2. Materials and Methods
In this prospective, non-randomized, single-center study, 179 consecutive patients were hospitalized and treated for acute STEMI at the Department of Cardiology at the Ufa City Hospital N21, Russian Federation offering 24/7 percutaneous coronary intervention (PCI) services. Patients were enrolled in the study between September 2020 and June 2021 and followed for 18 months (last patient, last follow-up January 2023). Initial diagnosis was established by 12-lead electrocardiogram (ECG) upon admission according to European Guidelines criteria [
13]. ST-segment elevation was measured at the J-point at least in two contiguous leads with ST-segment elevation of 2.5 mm (mm) in men < 40 years, 2 mm in men 40 years, or 1.5 mm in women in leads V2–V3 and/or 1 mm in the other leads in the absence of left ventricular hypertrophy or left bundle branch block. The diagnosis was verified during clinical FU by further ECG recordings (day two and/or day three of hospital stay), transthoracic echocardiographic (day two or day three of hospital stay), laboratory (hs-Troponin I and CK-MB at admission and during FU at day two and/or day three of hospital stay) and coronary angiography (CAG) according to the 2023 European Society of Cardiology (ESC) guidelines [
1].
Dependent on the time of STEMI diagnosis, acute CAG/PCI was performed if patient presentation to the cardiac catheterization laboratory was ≤120 min (min) or acute thrombolysis was given presentation by ≤12 h time from symptom presentation and no contraindication for thrombolytic therapy as established by and emergency doctor or primary care physician. If patients presented with signs of failed fibrinolysis, or by evidence of re-occlusion or re-infarction with ST-segment dynamics, rescue PCI was performed as soon as possible. Acute medical treatment, including an antiplatelet regimen and post-MI guideline-recommended medications were given according to the ESC guidelines (
Table 1) during baseline hospitalization and at discharge [
1]. Capture of further relevant diagnoses was obtained according to medical history, clinical findings, ECG, laboratory data and transthoracic echocardiography reports.
The study was approved by the Ethics Committee of Bashkir State Medical University (N1 from 23 January 2017) and was performed in accordance with standards of good clinical practice (GCP) and the principles of the Declaration of Helsinki. Prior to inclusion, all participants signed an informed consent.
The inclusion criteria were: age > 18 years and diagnosis of STEMI according to current ESC guidelines (see above). The exclusion criteria were: >48 h from start of typical symptoms of acute coronary syndrome (ACS), severe valvular dysfunction defined as severe regurgitation or stenosis of one or more of the cardiac valves, dilative cardiomyopathy, permanent atrial fibrillation (AF) and/or atrial flutter, AV block II–III according to medical history and ECG, implanted pacemaker, acute pulmonary embolism, active malignant disease defined as achieved tumor-free survival under three years, severe chronic obstructive pulmonary disease (GOLD 2009 stage III–IV), uncontrolled bronchial asthma (according to Global Initiative for Asthma, GINA 2019), acute infectious diseases at the time of STEMI defined as acute pyelonephritis, community-acquired pneumonia, acute bronchitis and/or flu/acute respiratory viral infection, and kidney failure defined as glomerular filtration rate (GFR) < 30 mL/min 1.73 m2, as well as pregnancy or lactation.
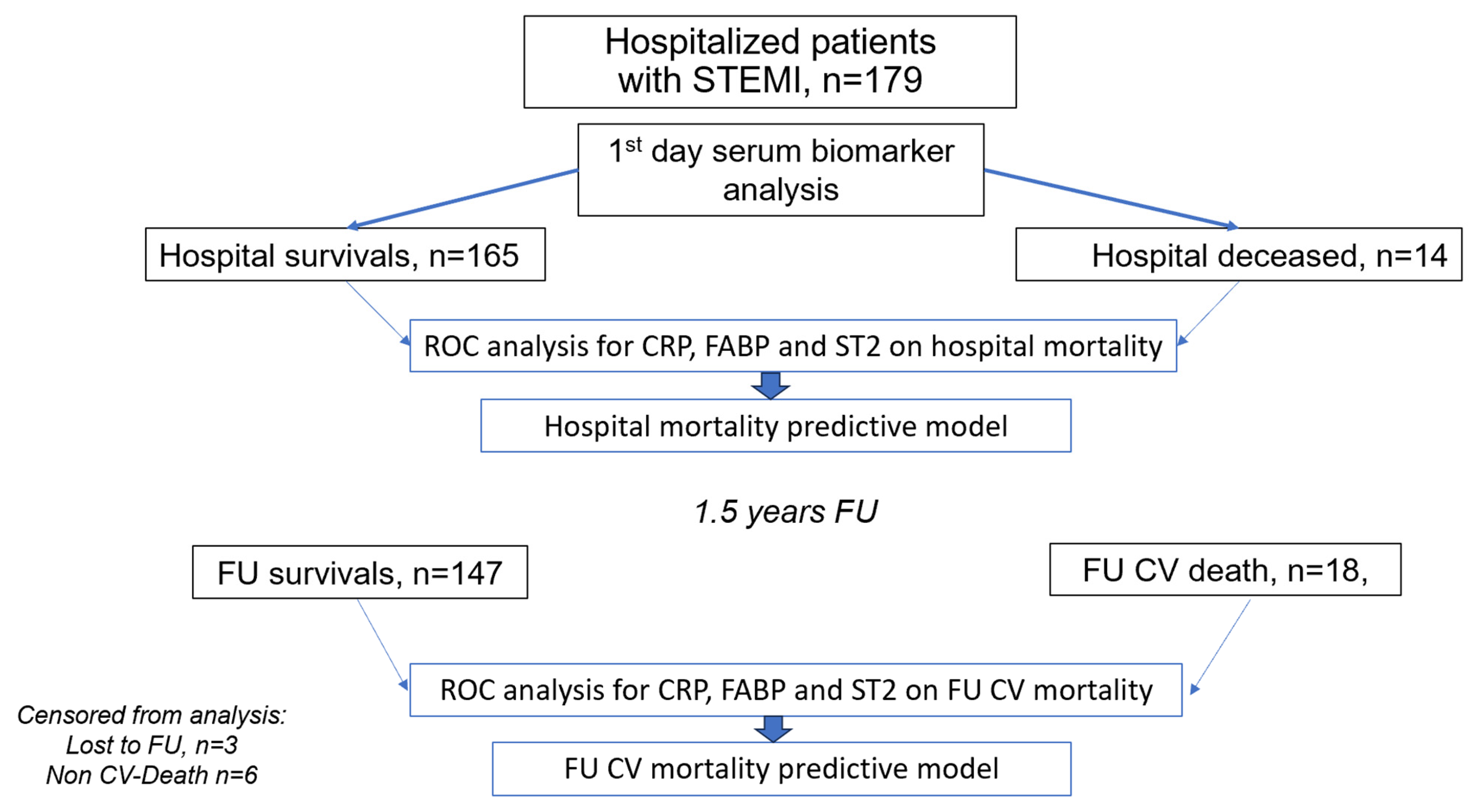
Patient enrollment and the design of the study are presented in
Figure 1. On the day of hospital admission, patients’ venous blood was drawn for the study prior to PCI (≤8 h), subsequently centrifuged and the serum was frozen for further analyses. Blood serum samples were anonymized, aliquoted, and frozen immediately at −70 °C.
The serum concentrations of biomarkers CRP, H-FABP, and sST2 were analyzed by enzyme immunoassay (ELISA) as indicated by the manufacturer “Vector-Best”, Russia using calibrated, certified, and maintained equipment with technicians blinded to study outcomes.
CRP was measured using the kit with the catalog number (cat. №A-9002 CRP-EIA-BEST). The analytical range of measurements was 0–10 mg/L. The coefficient of variation within a batch (intra-assay CV) was <8%, respectively. Lot number (lot): 115.
H-FABP was measured using the kit with the catalog number A-9104-H-FABP EIA-BEST. The analytical range of measurements was 0–15 ng/mL. The coefficient of variation within a batch (intra-assay CV) was <8%, respectively. Lot number (lot): 48.
sST2 was measured using the kit with the catalog number A-9110-sST2 EIA-BEST. The analytical range of measurements was 0–140 ng/mL. The coefficient of variation within a batch (intra-assay CV) was <8%, respectively. Batch number (lot): 12.
Samples were thawed only once. Storage was for no more than 6 months before analysis according to the manufacturer’s specification. All samples were analyzed in a single set of experiments to minimize variability. The inter-batch variation coefficient is not provided, since all samples were analyzed in one batch.
A detailed medical history was obtained at admission for all enrolled patients, including current clinical symptoms, as well as history of previous illnesses, and current medications.
All eligible patients were examined during baseline hospitalization and followed for 18 months to capture CV mortality. Survival status at 18 months (552 ± 42 days) was recorded using a remote data capture system “ProMed” (Program for Medical Cases Monitoring).
Demographic variables captured in the analysis included age, sex, Body Mass Index (BMI), concomitant CV disease, CHD, arterial hypertension (AH), diabetes mellitus (DM), chronic kidney disease (CKD), prior MI, acute/chronic HF, prior stroke, chronic obstructive pulmonary disease (COPD). The primary endpoints were in-hospital mortality and CV mortality at 18 months post-discharge.
Statistical analysis was carried out by our blinded statistical analytic team using SPSS software package 21 (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA: IBM Corp.) and R Studio version 4.4.3 (Posit team (2025). RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA, USA).
Preliminary Shapiro-Wilk testing showed non-normal distribution, therefore median and interquartile range (Q1; Q3) were used to describe the distribution of continuous variables. For the description of categorical variables, absolute and relative frequencies were used. The Mann-Whitney test was applied to determine the statistical significance of difference between medians of continuous variables, while Chi-square testing was applied for analysis of categorical variables and Yates correction was applied if necessary.
Kaplan-Meier multiple estimator methods were used to compare in-hospital survival between groups, and the Gahan-Wilcoxon test was used to evaluate survival outcomes defined significant at p < 0.05. The target variable defining the duration of the event state is given in days for in-hospital survival and in months for FU survival. Univariate Cox proportional hazards models for binarized by cut-off points of CRP, H-FABP, and sST2 biomarkers were estimated to identify predictors of the risk of in-hospital death. Model estimates were controlled using the likelihood ratio test (LR test) with the null hypothesis of the model as a whole and the difference from one of the values of the Harrell confidence interval.
Alternatively, Cox models with restricted cubic splines (RCS) were also built, where transformed values of baseline CRP, H-FABP, and sST2 levels were used as predictors. For right-skewed H-FABP and sST2, logarithm was used as a transformation, for left-skewed CRP levels, their values were inverted before re-characterization (baseline CRP levels were subtracted from the maximum value +1). The number of nodes for splines was selected by enumeration from 3 to 5, based on the minimum values of the Akaike information criterion. To visualize the results of Cox with RCS modeling, a graph of the dependence of HR on the baseline values of biomarker levels was constructed. For modeling hospital survival, the following R libraries were used: “rms”, “survival”, “ggplot2”, “survminer”.
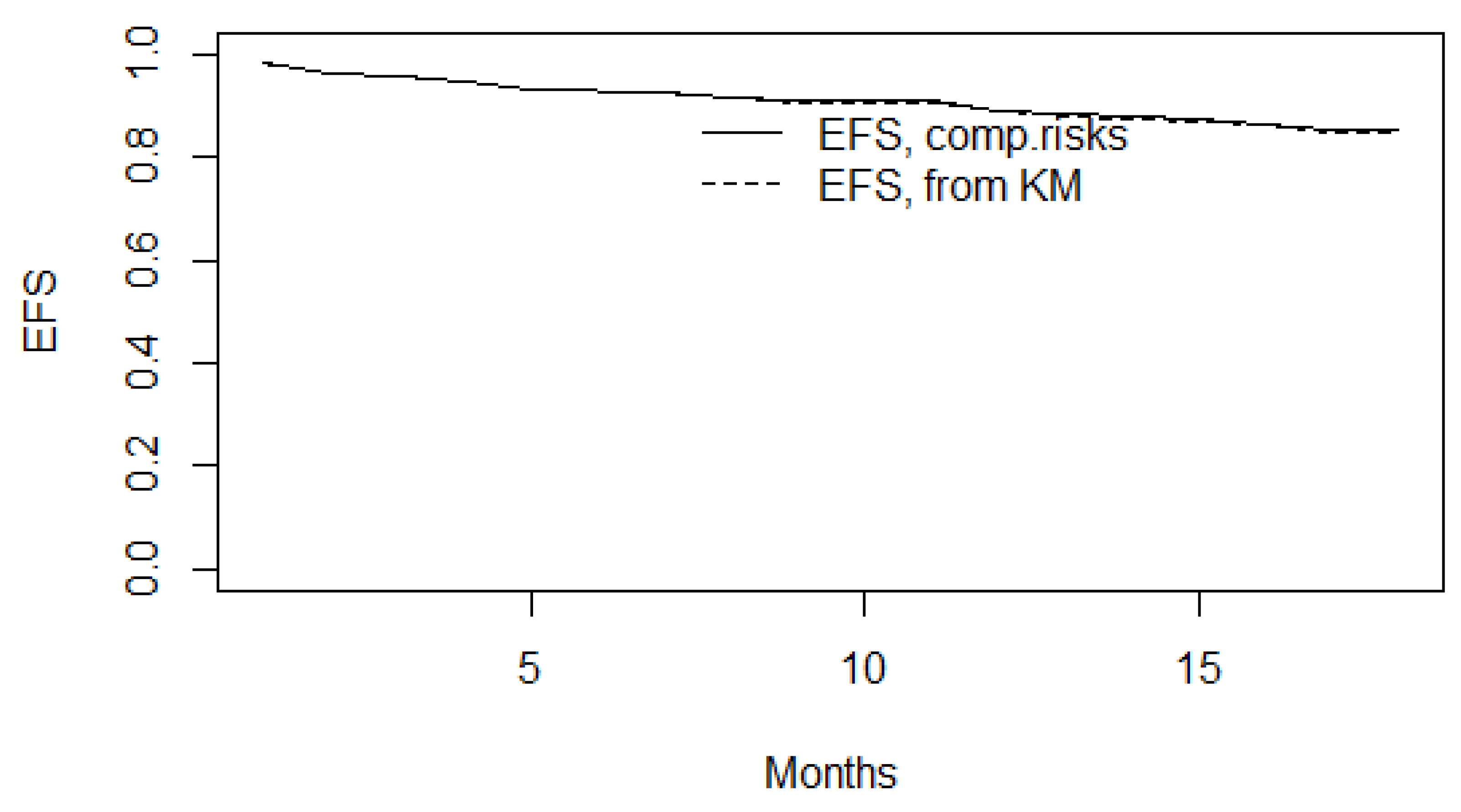
Since we had competing events (“Alive”, “Cardiovascular deaths”, “Non-cardiovascular deaths”) in the FU 18-month observation, their cumulative probability was calculated using Fine-Gray estimates for competing risks of death. The Fine test was also performed to assess the significance of the effect of biomarker levels exceeding their cutoff points on the risk of death (here the parameter of interest is cardiovascular deaths). Event-free survival (EFS) was also assessed. To assess the risk of death from cardiovascular causes, two-factor Fine-Gray models with competing risks were used, where biomarker levels (baseline, transformed, and binarized) and patient age were considered as factors. As with in-hospital survival, logarithmic transformation was performed as a transformation for right-skewed sST2 and H-FABP, and for left-skewed CRP, the values were “inverted” from higher to lower before logarithmic transformation. The interpretation of the results is based on the calculation of the hazard ratio (HR) and confidence interval with 95% reliability for each reliable risk predictor. The following R libraries were used to model post-hospital survival: “tidycmprsk”, “survival”, “ggsurvfit”.
4. Discussion
STEMI still represents a leading cause for CV morbidity and mortality worldwide and is thus also a considerable economic factor [
1]. Although STEMI patients exhibit high in-hospital mortality, they also face a significant risk of major adverse CV events and CV death in the post-acute phase [
14]. Identifying high-risk individuals after STEMI is therefore a key clinical objective. Despite this clear need, effective tools for risk stratification and prognosis remain limited, prompting ongoing research. Notably, many studies advocate for a multi-marker strategy, suggesting that combining biomarkers from diverse pathophysiological pathways may enhance diagnostic accuracy [
15,
16].
In this study, we aimed to assess the prognostic value of three CV biomarkers, CRP, H-FABP, and sST2, for mortality risk stratification in STEMI patients, both during hospitalization and over an 18-month FU period. Notably, these biomarkers represent distinct pathophysiological pathways, yet each has been associated with prognostic relevance in MI and related conditions, such as HF.
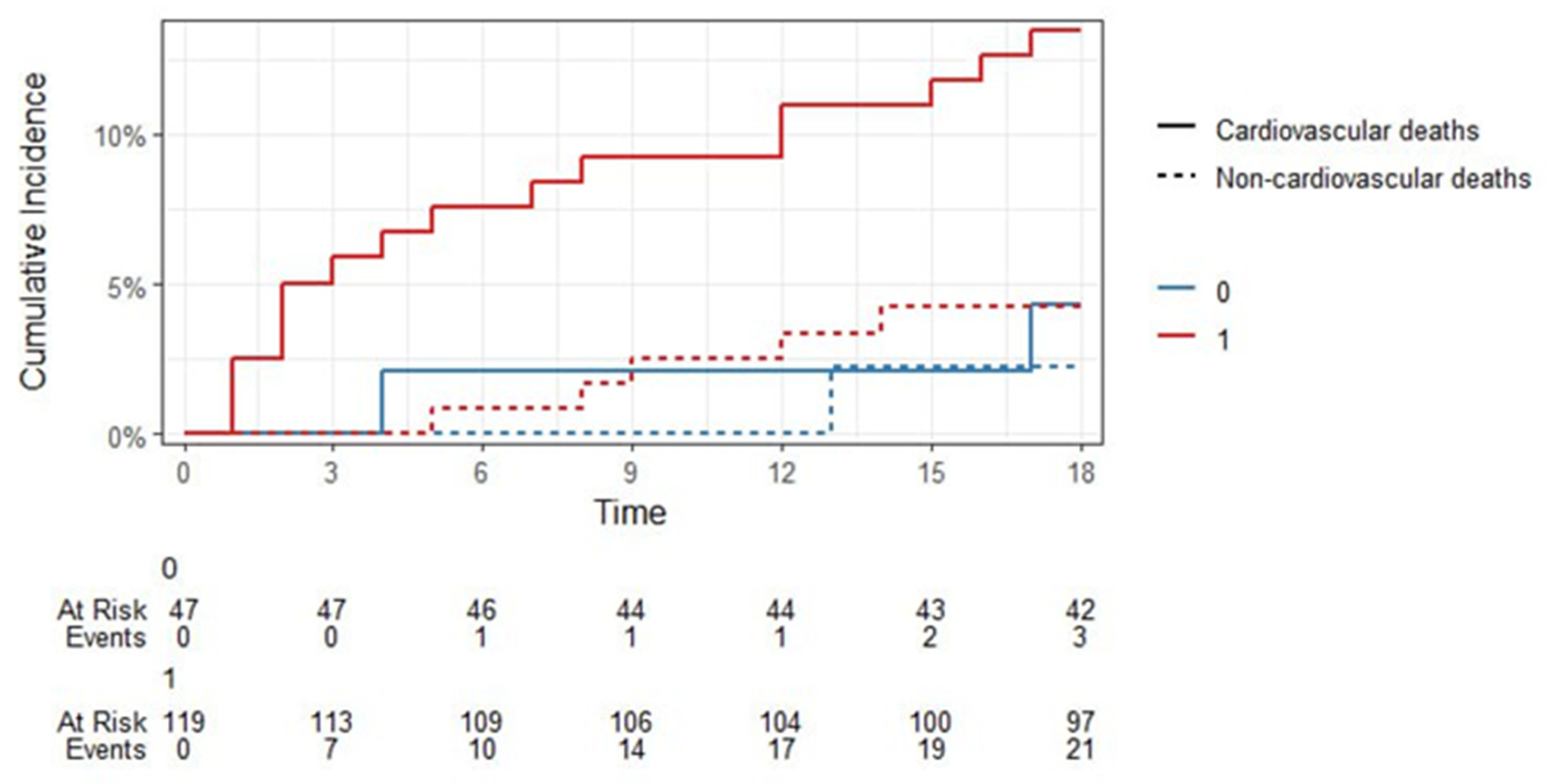
In our trial, 14 of the 179 patients died during baseline hospitalization for STEMI (7.8%) while the mortality rate in FU was much higher. Among the remaining 165 patients, 18 (10.9%) individuals died due to the CV reasons during the 18-month FU, while 6 died from non-CV causes.
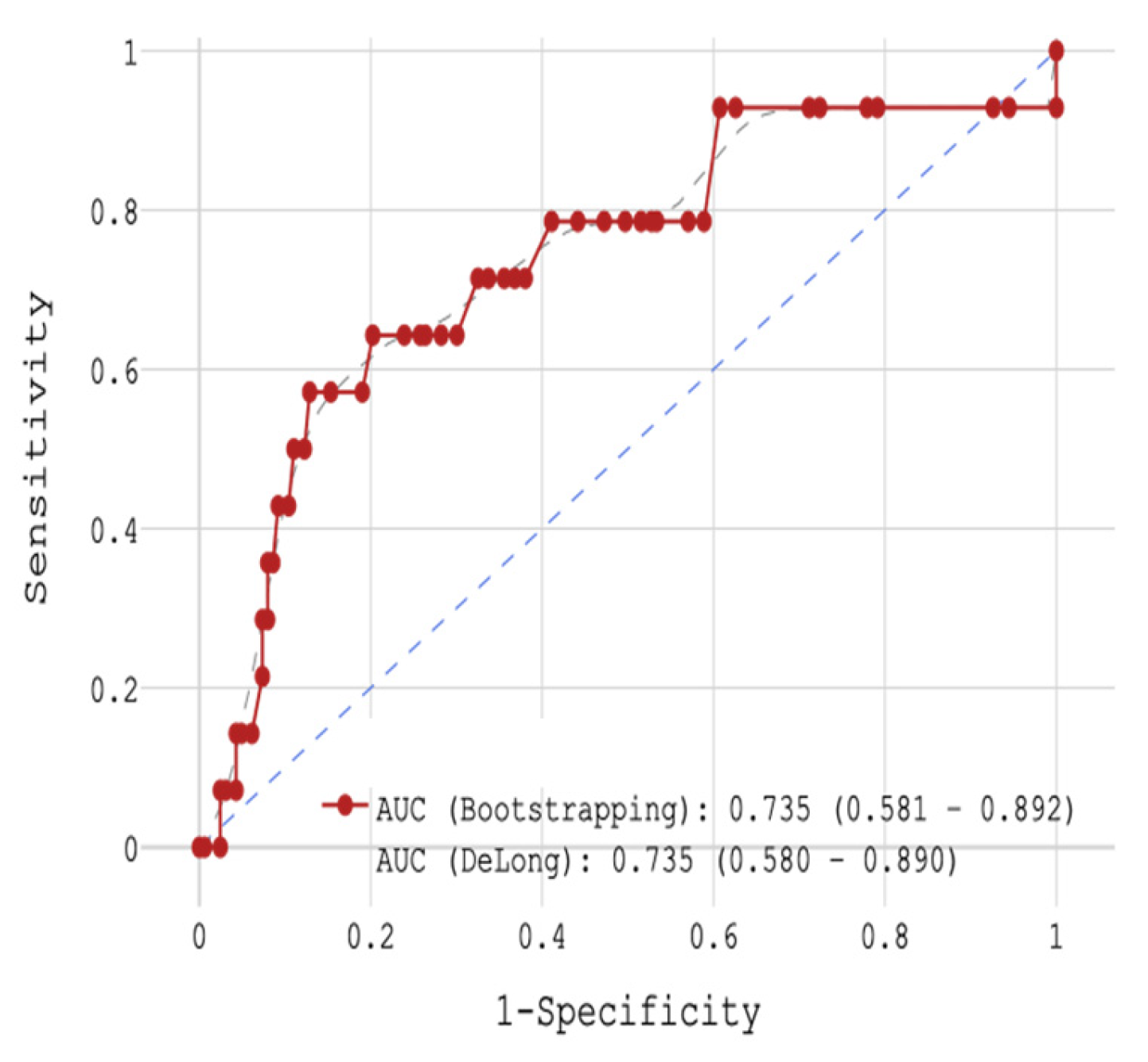
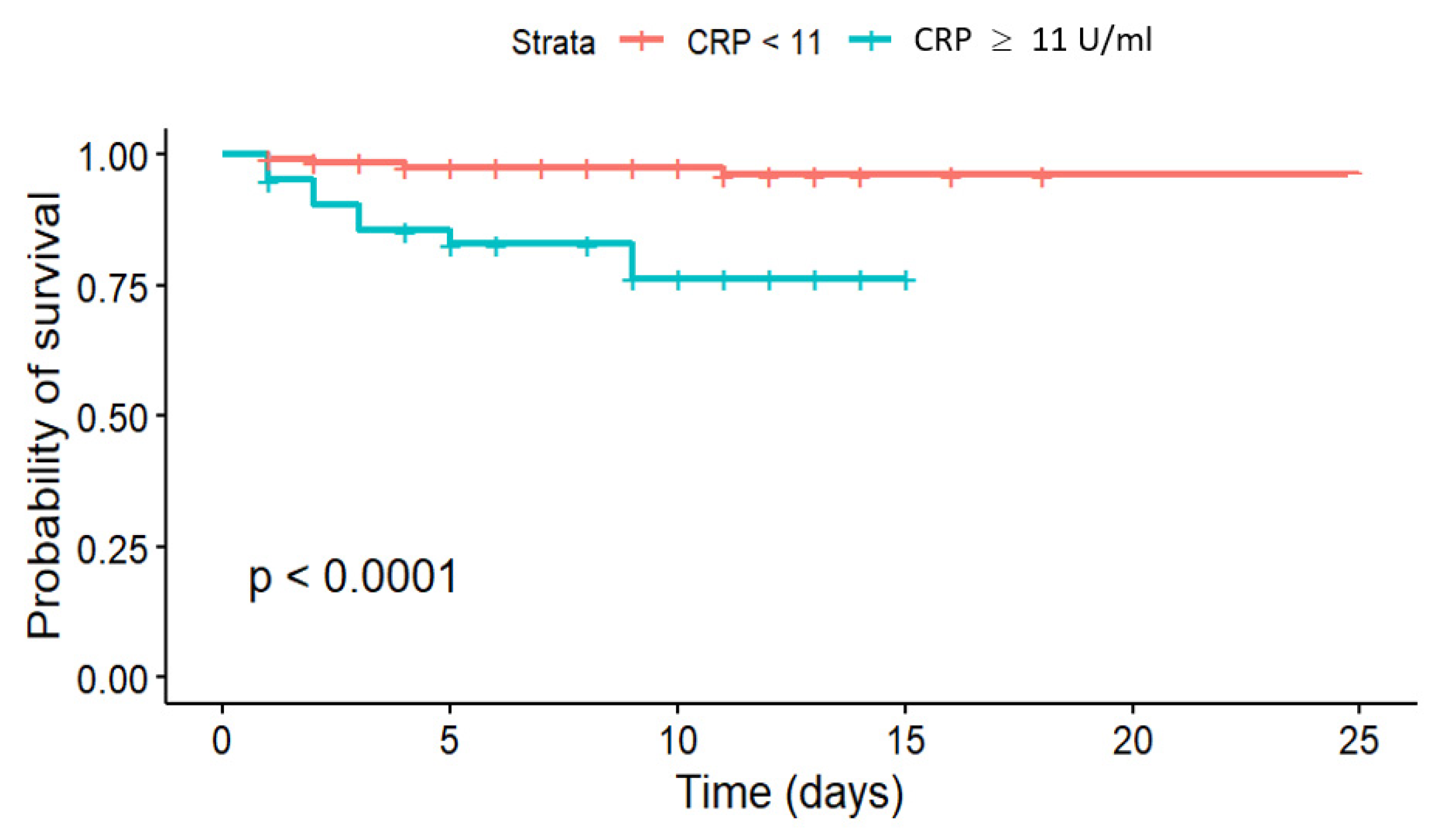
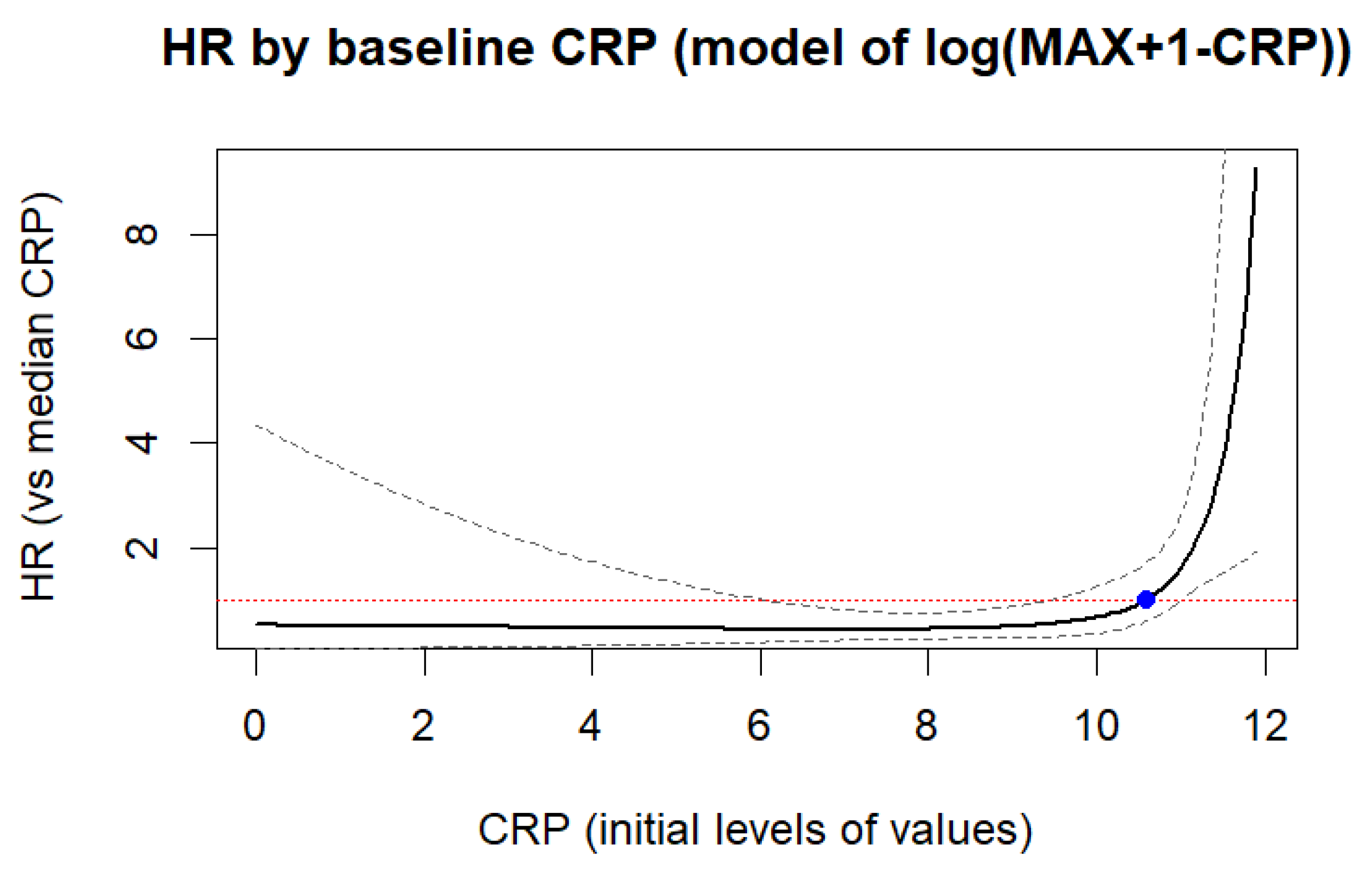
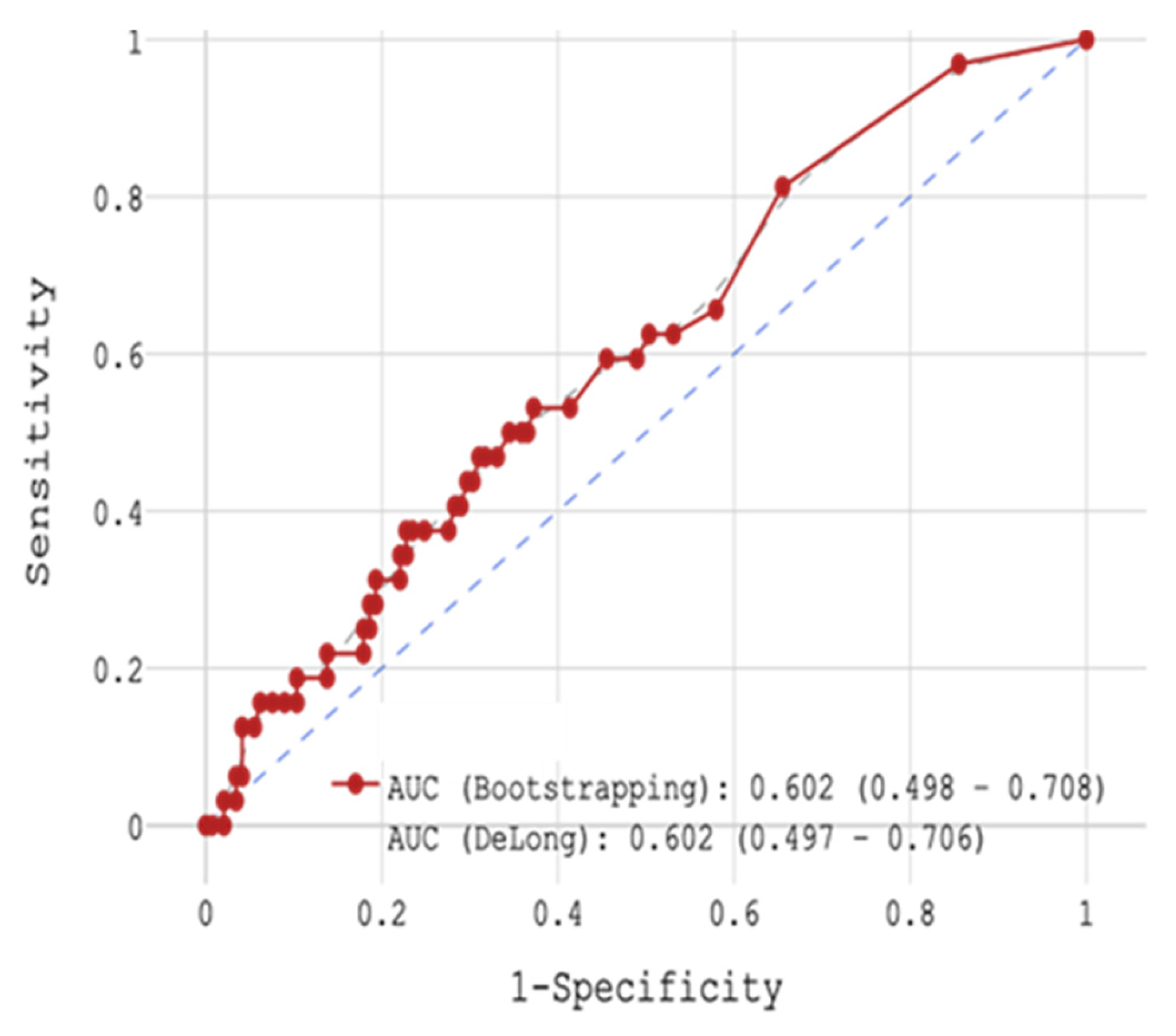
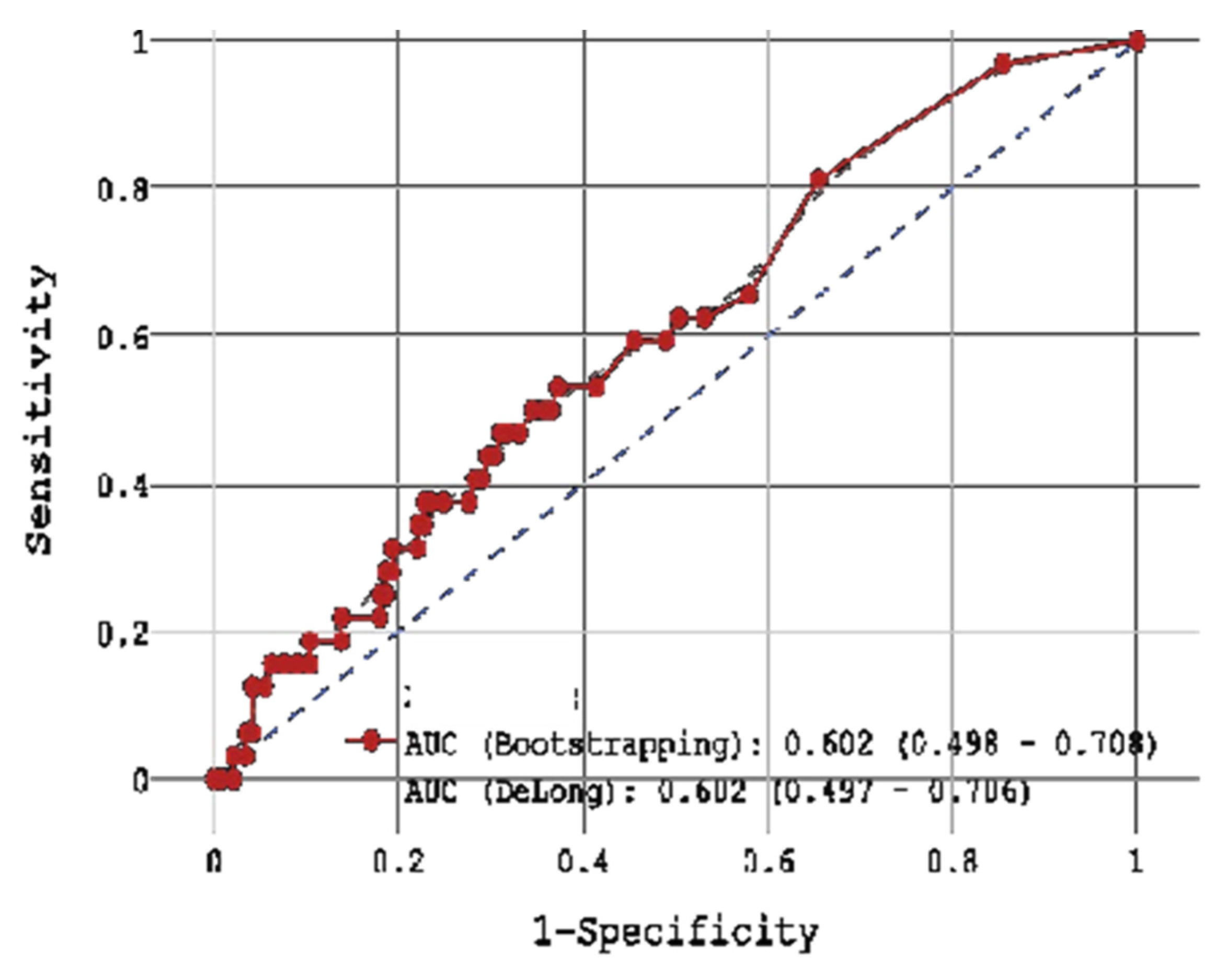
Of the three biomarkers examined in our trial, only CRP > 11 mg/L together with low GFR (HR = 4.928,
p = 0.004) and high glucose level (HR = 1.119,
p = 0.004) showed potential for the prediction of in-hospital mortality in STEMI patients (
Figure 2 and
Figure 5 and
Table 4 and
Table 9), yet CRP was not robustly associated with CV mortality during 18-month FU. Regarding the cumulative probability of mortality during FU, the results of the Fine-Gray test confirmed the significance of the effect of the CRP level exceeding 8.1 mg/L on mortality from cardiovascular causes during FU at the level of
p = 0.087, while for the event “Died from other causes” there was no significance of the effect (
p = 0.53).
CRP is a downstream marker of systemic inflammation, participating in atherogenesis and atherothrombosis. CRP promotes endothelial dysfunction and accelerates the formation and progression of atherosclerotic plaques. In addition, it stimulates the production of proinflammatory cytokines, including interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), enhances oxidative stress, and contributes to a prothrombotic state. At sites of vascular injury or inflammation, CRP induces platelet activation and aggregation, directly contributing to thrombus formation and plaque instability, which are critical in ACS and sudden CV death [
17]. Aligning with our findings, some prior studies have shown a correlation of increased CRP with in-hospital CV death. The Acuity multi-center US trial of 2974 patients with ACS linked higher baseline CRP levels to significantly higher incidence of CV mortality in patients with upper quartile baseline CRP values compared to those with the lowest quartile CRP values at 30 days post discharge (2.3 vs. 0.3%,
p = 0.0004) and also at 1-year post discharge (5.5 vs. 2.8%,
p = 0.0003) [
18]. Several meta-analyses have also confirmed the prognostic value of CRP in acute coronary syndromes. He et al. performed a meta-analyses of longitudinal studies and showed that higher CRP within 72 h of the onset of ACS was associated with a moderate increased long-term risk of recurrent CV events or death, especially among patients with CRP exceeding 10 mg/L with pooled RR of 2.18, (95% CI: 1.77–2.68) for adverse outcomes compared to patients with low CRP ≤ 3 mg/L [
19] One meta-analysis of seven heterogenous studies (six retrospective, one prospective) with more than 6000 STEMI patients observed that high pre-procedural (percutaneous coronary intervention) CRP values were associated with increased in-hospital all-cause mortality and CV events as well as increased post discharge all-cause mortality and CV events during 6-month FU. Due to the heterogeneity of the studies, however, CV mortality could not be ascertained [
20], thus our study offers insight into CV mortality during hospitalization for STEMI.
In our study, association of elevated CRP with CV mortality in midterm FU was less robust contrasting with some studies found in the literature. A 2010 meta-analysis of 54 long-term studies of 160,309 patients without history of vascular disease demonstrated the association of CRP concentrations with the risk of later developing CHD, ischemic stroke and vascular mortality [
21]. A recent analysis published in 2022 of high-risk vascular patients showed that higher longitudinal high-sensitivity CRP (hsCRP) levels over 30 months were independently associated with increased risk of major adverse CV events and all-cause death even in patients on optimal primary and secondary preventive therapies [
22]. A cohort study performed in 2023 in patients with established CV disease with median 9.5-year FU found that higher CRP was independently associated with recurrent CV events and all-cause mortality, incidence also increasing with higher CRP levels and persisting in long-term FU to 15 years [
21].
Although not measured in our study and a potential limitation, the use and predictive utility of the biomarker hsCRP must be mentioned. hsCRP is an independent predictor of short- and long-term CV events and CV mortality, yet its incremental predictive value compared to risk models using established risk factors is modest (i.e., risk models incorporating age, blood pressure, LDL-C, smoking status) with benefit shown when used to reclassify intermediate risk patients. In addition, hsCRP can be elevated in a range of non-CV conditions (i.e., infection, trauma, inflammatory diseases) limiting its specificity for CV risk assessment [
23]. Standard CRP and hsCRP highly correlate in the acute phase at time of MI and demonstrate comparable diagnostic accuracy for predicting in-hospital mortality and major CV events following MI. Furthermore, several studies have demonstrated that elevated CRP measured within the first 24 h post-MI is independently associated with increased risk of long-term mortality and HF [
24,
25,
26].
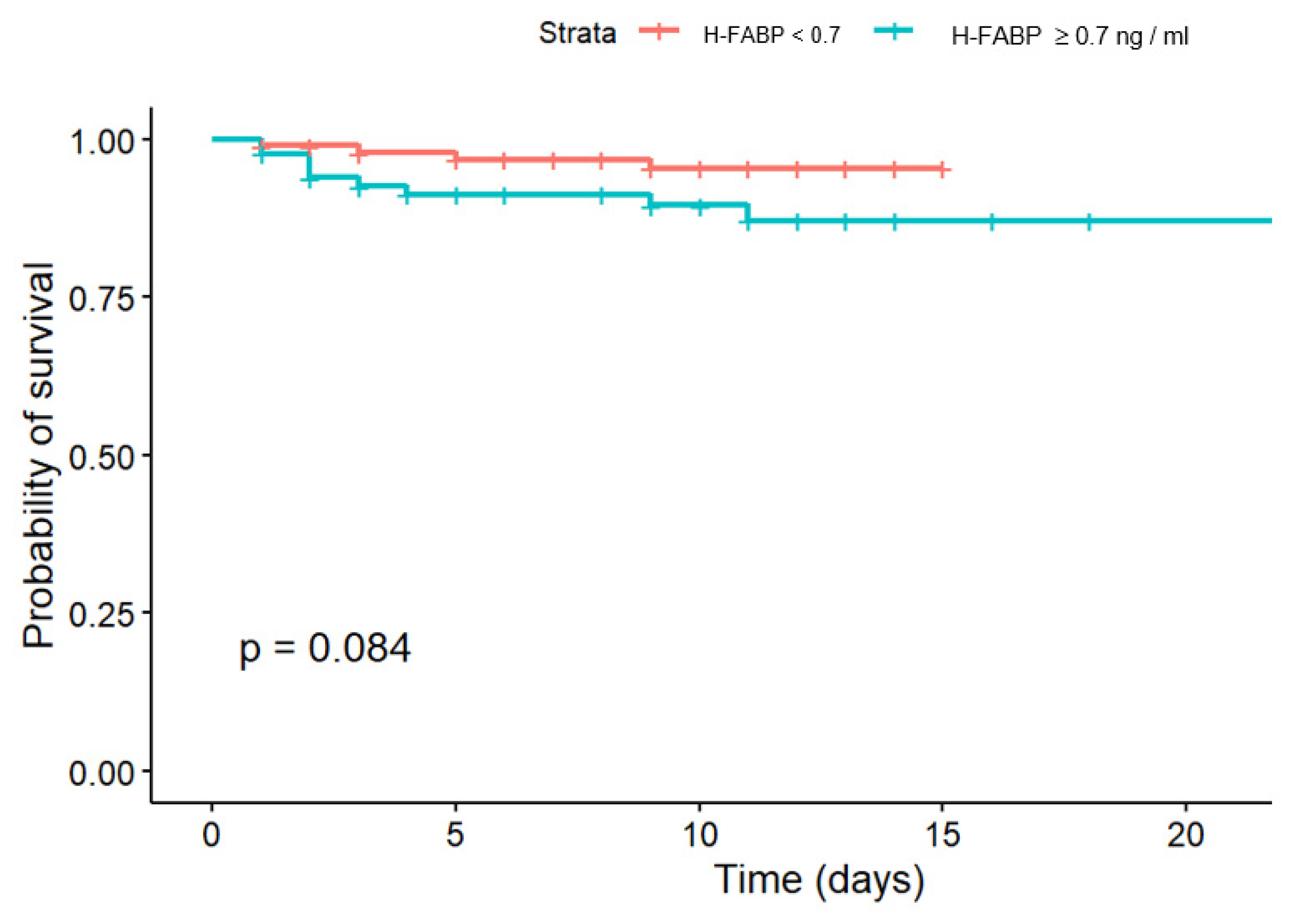
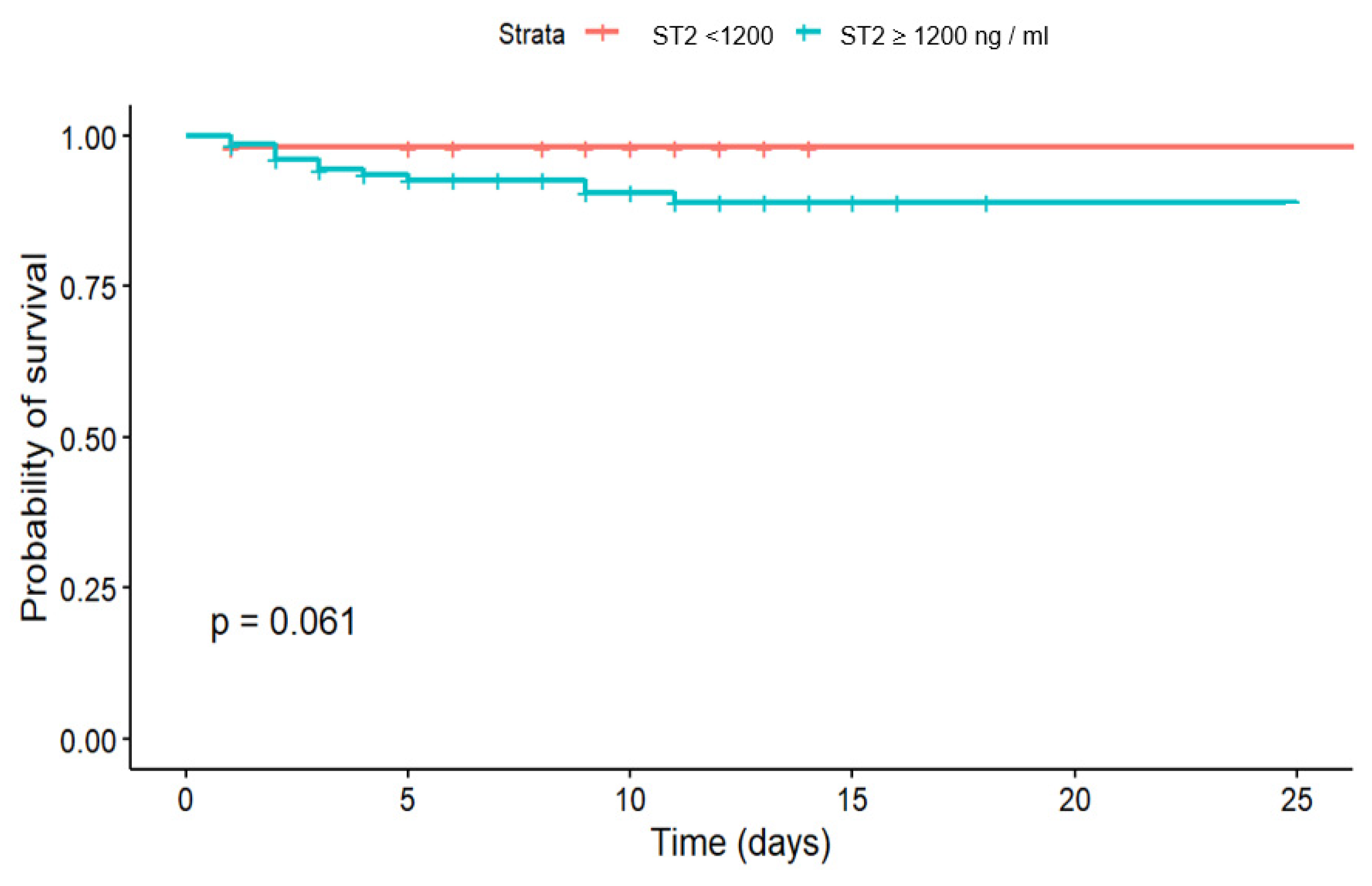
The second biomarker evaluated in our study for its potential association with in-hospital CV mortality and post-discharge CV mortality was H-FABP. In our study, the effectivity of H-FABP in prediction of in-hospital CV mortality risk was not confirmed, while in midterm FU differences in survival according to cut-off could be observed. The results of the Fine-Gray test did not confirm the significance of the effect of exceeding the H-FABP cut-off > 0.2 ng/mL on 18-months FU survival after STEMI (p = 0.45), and there was also no significance of the effect for the event “Died from other causes” (p = 0.34).
In the literature in contrast, H-FABP has been shown to have predictive value for CV events and CV mortality in several disease entities. Compared to established biomarkers, H-FABP provides complementary prognostic information to high-sensitivity cardiac troponin and NT-proBNP. H-FABP is more specific for myocardial injury and less confounded by non-cardiac inflammation than CRP. [
27,
28] While NT-proBNP and troponin remain superior for long-term risk stratification, H-FABP is particularly useful for early risk assessment. Contrasting with our results, elevated H-FABP levels measured within 12–24 h after ACS symptom onset in the literature are associated with a significantly increased risk of all-cause and CV mortality (4.1-fold higher HR), recurrent MI (HR 1.6), and developing HF (HR 4.5) over 10-months, independent of established risk factors and cardiac troponins, findings in contrast with our results [
27,
29,
30]. In ACS, the prognostic value of H-FABP in troponin-negative patients has been described, identifying high-risk individuals missed by troponin alone [
31]. In stable CAD, higher H-FABP also identifies individuals at higher risk for CV events and CV mortality, predicting a twofold increase in composite cardiovascular events and mortality over 24 months, independent of other clinical variables [
32].
The predictive utility of H-FABP is varied and among patients with CKD, H-FABP is associated with major adverse CV events and increased non-CV mortality, although its association with CV mortality is less robust [
28]. H-FABP has also been shown to be useful for the prediction of post-operative mortality and ventricular dysfunction following coronary artery bypass grafting [
33]. In addition, H-FABP has been shown to be an independent predictor of all-cause as well as CV mortality in patients with chronic HF and DM type 2, with the strongest predictive utility among those with diabetes [
34]. In our study, the effectivity of H-FABP in prediction of CV mortality risk was less robust, which we speculate may have been due to the relatively small sample size and/or the high rate of systemic thrombolysis.
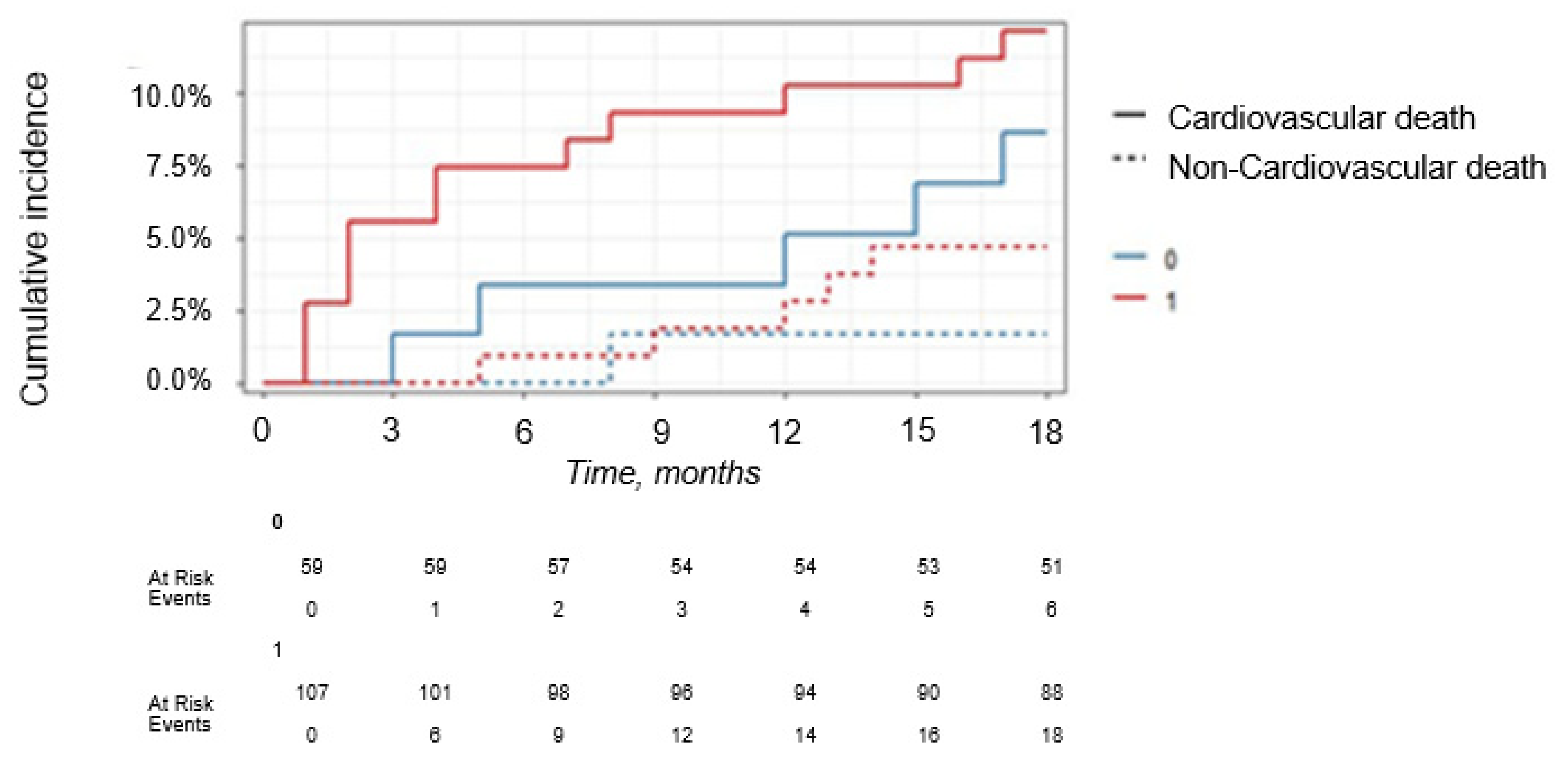
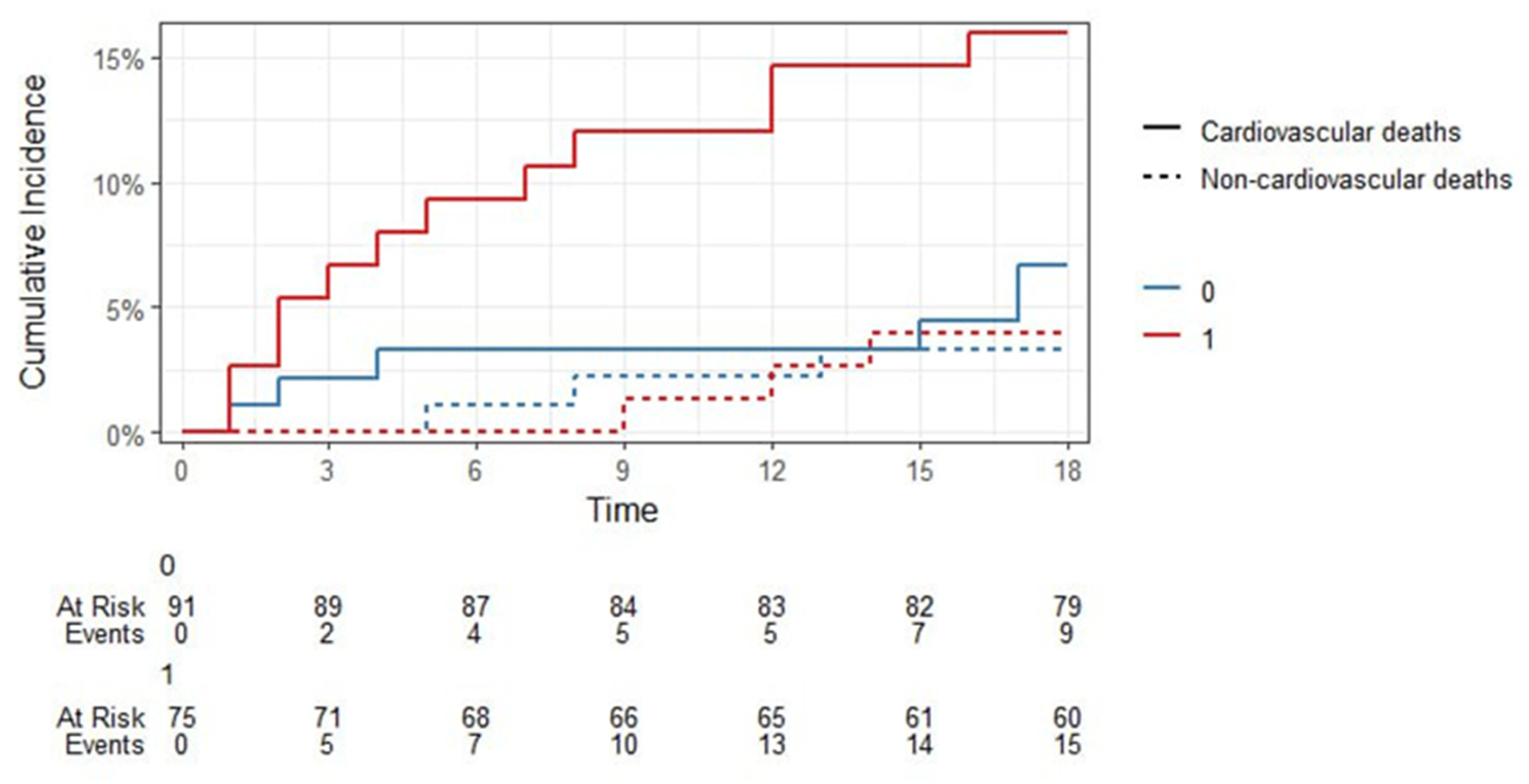
One of the most important findings during the 18-month FU in our study was the predictive value of sST2 in predicting midterm CV mortality. sST2 > 11 ng/mL (HR = 5.101,
p = 0.011) together with older age (HR = 1.022,
p = 0.261), DM type 2 (HR = 6.433,
p = 0.006) and decreased CKD (HR = 0.991,
p = 0.991) showed a promising potential for the prediction of post-discharge mortality in 18-month FU among our STEMI patients (
Figure 12 and
Figure 13 and
Table 16,
Table 17 and
Table 18). ST2 represents a marker of inflammation and cardiac stress and has two known isoforms: a membrane-bound ST2L and a soluble form, sST2 [
35]. A ligand to both isoforms is interleukin-33 (IL-33), which is known to mediate cardioprotective effects on a molecular level through binding to the ST2L [
36,
37]. In contrast, sST2 acts as a decoy receptor, binding IL-33 and making it unavailable for cardioprotective signaling through the ST2L. This biomarker is elevated in numerous CV pathologies, such as HF, but also in MI [
35].
sST2 was specifically analyzed in the multicenter, prospective PRIDE study (ProBNP Investigation of Dyspnea in the Emergency department) evaluating the diagnostic and prognostic utility of NT-proBNP in patients presenting with acute dyspnea. The study demonstrated that sST2 is a strong, independent predictor of mortality and heart failure hospitalization in patients with acute dyspnea, including those with acute heart failure and acute coronary syndrome [
38]. In the context of ischemic heart disease, subsequent analyses of PRIDE showed that elevated sST2 levels at presentation were associated with increased risk of adverse outcomes, including all-cause mortality and incident heart failure, independent of traditional risk factors and established cardiac biomarkers. sST2 provided additive prognostic information to NT-proBNP and troponins, supporting its role in risk stratification for patients with acute coronary syndromes and heart failure [
38] The additive prognostic value of sST2 to NT-proBNP and hs-TnT may reflect the pathophysiology underlying each biomarker. In contrast to NT-pBNP and troponin produced by cardiomyocytes, the production of sST2 finds its source in endothelial cells, and is promoted by tissue damage, inflammation, and extracellular matrix remodeling [
39]. In PRIDE, patients with hemodynamic overload, expression of cardiomyocyte damage, and tissue remodeling showed an 8-fold increased risk of all-cause mortality and a 5-fold increased risk of CV mortality [
39].
Several meta-analyses have demonstrated the predictive potential of sST2 not just in acute HF but in patients suffering from acute coronary syndromes [
6,
40]. A meta-analysis by Liu et comprised of 22 articles and 17,432 enrolled CAD patients showed that higher concentrations of baseline sST2 are associated with a higher risk of MACEs, all-cause mortality, CV death, and HF in patients with CAD. Elevated sST2 levels significantly predicted future MACEs in the ACS yet not in the non-ACS population [
41]. However, sST2 in isolation cannot be considered as a risk factor. Its low specificity in relation to endpoints during MI was confirmed in the CLARITY-TIMI study [
29,
42]. However, as indicated by a sub-analysis of this trial, when sST2 is combined with NT-proBNP, the predictive prognostic power of short-term risk stratification in this population is enhanced. Nevertheless, the prognostic power of sST2 during a longer observation period has not yet been fully elucidated.
Furthermore, in our trial sST2 levels during baseline hospitalization were associated with less predictive accuracy when matched with the other two evaluated biomarkers aligning with the findings of the CLARITY-TIMI trial [
37], although sST2 has shown efficacy in diagnosis of later CV events, CHF and even CV events after COVID-19 in FU [
43]. Indeed, these findings support previous speculations, proposing a combination of cardiac biomarkers from different pathogenic backgrounds for improvement of risk stratification in different CV pathologies [
11,
21,
25,
38].
While our study focused on a STEMI population, some authors have noted comparable 1-year outcomes for STEMI and NSTEMI patients alike [
44]. sST2 has also been shown to be an independent predictor of adverse outcomes in long-term follow-up after NSTEMI, demonstrating its predictive utility, especially when combined with traditional risk scores (e.g., GRACE). However, its value in improving outcomes over established risk models in a NSTEMI population is negligible [
45]. In our study, the CV mortality rate of 22.1% during 18-month FU seems high. However, when compared to previous registry results, it is only slightly higher than CV endpoint rates in the average European population [
39]. Our high CV mortality rate may be mainly attributed to our patient population stemming from large, rural regions with inadequate access to timely acute medical care and appropriate FU, also demonstrated by the high number of patients undergoing primary thrombolytic therapy (35/147, 23.8%) in our study. Additionally, high alcohol consumption, an unhealthy diet, a high incidence of metabolic syndrome and social-economic factors must be taken into account in this regard [
40,
41]. Nevertheless, high-risk patients can be effectively identified retrospectively, and our data emphasize the potential need for application of multimarker approaches in populations at increased risk for CV events.
Compared to previous trials, the cut-off levels for biomarkers proposed in our study are relatively divergent. Indeed, sST2 was shown to be a prognostic marker in the FU of HF patients, with a cut-off level of >35 ng/mL indicating a worse prognosis [
21,
23]. However, the calculated cut-off for sST2 for our study endpoint CV mortality was 27 ng/mL. The potential reasons for this finding are varied. First, differing results between labs may be attributed to the type of ELISA kits for sST2 analysis available, potentially accounting for variation in the sST2 values. Second, given the inclusion of patients up to 48 h after onset of symptoms, a delay in blood sampling may be a potential confounder in this regard, with sST2 levels peaking about 6–18 h after the onset of symptoms in MI [
22,
35]. Additionally, the young mean age of our study collective may have had an influence on the relatively low cut-off. Nevertheless, while dealing with CV mortality in a STEMI population with higher LVEF, considering the proposed cut-off value of 35 ng/mL in the FU of stable HF with reduced ejection fraction patients in numerous studies, our cut-off value seems reasonable [
21,
23]. On the other hand, regarding NT-pro-BNP, given the time between blood sampling and onset of symptoms as well as the ongoing secretion of NT-pro-BNP following MI, these findings also seem adequate.
In conclusion, our study proposes a significant correlation of biomarkers with 18-month post-discharge CV mortality in STEMI patients. While CRP > 11 mg/L has demonstrated prognostic efficacy predicting in-hospital mortality, our study could show that sST2 > 11 ng/mL is a promising predictive tool for CV mortality during 18-month FU post STEMI. In addition to CRP and sST2, the risk factors associated with in-hospital mortality were high glucose and low GFR, while older age and CKD were associated with higher mortality in midterm FU, which were in line with previous studies.
4.1. Future Perspectives
Currently, the American College of Cardiology/American Heart Association and European Society of Cardiology do not endorse use of CRP, H-FABP, or sST2 for risk stratification or prediction in acute coronary syndrome. However, our study and the aforementioned trials suggest the potential value of CRP and sST2 for targeted short- and intermediate CV event prediction, which in turn could guide the intensity of follow-up and therapeutic interventions.
CRP reflects residual inflammatory risk, and tailored post-discharge management of patients with higher CRP levels could thus include more intensive follow-up, aggressive risk factor modification, and consideration of anti-inflammatory therapies or statin intensification. sST2 reflects myocardial stress and fibrosis, and elevated sST2 levels are associated with a higher risk of heart failure, adverse remodeling, and mortality. As sST2 is not confounded by age or renal function, it may be valuable for use in elderly and comorbid populations.
Combining biomarkers with clinical risk scores (e.g., GRACE, TIMI), may enable more precise risk stratification, allowing clinicians to tailor follow-up intensity and guide therapeutic decisions for secondary prevention in the individual ischemic heart disease patient. Future, large prospective studies are needed to better determine the combined prognostic value of these biomarkers among specific STEMI subgroups by age, sex, ethnicity, and comorbidity profiles.
4.2. Limitations
Several limitations in our study must be considered. Our single-center study contained a relatively small sample size of n = 179. In this regard, the obtained sample of participants cannot be considered sufficiently representative, limiting the interpretation of results and its applicability to the general population. Furthermore, 9/156 patients (5.8%) in the post-discharge cohort were censored in Kaplan-Meier analysis: 6 due to non-CV death and 3 due to lost to follow-up. The survival status of the 3 lost to follow-up patients may only be speculated, although no information was found in the death registry.
A major limitation in our study was the lack of serialized biomarker measurements and our study used a single sample only which may not capture biomarker peaks at later timepoints. In our study, biomarkers were drawn within the first 8 h of admission prior to PCI, however, as CRP levels may peak later, the inflammatory signal in our study, therefore may be underrepresented. As previously mentioned, patients were included up to 48 h after onset of symptoms, therefore, a delay in blood sampling may be a potential confounder. While CRP was analyzed, our study also lacked hs-CRP analysis.
Another limitation of our study concerned the timing of the study blood sampling with respect to PCI or lytic therapies. In our study, there were high rates of thrombolytic therapy (35/147 patients, 23.8%), which is explained by longer patient transfer times from distant rural regions to our cardiac catheterization center. However, more specific information on the timing of the blood draw in the subgroup of patients undergoing lytic therapy was not analyzed, which is also a weakness of our paper. However, our data represent a real-life scenario, which in daily clinical practice is often characterized by testing and treatments done at various time points after symptom start/after presentation for STEMI. While hs-Troponin I and CK-MB were used to confirm the diagnosis of STEMI, hs-Tropinin T levels were not routinely used to facilitate the diagnosis of acute MI which may be considered a limitation. In addition, fast and routine applications of measurements of sST2 are currently still lacking. Therefore, despite promising results, routine application of the proposed biomarkers H-FABP and sST2 is still limited. It must be stated that due to our small sample size, our results are not intended to replace established risk scores (e.g., GRACE) but instead offer further insight into the prognostic potential of these biomarkers in STEMI patients.