Combined Robotic VErticalization and Lower Limb Mobilization in Patients with Severe Acquired Brain Injury: Protocol of a Multicenter Randomized Controlled Trial (VEM-sABI) †
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Participating Centers
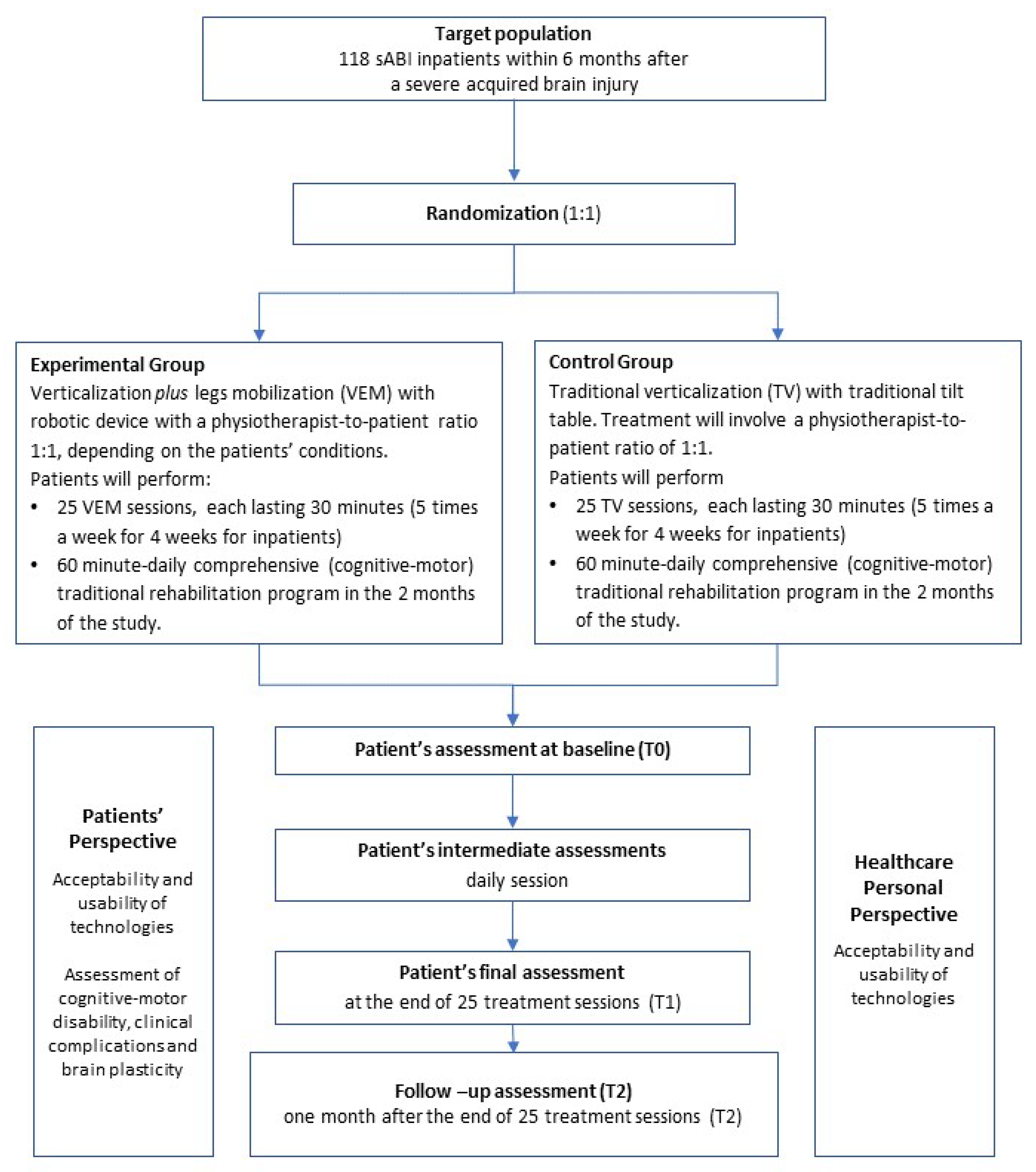
2.2. Study Population
Sample Size
2.3. Randomization and Trial Intervention
2.3.1. Randomization
2.3.2. Trial Protocol
- -
- Mild AE: No session suspension required. Symptoms are transient and resolve without intervention (e.g., mild and temporary facial pallor without changes in vital signs);
- -
- Moderate AE: Interruption of a single session is required. Symptoms resolve within the same day (e.g., oxygen desaturation resolving after endotracheal suctioning);
- -
- Severe AE: Permanent discontinuation of treatment is considered in case of repeated or persistent AE unresponsive to intervention (e.g., hypotension not responding to reduction in tilt angle), or based on medical judgment prioritizing patient safety.
2.4. Outcome Measures
- -
- -
- The Coma Recovery Scale-Revised (CRS-R) [23,26] is an observer-rated tool designed to assess patients with disorders of consciousness. It evaluates auditory, visual, motor, oromotor/verbal, communication, and arousal functions to help differentiate between vegetative state, minimally conscious state, and emergence from these conditions.
- -
- The Disability Rating Scale (DRS) [27], an observer-rated, 30-point continuous scale that provides quantitative information to document the progress of patients with severe brain injury from coma to community reintegration. It evaluates 8 areas of functioning, organized into 4 categories: (1) consciousness (eye opening, verbal response, motor response); (2) cognitive ability (feeding, toileting, grooming); (3) dependence on others; (4) employability. Each area of functioning is rated on a scale of 0 to either 3, 4, or 5 (maximum score = 30-death, minimum score = 0-person without disability) with the highest scores representing the higher level of disability.
- -
- The Modified Ashworth Scale (MAS) [28], an observer-rated scale used to measure muscle spasticity by assessing resistance during passive soft-tissue stretching. Scores range from 0 (no increase in muscle tone) to 4 (rigid in flexion or extension).
- -
- The Medical Research Council (MRC) scale [29], an observer-rated scale for assessing muscle strength, scoring from 0 (no contraction) to 5 (normal strength) in specific muscle groups.
- -
- The Fondazione Don Gnocchi Clinical Complication Scale (FDG-CCS) [30], an observer-rated tool for recording and grading clinical complications during rehabilitation of patients with sABI, covering medical, nursing, and functional events to support patient monitoring and care planning.
- -
- The modified Barthel Index (mBI) [31], an observer-rated continuous scale for evaluation of ability to perform autonomously personal activities of daily living. It measures physical disability across 10 categories that are scored from 0 to either 5, 10, or 15 (maximum score = 100-independence, minimum score = 0-complete dependence) with the highest scores representing the higher level of independence.
- -
- The System Usability Scale (SUS) [32] is a simple, ten-item 5-point Likert scale giving a global view of subjective assessments of usability, including effectiveness, efficiency, and satisfaction of a device. Higher scores correspond to higher usability.
- -
- The Nociception Coma Scale–Revised (NCS-R) [33], an observer-rated tool to assess pain-related behaviors in patients with disorders of consciousness. It evaluates motor, verbal, and facial responses to noxious stimulation, with higher scores indicating stronger nociceptive responses.
- -
- The Agitated Behaviour Scale (ABS) [34], an observer-rated scale measuring the nature and extent of agitation during recovery from brain injury. It assesses behavioral, emotional, and cognitive components, with higher scores indicating greater agitation severity.
2.4.1. Blood Biomarkers and Sampling Procedure
2.4.2. EEG Recording and Analysis
- “resting-state” EEG (duration 15 min).
- “reactivity” EEG (duration 15–17 min) using the following randomized stimuli (each stimulus twice; interstimulus interval ≥ 1 min):
- eye opening and (forced) eye closing;
- proximal noxious stimulation (deep pressure applied to the trapezius muscle on each side);
- distal noxious stimulation (pressing fingernail beds on each hand);
- acoustic stimulation (hand clapping);
- personalized acoustic stimulation (patient’s name);
- intermittent photic stimulation (5 s trains of flashes at 1-2-8-10-15-18-20-25-40-50-60 Hz)
2.5. Data Collection and Management
2.6. Statistical Plan
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Agitated Behavior Scale |
| AE | Adverse Events |
| AER | Adverse Events Report |
| ANCOVA | Analysis of Covariance |
| BDNF | Brain-Derived Neurotrophic Factor |
| BP | Blood Pressure |
| CRS-R | Coma Recovery Scale-Revised |
| CT | Computerized Tomography |
| DRS | Disability Rating Scale |
| EEG | Electroencephalogram |
| eDoC | emergence from Disorder of Consciousness |
| FDG-CCS | Fondazione Don Gnocchi-Clinical Complication Scale |
| GFAP | Glial Fibrillary Acid Protein |
| HR | Heart Rate |
| IQR | Inter-Quartile Range |
| LCF | Levels of Cognitive Functioning |
| LMM/GLMM | Linear Mixed-effects Models/Generalized Linear Mixed-effects Models |
| LP-HP | Low Pass-High Pass |
| MAR | Missing at Random |
| MAS | Modified Ashworth Scale |
| MCS | Minimally Conscious State |
| MNAR | Missing Not At Random |
| MRC | Medical Research Council scale |
| mBI | modified Barthel Index |
| NCS-R | Nociception Coma Scale-Revised |
| NF-L | Neurofilament Light chain |
| pDoC | prolonged Disorder of Consciousness |
| RCT | Randomized Controlled Trial |
| REDCap | Research Electronic Data Capture |
| sABI | severe Acquired Brain Injury |
| SPIRIT | Standard Protocol Items: Recommendations for Interventional Trials |
| SUS | System Usability Scale |
| TPI | Time Post-Injury |
| TV | Traditional Verticalization |
| VEM | robotic VErticalization and Mobilization |
| VS/UWS | Vegetative State/Unresponsive Wakefulness Syndrome |
Appendix A
| Original Name | English Name | Description |
|---|---|---|
| Sinus | Sinus | Walking with additional high strike phase, step per minute range from 8 to 80 |
| Andatura | Walking | Walking, step per minute range from 8 to 80 |
| Alternato | Alternate | Simple stepping, step per minute range from 8 to 80 |
Appendix B
| Drug Class | Examples | Estimated Half-Life | Recommended Action |
|---|---|---|---|
| Loop diuretics | Furosemide | 1–1.5 h (up to 24 h in renal failure) | Consider holding 12–24 h prior |
| Beta-blockers | Metoprolol, Atenolol | Metoprolol: 1–9 h Atenolol: Up to 24 h | Reduce dose or hold 24 h prior |
| Alpha-blockers | Doxazosin, Prazosin | ~22 h | Stop 48 h before |
| ACE inhibitors | Enalapril, Lisinopril | 11–24 h | Consider holding 24–48 h before |
| ARBs | Losartan | up to 24 h | Consider holding 24–48 h before |
| Tricyclic antidepressants | Amitriptyline, Nortriptyline | 25 h | Hold 48–72 h before |
| Antipsychotics | Risperidone, Quetiapine, Olanzapine | 3–30 h (depends on drug) | Review or suspend 48 h before |
| Dopaminergic agents | Pramipexole, Ropinirole | >6 h | Taper or review |
| Benzodiazepines/Sedatives | Lorazepam, Diazepam | Lorazepam: 12–16 h; Diazepam: >30 h | Hold 24–72 h before |
| Nitrates | Nitroglycerin, Isosorbide | 1–5 h | Suspend the day before |
| Opioids | Morphine, Oxycodone | Morphine: 2–4 h; Oxycodone: >12 h | Hold 24–48 h before if possible |
| Drug Class | Examples | Half-Life (approx.) | Cognitive/CNS Effects | When to Stop Before Cognitive Tasks/Intervention |
|---|---|---|---|---|
| Z-drugs | Zolpidem | 2–3 h | Memory alteration, confusion | 12–24 h before |
| Typical Antipsychotics | Haloperidol | 14–26 h | Rigidity, slowed cognition, delirium | ≥3–4 days before |
| Sedating Antidepressants | Mirtazapine, Trazodone | Mirtazapine: 20–40 h; Trazodone: 5–13 h | Sedation, psychomotor slowing | Mirtazapine: 2–3 days; Trazodone: 24–48 h before |
| Anticholinergics | Oxybutynin, Trihexyphenidyl | 2–16 h | Confusion, disorientation | ≥2–3 days before |
| Antihistamines | Diphenhydramine | 4–9 h | Sedation, attention deficit | 24–48 h before |
| Antiepileptics | Levetiracetam, Carbamazepine | Levetiracetam: 6–8 h; Carbamazepine: 12–17 h | Slowed processing, dizziness | ≥2–4 days before |
| Parkinson’s Drugs | Levodopa | 1–2 h | Hallucinations, confusion | Taper 24–48 h before if possible |
| Corticosteroids | Dexamethasone, Prednisone | Dexamethasone: 3–4.5 h; Prednisone: 3–6 h | Insomnia, psychosis, delirium (high doses) | Avoid night doses; consider tapering 48 h before |
| Chemotherapeutics | Methotrexate, Cisplatin | Methotrexate: ~3–10 h Cisplatin: variable | Confusion, memory loss | Not suspendable; monitor symptoms |
| Barbiturates | Phenobarbital | 80–100 h | Deep sedation, cognitive decline | ≥7–10 days before |
| Melatonin (high dose) | Melatonin | 20–50 min | Mild sedation, drowsiness | ≥6–8 h before |
| Category | Intervention | Evidence Level |
|---|---|---|
| Pharmacological | Amantadine | ★★★★☆—Supported by one RCT in subacute traumatic brain injury |
| Zolpidem | ★★☆☆☆—Case reports and EEG, PET studies; paradoxical responders | |
| Baclofen, Midazolam | ★☆☆☆☆—Theoretical rationale; anecdotal use; limited case data | |
| Amitriptyline, Desipramine, Protriptyline | ★☆☆☆☆—Sparse data; potential cognitive modulation via noradrenergic/cholinergic systems | |
| Modafinil | ★★☆☆☆—Case reports and early-phase trials suggest increased arousal | |
| Psilocybin | ☆☆☆☆☆—Experimental stage; partial agonist of the 5-HT2A serotonergic receptor in the central nervous system. | |
| Neuromodulation | Deep Brain Stimulation | ★★☆☆☆—Positive results in small case series; no large RCTs |
| Transcranial Electrical Stimulation | ★★★☆☆—Some positive RCTs (30–50% response in MCS patients) | |
| Transcranial Magnetic Stimulation | ★☆☆☆☆—Inconsistent results; limited RCT evidence | |
| Mechanical | Low-Intensity Focused Ultrasound Pulsation | ★★☆☆☆—Early-phase studies and case reports; promising but experimental |
| Sensory | Auditory, tactile, vestibular stimulation | ★★☆☆☆—Some RCTs show behavioral or autonomic improvements |
| Personalized music therapy | ★★☆☆☆—Small studies and clinical reports suggest potential benefits | |
| Regenerative | Stem cell therapies (IV or intrathecal) | ★★☆☆☆—Phase I studies suggest safety and preliminary efficacy |
| Neurogenesis, gliogenesis, axonal regrowth | ☆☆☆☆☆—Preclinical and conceptual stage |
References
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef]
- Linee di Indirizzo per L’assistenza alle Persone in Stato Vegetativo e Stato di Minima Coscienza; Ministero della Salute: Roma, Italy, 2011.
- Harper, C.M.; Lyles, Y.M. Physiology and Complications of Bed Rest. J. Am. Geriatr. Soc. 1988, 36, 1047–1054. [Google Scholar] [CrossRef]
- Griffin, J.E. The Physiology and Pathology of Bed Rest. Phys. Ther. 1971, 51, 592–593. [Google Scholar] [CrossRef]
- Ng, H.; King, A. A Systematic Review of Head-up Tilt to Improve Consciousness in People with a Prolonged Disorder of Consciousness. Clin. Rehabil. 2021, 35, 13–25. [Google Scholar] [CrossRef]
- Kuznetsov, A.N.; Rybalko, N.V.; Daminov, V.D.; Luft, A.R. Early Poststroke Rehabilitation Using a Robotic Tilt-Table Stepper and Functional Electrical Stimulation. Stroke Res. Treat. 2013, 946056. [Google Scholar] [CrossRef]
- Czell, D.; Schreier, R.; Rupp, R.; Eberhard, S.; Colombo, G.; Dietz, V. Influence of Passive Leg Movements on Blood Circulation on the Tilt Table in Healthy Adults. J. Neuroeng. Rehabil. 2004, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Huebner, L.; Warmbein, A.; Scharf, C.; Schroeder, I.; Manz, K.; Rathgeber, I.; Gutmann, M.; Biebl, J.; Mehler-Klamt, A.; Huber, J.; et al. Effects of Robotic-Assisted Early Mobilization versus Conventional Mobilization in Intensive Care Unit Patients: Prospective Interventional Cohort Study with Retrospective Control Group Analysis. Crit. Care 2024, 28, 112. [Google Scholar] [CrossRef] [PubMed]
- Riberholt, C.G.; Lindschou, J.; Gluud, C.; Mehlsen, J.; Møller, K. Early Mobilisation by Head-up Tilt with Stepping versus Standard Care after Severe Traumatic Brain Injury—Protocol for a Randomised Clinical Feasibility Trial. Trials 2018, 19, 612. [Google Scholar] [CrossRef] [PubMed]
- Frazzitta, G.; Valsecchi, R.; Zivi, I.; Sebastianelli, L.; Bonini, S.; Zarucchi, A.; Matteri, D.; Molatore, K.; Maestri, R.; Saltuari, L. Safety and Feasibility of a Very Early Verticalization in Patients with Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, 290–292. [Google Scholar] [CrossRef]
- Turolla, A.; Kiper, P.; Mazzarotto, D.; Cecchi, F.; Colucci, M.; D’Avenio, G.; Facciorusso, S.; Gatti, R.; Giansanti, D.; Iosa, M.; et al. Reference Theories and Future Perspectives on Robot-Assisted Rehabilitation in People with Neurological Conditions: A Scoping Review and Recommendations from the Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE). NeuroRehabilitation 2022, 51, 681–691. [Google Scholar] [CrossRef]
- Garlet, A.B.; Righi, N.C.; Schardong, J.; Della Méa Plentz, R. Effects of Robotic Rehabilitation Using the Erigo® Device on Patients with Neurological Injury: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Disabil. Rehabil. Assist. Technol. 2024, 19, 1135–1144. [Google Scholar] [CrossRef]
- Rosenfelder, M.J.; Helmschrott, V.C.; Willacker, L.; Einhäupl, B.; Raiser, T.M.; Bender, A. Effect of Robotic Tilt Table Verticalization on Recovery in Patients with Disorders of Consciousness: A Randomized Controlled Trial. J. Neurol. 2023, 270, 1721–1734. [Google Scholar] [CrossRef] [PubMed]
- Ancona, E.; Quarenghi, A.; Simonini, M.; Saggini, R.; Mazzoleni, S.; De Tanti, A.; Saviola, D.; Salvi, G. Pietro Effect of Verticalization with Erigo® in the Acute Rehabilitation of Severe Acquired Brain Injury. Neurol. Sci. 2019, 40, 2073–2080. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; Balletta, T.; Saccá, I.; De Luca, R.; Bramanti, P. Do Post-Stroke Patients Benefit from Robotic Verticalization? A Pilot-Study Focusing on a Novel Neurophysiological Approach. Restor. Neurol. Neurosci. 2015, 33, 671–681. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Chan, A.W.; Boutron, I.; Hopewell, S.; Moher, D.; Schulz, K.F.; Collins, G.S.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. SPIRIT 2025 statement: Updated guideline for protocols of randomised trials. BMJ 2025, 389, e081477. [Google Scholar] [CrossRef]
- De Luca, R.; Gangemi, A.; Bonanno, M.; Fabio, R.A.; Cardile, D.; Maggio, M.G.; Rifici, C.; Vermiglio, G.; Di Ciuccio, D.; Messina, A.; et al. Improving Neuroplasticity through Robotic Verticalization Training in Patients with Minimally Conscious State: A Retrospective Study. Brain Sci. 2024, 14, 319. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)-A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011, 161, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Edlow, B.L.; Sanz, L.R.D.; Polizzotto, L.; Pouratian, N.; Rolston, J.D.; Snider, S.B.; Thibaut, A.; Stevens, R.D.; Gosseries, O.; Curing Coma Campaign and its contributing members. Therapies to Restore Consciousness in Patients with Severe Brain Injuries: A Gap Analysis and Future Directions. Neurocrit Care 2021, 35 (Suppl. S1), 68–85. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, F.; Savva, G.M.; Grossi, C.M.; Bennett, K.; Fox, C.; Maidment, I.; Loke, Y.K.; Steel, N.; Kenny, R.A.; Richardson, K. Cognitive decline associated with anticholinergics, benzodiazepines and Z-drugs: Findings from The Irish Longitudinal Study on Ageing (TILDA). Br. J. Clin. Pharmacol. 2021, 87, 2818–2829. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement Characteristics and Diagnostic Utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef]
- Hagen, C.; Malkmus, D.; Durham, P. Levels of Cognitive Functioning. In Rehabilitation of the Head Injured Adult: Comprehensive Physical Management; Professional Staff Association of Rancho Los Amigos Hospital: Downey, CA, USA, 1972. [Google Scholar]
- Galeoto, G.; Turriziani, S.; Berardi, A.; Sansoni, J.; Santilli, V.; Mascio, M.; Paoloni, M. Levels of Cognitive Functioning Assessment Scale: Italian Cross-Cultural Adaptation and Validation. Ann. Ig. 2020, 32, 16–26. [Google Scholar] [CrossRef]
- Estraneo, A.; Moretta, P.; Cardinale, V.; De Tanti, A.; Gatta, G.; Giacino, J.T.; Trojano, L. A Multicentre Study of Intentional Behavioural Responses Measured Using the Coma Recovery Scale-Revised in Patients with Minimally Conscious State. Clin. Rehabil. 2015, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, M.; Hall, K.M.; Hopkins, K.; Belleza, T.; Cope, D.N. Disability Rating Scale for Severe Head Trauma: Coma to Community. Arch. Phys. Med. Rehabil. 1982, 63, 118–123. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council: Nerve Injuries Research Committee. Aids to the Investigation of Peripheral Nerve Injuries; His Majesty’s Stationery Office: Norwich, UK, 1942; Volume 48. [Google Scholar]
- Estraneo, A.; Fiorentino, M.R.; Cibellis, T.; Campana, B.; Balbi, P.; Carli, V.; Vatteroni, E.; Devalle, G.; Mantelli, F.; Villa, M.; et al. The Clinical Complication Scale of Fondazione Don Gnocchi for Classifying Clinical Complications in Patients with Severe Acquired Brain Injury: Development and Multicenter Validation. Front. Neurol. 2025, 16, 1537093. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the Sensitivity of the Barthel Index for Stroke Rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Brooke, J. SUS—A Quick and Dirty Usability Scale. Usability Eval Ind. 1996, 4, 189–194. [Google Scholar]
- Chatelle, C.; Majerus, S.; Whyte, J.; Laureys, S.; Schnakers, C. A Sensitive Scale to Assess Nociceptive Pain in Patients with Disorders of Consciousness. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1233–1237. [Google Scholar] [CrossRef]
- Derchi, C.C.; Arcuri, P.; Comanducci, A.; Caronni, A.; Pagliari, C.; Viganò, A.; Volpato, E.; Navarro, J.; Trimarchi, P.D. Italian translation and cultural adaptation of the Agitated Behavior Scale (ABS-I) in patients with acquired brain injuries. J. Rehabil. Med. 2024, 56, 11663. [Google Scholar] [CrossRef]
- Coppola, L.; Mirabelli, P.; Baldi, D.; Smaldone, G.; Estraneo, A.; Soddu, A.; Grimaldi, A.M.; Mele, G.; Salvatore, M.; Cavaliere, C. An Innovative Approach for the Evaluation of Prolonged Disorders of Consciousness Using NF-L and GFAP Biomarkers: A Pivotal Study. Sci. Rep. 2022, 12, 18446. [Google Scholar] [CrossRef]
- Bagnato, S.; Galardi, G.; Ribaudo, F.; Boccagni, C.; Fiorilla, T.V.; Rubino, F.; D’Ippolito, M.E.; Andriolo, M. Serum BDNF Levels Are Reduced in Patients with Disorders of Consciousness and Are Not Modified by Verticalization with Robot-Assisted Lower-Limb Training. Neural Plast 2020, 2020, 5608145. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, S.; D’Ippolito, M.E.; Boccagni, C.; De Tanti, A.; Lucca, L.F.; Nardone, A.; Salucci, P.; Fiorilla, T.; Pingue, V.; Gennaro, S.; et al. Sustained Axonal Degeneration in Prolonged Disorders of Consciousness. Brain Sci. 2021, 11, 1068. [Google Scholar] [CrossRef]
- Pei, Y.; Tang, X.; Zhang, E.; Lu, K.; Xia, B.; Zhang, J.; Huang, Y.; Zhang, H.Z.; Dong, L. The Diagnostic and Prognostic Value of Glial Fibrillary Acidic Protein in Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Eur. J. Trauma Emerg. Surg. 2023, 49, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, P.B.; Conte, M.M.; Fridman, E.A.; Voss, H.U.; Victor, J.D.; Schiff, N.D. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann. Neurol. 2014, 76, 869–879. [Google Scholar] [CrossRef]
- Estraneo, A.; Loreto, V.; Guarino, I.; Boemia, V.; Paone, G.; Moretta, P.; Trojano, L. Standard EEG in Diagnostic Process of Prolonged Disorders of Consciousness. Clin. Neurophysiol. 2016, 127, 2379–2385. [Google Scholar] [CrossRef]
- Hirsch, L.J.; Fong, M.W.K.; Leitinger, M.; LaRoche, S.M.; Beniczky, S.; Abend, N.S.; Lee, J.W.; Wusthoff, C.J.; Hahn, C.D.; Westover, M.B.; et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J. Clin. Neurophysiol. 2021, 38, 1–29. [Google Scholar] [CrossRef]
- Estraneo, A.; Loreto, V.; Masotta, O.; Pascarella, A.; Trojano, L. Do Medical Complications Impact Long-Term Outcomes in Prolonged Disorders of Consciousness? Arch. Phys. Med. Rehabil. 2018, 99, 2523–2531.e3. [Google Scholar] [CrossRef] [PubMed]
- Estraneo, A.; Masotta, O.; Bartolo, M.; Pistoia, F.; Perin, C.; Marino, S.; Lucca, L.; Pingue, V.; Casanova, E.; Romoli, A.; et al. Multi-Center Study on Overall Clinical Complexity of Patients with Prolonged Disorders of Consciousness of Different Etiologies. Brain Inj. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Estraneo, A.; Briand, M.M.; Noé, E. Medical Comorbidities in Patients with Prolonged Disorder of Consciousness: A Narrative Review. NeuroRehabilitation 2024, 54, 61–73. [Google Scholar] [CrossRef]
- Moretta, P.; Estraneo, A.; De Lucia, L.; Cardinale, V.; Loreto, V.; Trojano, L. A Study of the Psychological Distress in Family Caregivers of Patients with Prolonged Disorders of Consciousness during In-Hospital Rehabilitation. Clin. Rehabil. 2014, 28, 717–725. [Google Scholar] [CrossRef]
- Soeterik, S.M.; Connolly, S.; Playford, E.D.; Duport, S.; Riazi, A. The Psychological Impact of Prolonged Disorders of Consciousness on Caregivers: A Systematic Review of Quantitative Studies. Clin. Rehabil. 2017, 31, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Knox, L.; Douglas, J.M. A Scoping Review of the Nature and Outcomes of Extended Rehabilitation Programmes after Very Severe Brain Injury. Brain Inj. 2018, 32, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Di Muro, G.; Tessarolo, C.; Cagnotti, G.; Favole, A.; Ferrini, S.; Ala, U.; Favole, A.; Ferrini, S.; Ala, U.; Bellino, C.; et al. Neurofilament light chain (Nf-L) in cerebrospinal fluid and serum as a potential biomarker in the differential diagnosis of neurological diseases in cattle. Vet Res. 2025, 56, 6. [Google Scholar] [CrossRef] [PubMed]
- Baldez, D.P.; Biazus, T.B.; Rabelo-da-Ponte, F.D.; Nogaro, G.P.; Martins, D.S.; Kunz, M.; Czepielewski, L.S. The effect of antipsychotics on the cognitive performance of individuals with psychotic disorders: Network meta-analyses of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 126, 265–275. [Google Scholar] [CrossRef]
| Time Point/ Measure | Pre-Randomization | Randomization | Post-Randomization | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Enrolment | T0 (At the Start of Intervention) | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | T1 (After the End of the Intervention) | T2 (1 Month After T1) | |
| Informed consent | X | ||||||||
| Eligibility screening | X | ||||||||
| Allocation | X | ||||||||
| Clinical and anamnestic data collection | X | ||||||||
| Interventions: | |||||||||
| VEM | X | X | X | X | X | ||||
| TV | X | X | X | X | X | ||||
| Outcomes: | |||||||||
| LCF | X | X | X | ||||||
| CRS-R | X | X | X | ||||||
| mBI | X | X | X | ||||||
| DRS | X | X | X | ||||||
| SUS | X | ||||||||
| MAS | X | X | X | ||||||
| MRC | X | X | X | ||||||
| FDG-CCS | X | X | X | ||||||
| ABS | X | X | X | X | X | ||||
| NCS-R | X | X | X | X | X | ||||
| 30 min standard EEG | X | X | X | ||||||
| Blood sampling | X | X | |||||||
| AER | X | X | X | X | X | X | X | ||
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Outcome | Timing, Measure |
|---|---|
Primary outcome:
|
|
| Secondary outcomes: | |
|
|
| Exploratory outcomes: | |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estraneo, A.; Fiorentino, M.R.; Magliacano, A.; Puopolo, M.A.; Rivetti, I.; Messa, M.C. Combined Robotic VErticalization and Lower Limb Mobilization in Patients with Severe Acquired Brain Injury: Protocol of a Multicenter Randomized Controlled Trial (VEM-sABI). J. Clin. Med. 2025, 14, 6628. https://doi.org/10.3390/jcm14186628
Estraneo A, Fiorentino MR, Magliacano A, Puopolo MA, Rivetti I, Messa MC. Combined Robotic VErticalization and Lower Limb Mobilization in Patients with Severe Acquired Brain Injury: Protocol of a Multicenter Randomized Controlled Trial (VEM-sABI). Journal of Clinical Medicine. 2025; 14(18):6628. https://doi.org/10.3390/jcm14186628
Chicago/Turabian StyleEstraneo, Anna, Maria Rosaria Fiorentino, Alfonso Magliacano, Maria Assunta Puopolo, Ilaria Rivetti, and Maria Cristina Messa. 2025. "Combined Robotic VErticalization and Lower Limb Mobilization in Patients with Severe Acquired Brain Injury: Protocol of a Multicenter Randomized Controlled Trial (VEM-sABI)" Journal of Clinical Medicine 14, no. 18: 6628. https://doi.org/10.3390/jcm14186628
APA StyleEstraneo, A., Fiorentino, M. R., Magliacano, A., Puopolo, M. A., Rivetti, I., & Messa, M. C. (2025). Combined Robotic VErticalization and Lower Limb Mobilization in Patients with Severe Acquired Brain Injury: Protocol of a Multicenter Randomized Controlled Trial (VEM-sABI). Journal of Clinical Medicine, 14(18), 6628. https://doi.org/10.3390/jcm14186628






