison of Liquid-Based Cytology with Conventional Smear Cytology for Endoscopic Ultrasound-Guided Fine Needle Aspiration of Solid Pancreatic Masses: Prospective Randomized Noninferiority Study. Gut Liver 2019, 13, 19. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02661096/full (accessed on 17 November 2024).
- Chun, J.W.; Lee, S.H.; Kim, H.; Huh, G.; You, M.S.; Hwang, Y.J.; Lee, K.; Lee, K.; Paik, W.-H.; Ryu, J.-K.; et al. A randomised prospective comparison of liquid-based cytology with conventional smear cytology for endoscopic ultrasound-guided fine needle aspiration of solid pancreatic masses. United Eur. Gastroenterol. J. 2019, 7, 119–120. [Google Scholar]
- Han, J.H.; Park, B.K.; Seo, J.H.; Park, Y.; Jeong, H.S.; Yeon, M.H.; Park, S.M.; Lee, H.C. Comparison of liquid-based cytology (CellPrepPlus®) and conventional smears in pancreaticobiliary cytology. Pancreatology 2017, 17, S114. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L620414653&from=export (accessed on 17 November 2024). [CrossRef]
- Hashimoto, S.; Taguchi, H.; Higashi, M.; Fujita, T.; Arima, S.; Nakazawa, J.; Iwaya, H.; Iwashita, Y.; Sasaki, F.; Nasu, Y.; et al. Diagnostic efficacy of liquid-based versus smear cytology for pancreatic solid lesions obtained by endoscopic ultrasound-guided fine-needle aspiration. United Eur. Gastroenterol. J. 2015, 3, A571. [Google Scholar] [CrossRef]
- Hashimoto, S.; Taguchi, H.; Higashi, M.; Hatanaka, K.; Fujita, T.; Iwaya, H.; Nakazawa, J.; Arima, S.; Iwashita, Y.; Sasaki, F.; et al. Diagnostic efficacy of liquid-based cytology for solid pancreatic lesion samples obtained with endoscopic ultrasound-guided fine-needle aspiration: Propensity score-matched analysis. Dig. Endosc. 2017, 29, 608–616. [Google Scholar] [CrossRef]
- Itonaga, M.; Murata, S.I.; Hatamaru, K.; Tamura, T.; Nuta, J.; Kawaji, Y.; Maekita, T.; Iguchi, M.; Kato, J.; Kojima, F.; et al. Diagnostic efficacy of smear plus liquid-based cytology for EUS-FNA of solid pancreatic lesions: A propensity-matched study. Medicine 2019, 98, e15575. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, M.; Nuta, J.; Tamura, T.; Kitano, M. Diagnostic efficacy of combination smear cytology and liquid-based cytology in EUS-guided fine needle aspiration of pancreatic solid lesions. Gastrointest. Endosc. 2017, 85, AB334–AB335. [Google Scholar] [CrossRef]
- Jiang, H.; Qin, S.Y.; Tao, L.; Luo, W.; Su, S.-B.; Lu, X.-P.; Lei, R.-E. Diagnostic efficiency of cell-block with immunostaining, smear cytology, liquid-based cytology in EUS-FNA on pancreatic lesions: An institution’s experience. United Eur. Gastroenterol. J. 2014, 2, A156–A157. [Google Scholar] [CrossRef][Green Version]
- Jun, J.C.; Lee, S.H.; Lee, H.M.; Kim, S.G.; Chung, H.; Kim, J.S.; Park, N.; Choi, J.H.; Kwak, Y.; Cho, S.J. A prospective randomized noninferiority trial comparing conventional smears and SurePathTM liquid-based cytology in endoscopic ultrasound-guided sampling of esophageal, gastric, and duodenal lesions. Medicine 2023, 102, e34321. [Google Scholar] [CrossRef]
- Lee, S.H.; Chun, J.W.; Huh, G.; You, M.S.; Ahn, D.W.; Paik, W.H.; Ryu, J.K.; Kim, Y.T.; Kim, H.; Lee, K.; et al. A randomized prospective comparison of surepath with conventional smear cytology for endoscopic ultrasound-guided fine needle aspiration of solid pancreatic masses. Pancreas 2019, 48, 1472. [Google Scholar] [CrossRef]
- Min, C.; Xing, J. Cancer (IPMN, pancreas cancer, cystic neoplasms) Evaluation of endoscopic ultrasound-guided fine-needle aspiration biopsy for pancreatic lesions. J. Gastroenterol. Hepatol. 2013, 28, 880. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, H. Cell block technique and cytological smears for pancreatic neoplasms after EUS-FNA. J. Gastroenterol. Hepatol. 2013, 28, 250–251. [Google Scholar] [CrossRef]
- Qin, S.Y.; Jiang, H.X.; Zhang, X.L.; Yang, X.W.; Lei, R.E. Evaluation of endoscopic ultrasound-guided fine-needle aspiration biopsy of cell block with immunostaining for panceatic lesions. J. Dig. Dis. 2014, 15, 109. Available online: https://www.embase.com/records?subaction=viewrecord&id=L71763870 (accessed on 17 November 2024).
- Qin, S.Y.; Zhou, Y.; Li, P.; Jiang, H.X. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: A single-institution experience. PLoS ONE 2014, 9, e108762. [Google Scholar] [CrossRef]
- Schmidt, C.; Besser, A.; Petersen, I.; Stallmach, A. Increased accuracy of FNA based cytological diagnosis of solid pancreatic lesions by use of an ethanol based fixative system. Gastrointest. Endosc. 2015, 81, AB537. [Google Scholar] [CrossRef]
- van Riet, P.A.; Quispel, R.; Cahen, D.L.; Snijders-Kruisbergen, M.C.; van Loenen, P.; Erler, N.S.; Poley, J.W.; van Driel, L.M.J.W.; Mulder, S.A.; Veldt, B.J. Diagnostic yield and agreement on fine-needle specimens from solid pancreatic lesions: Comparing the smear technique to liquid-based cytology. Endosc. Int. Open 2020, 8, E155–E162. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, G.; Ji, J.; Gui, Y.; Chang, X.; Zhang, J.; Lv, K.; Tan, L. Evaluation of the diagnostic efficacy of liquid-based cytology obtained via percutaneous ultrasound-guided fine-needle aspiration for pancreatic masses: A large tertiary center’s 8-year experience. J. Cancer Res. Clin. Oncol. 2023, 149, 17189–17197. [Google Scholar] [CrossRef]
- Yeon, M.H.; Jeong, H.S.; Lee, H.S.; Jang, J.S.; Lee, S.; Yoon, S.M.; Chae, H.B.; Park, S.M.; Youn, S.J.; Han, J.H.; et al. Comparison of liquid-based cytology (CellPrepPlus) and conventional smears in pancreaticobiliary disease. Korean J. Intern. Med. 2018, 33, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, L.; Wang, S.M.; Li, F.; Li, J.; Li, S.Y.; Wang, P.; Jia, F.Z.; Xu, J.J.; Zhou, C.H.; et al. Comparison of smear cytology and liquid-based cytology in EUS-guided FNA of pancreatic lesions: Experience from a large tertiary center. Gastrointest. Endosc. 2020, 91, 932–942. [Google Scholar] [CrossRef]
- de Luna, R.; Eloubeidi, M.A.; Sheffield, M.V.; Eltoum, I.; Jhala, N.; Jhala, D.; Chen, V.K.; Chhieng, D.C. Comparison of ThinPrep and conventional preparations in pancreatic fine-needle aspiration biopsy. Diagn. Cytopathol. 2004, 30, 71–76. [Google Scholar] [CrossRef]
- Leblanc, J.K.; Emerson, R.E.; DeWitt, J.M.; Symms, M.; Cramer, H.M.; McHenry, L.; Wade, C.L.; Wang, X.; Musto, P.; Eichelberger, L.; et al. A prospective study comparing rapid assessment of smears and ThinPrep® for endoscopic ultrasound-guided fine-needle aspirates. Endoscopy 2010, 42, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kang, Y.S.; Cho, M.Y.; Kim, J.W. Comparison of cytologic preparation methods in endoscopic ultrasound-guided fine needle aspiration for diagnosis of pancreatic adenocarcinoma. Pancreatology 2016, 16, 824–828. [Google Scholar] [CrossRef]
- Weynand, B.; Borbath, I.; Galant, C.; Piessevaux, H.; Deprez, P.H. Optimizing specimen collection and laboratory procedures reduces the non-diagnostic rate for endoscopic ultrasound-guided fine-needle aspiration of solid lesions of the pancreas. Cytopathology 2013, 24, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fischler, D.F.; Toddy, S.M. Nongynecologic cytology utilizing the ThinPrep Processor. Acta Cytol. 1996, 40, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Chhieng, D.C.; Jhala, D.; Jhala, N.; Ltoum, I.; Chen, V.K.; Vickers, S.; Heslin, M.J.; Wilcox, C.M.; Eloubeidi, M.A. Endoscopic ultrasound-guided fine-needle aspiration biopsy: A study of 103 cases. Cancer 2002, 96, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Stasi, E.; Di Maso, M.; Serviddio, G.; Ali Hussein, M.S.; Muscatiello, N. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United Eur. Gastroenterol. J. 2017, 5, 846–853. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Study Period | Country | Population | Mass | Needle Type | Reference | Description of Assessments | Staining | ROSE | Sample |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chun 2020 [27] | Randomized, crossover | April 2018–March 2019 | Republic of Korea | N: 170, Female: 44.1%, age: 64.8 ± 10.6 (37–88) | Pancreatic | 19/22G EZ Shot 3 Plus (Olympus Medical Systems, Tokyo, Japan) | LBC, CS; EUS-FNA core biopsy or surgical specimen, 6-month clinical/radiological follow-up | CS: alcohol, LBC: CytoRich Red, SurePath | Papanicolaou | Unclear/No | Individual |
| de Luna 2004 [47] | Retrospective, crossover | August 2000 to February 2002 | USA | N: 67, Female: 32.8%, age: 64 (39–87) | Pancreatic | NA | Histologic and clinical follow-up | CS: alcohol/air, LBC: PreservCyt, ThinPrep | Modified Giemsa for air-dried smears, Papanicolaou for alcohol-fixed and LBC samples | Unclear | Split |
| Hashimoto 2017 [32] | PSM | January 2009–August 2014 | Japan | N: 126, Female: 49.2%, age: CS: 65 (35–93), LBC: 66 (33–85) | Pancreatic | 19G/22G/25G | Surgical resection, additional EUS-FNA procedure, 6 months clinical/imaging follow-up | CS: alcohol, LBC: SurePath | HE for smears, Papanicolaou for LBC samples | No | Individual |
| Itonaga 2019 [33] | PSM | December 2011 to October 2017 | Japan | N: 311, Female: 42.8%, age: NA | Pancreatic | 19G/22G/25G Expect (Boston Scientific) or EZ Shot2 (Olympus Medical) | Surgical resection, 12-month clinical follow-up | LBC: ThinPrep, CS: Air/alcohol | Air-dried: Diff-quick, alcohol-fixed and LBC Papanicolaou | Yes | Split |
| Jun 2023 [36] | Randomized crossover | January 2019 to August 2022 | Republic of Korea | N: 60, Female: 53.3%, age: 60.7 ± 12.8 (24–85) | Abdominal | 19G/22G EZ Shot 3 Plus (Olympus) | Core biopsy, LBC; CS and surgical specimens, plus 6-month follow-up | LBC: CytoRich, SurePath vials, PrepStain processor, CS: alcohol | NA | No | Individual |
| LeBlanc 2010 [48] | Prospective crossover | April 2005 through April 2007 | USA | N: 50, Female: 36% (whole population), age: mean 63 | Subepithelial masses | 22G EUSN-3 or echo-1-22 | Final cytological diagnosis, surgical pathology, or follow-up | CS: air/alcohol, LBC: ThinPrep | Air-dried: Diff-quick, alcohol-fixed and LBC Papanicolaou | Yes | Individual |
| van Riet 2010 [43] | Prospective crossover | April 2016–2017 | The Netherlands | N: 71, Female: NA, age: NA | Solid pancreatic lesions | 19G, 22G, 25G Echotip/Expect | Surgical resection, 12-month clinical follow-up | CS: no information, LBC: Thinprep | Different per center: Hemocolor, no stain, Diff quick, or Giemsa | No | Split |
| Yan 2023 [44] | Retrospective crossover | January 2014 to February 2022 | China | N: 251, Female: 41%, age: Median 60 (IQR: 13) | Pancreatic | 20G FNA | Needle biopsy/surgery sample, FNA sample, 6-month clinical/imaging follow-up | CS: alcohol, LBC: ThinPrep | NA | Not all | Individual |
| Yeon 2018 [45] | Prospective crossover | June 2012–October 2013 | Republic of Korea | N: 43, Female: 32.6%, age: 65.5 ± 12.5 | Pancreatic | 22G Echotip | Biopsy, surgery, or 6-month clinical follow-up | CS: 95% alcohol fixation, LBC: CellPrepPlus | NA | Unclear | Individual |
| Zhou 2020 [46] | Retrospective crossover | January 2015 to January 2019 | China | N: 514, Female: 37.2%, age: Median 60 (IQR 50–67) | Pancreatic | 22G/25G | FNA, surgical pathology, 6-month clinical follow-up | CS: alcohol, LBC: CytoRich | CS: HE, LBC: Papanicolaou | Not all | Split |
| Qin 2014 [41] | Prospective crossover | January 2011 to January 2014 | China | N: 72, Female: 19.4%, age: 54.6 (24–70) | Pancreatic | 22G FNA | Surgery, 9-month clinical follow-up | CS: alcohol/air, LBC: ThinPrep | Modified Diff-Quick, Papanicolaou | No | Individual |
| Cheng 2020 [25] * | NA | December 2016 to January 2018 | China | N: 52, Female: NA, age: NA | Abdominal | NA | Pathology, follow-up | NA | NA | NA | NA |
| Schmidt 2015 [42] * | NA | 2008 to 2011 | Germany | N: 172, Female: 39%, age: 64.8 (±12.4) | Pancreatic | NA | Surgical histology, 12-month follow-up | NA | NA | NA | NA |
| Min 2013 [38] * | prospective crossover | November 2010 to February 2013 | China | N: 32, Female: NA, age: NA | Pancreatic | NA | NA | NA | NA | NA | NA |
| Lee 2016 [49] | Retrospective crossover | July 2010 to June 2015 | Republic of Korea | N: 48, Female: 50%, age: Median 67, range 39–84 | Pancreatic | 22G FNA | Clinical and imaging follow-up of 12 months, CS, LBC, and pathology from metastatic sites | CS: alcohol, LBC: ThinPrep | Papanicolaou | No | Individual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engh, M.A.; Teutsch, B.; Schulze Wenning, A.; Kói, T.; Hegyi, P.; Erőss, B. Combined Liquid-Based Cytology and Conventional Smear Provides Better Sensitivity and Adequacy Rates After Endoscopic Ultrasound-Guided Tissue Acquisition of Abdominal Masses: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6685. https://doi.org/10.3390/jcm14186685
Engh MA, Teutsch B, Schulze Wenning A, Kói T, Hegyi P, Erőss B. Combined Liquid-Based Cytology and Conventional Smear Provides Better Sensitivity and Adequacy Rates After Endoscopic Ultrasound-Guided Tissue Acquisition of Abdominal Masses: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(18):6685. https://doi.org/10.3390/jcm14186685
Chicago/Turabian StyleEngh, Marie Anne, Brigitta Teutsch, Alexander Schulze Wenning, Tamás Kói, Péter Hegyi, and Bálint Erőss. 2025. "Combined Liquid-Based Cytology and Conventional Smear Provides Better Sensitivity and Adequacy Rates After Endoscopic Ultrasound-Guided Tissue Acquisition of Abdominal Masses: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 18: 6685. https://doi.org/10.3390/jcm14186685
APA StyleEngh, M. A., Teutsch, B., Schulze Wenning, A., Kói, T., Hegyi, P., & Erőss, B. (2025). Combined Liquid-Based Cytology and Conventional Smear Provides Better Sensitivity and Adequacy Rates After Endoscopic Ultrasound-Guided Tissue Acquisition of Abdominal Masses: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(18), 6685. https://doi.org/10.3390/jcm14186685