Medication-Induced Xerostomia: Cross-Sectional Analysis of Salivary Flow, Intraoral Aching, and Anxiety
Abstract
1. Introduction
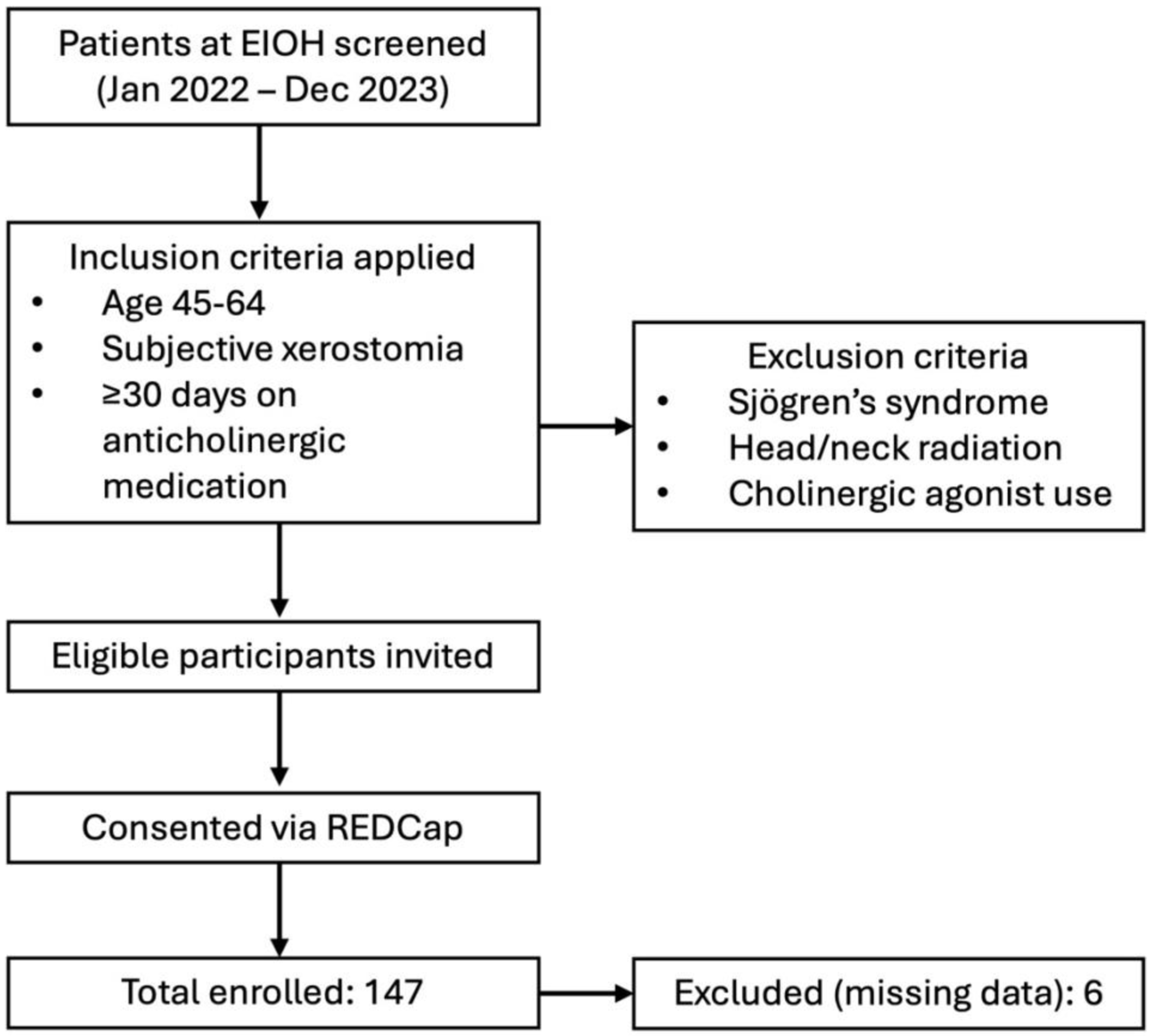
2. Materials and Methods
Statistical Analysis
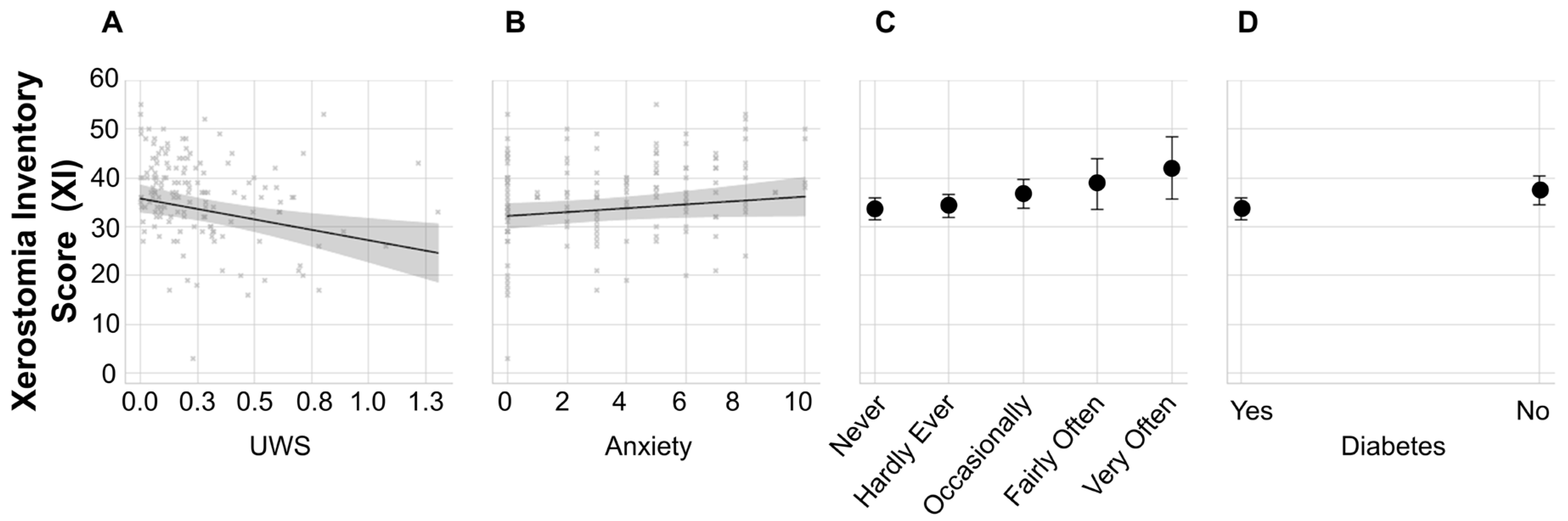
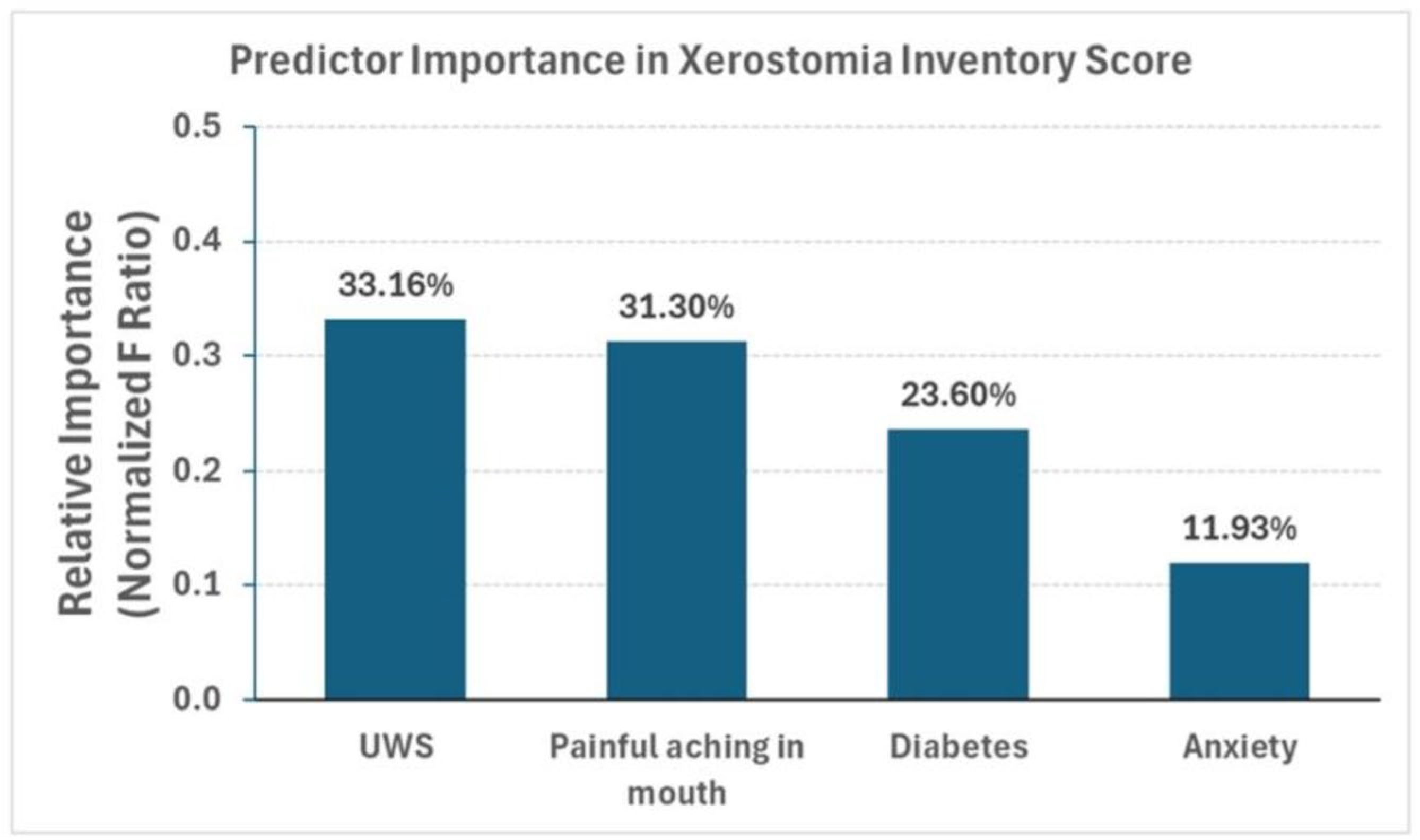
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbe, A.G. Medication-Induced Xerostomia and Hyposalivation in the Elderly: Culprits, Complications, and Management. Drugs Aging 2018, 35, 877–885. [Google Scholar] [CrossRef]
- Villa, A.; Wolff, A.; Aframian, D.; Vissink, A.; Ekstrom, J.; Proctor, G.; McGowan, R.; Narayana, N.; Aliko, A.; Sia, Y.W.; et al. World Workshop on Oral Medicine VI: A systematic review of medication-induced salivary gland dysfunction: Prevalence, diagnosis, and treatment. Clin. Oral. Investig. 2015, 19, 1563–1580. [Google Scholar] [CrossRef]
- Sreebny, L.M.; Schwartz, S.S. A reference guide to drugs and dry mouth—2nd edition. Gerodontology 1997, 14, 33–47. [Google Scholar] [CrossRef]
- Arany, S.; Kopycka-Kedzierawski, D.T.; Caprio, T.V.; Watson, G.E. Anticholinergic medication: Related dry mouth and effects on the salivary glands. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 132, 662–670. [Google Scholar] [CrossRef]
- Charlesworth, C.J.; Smit, E.; Lee, D.S.; Alramadhan, F.; Odden, M.C. Polypharmacy Among Adults Aged 65 Years and Older in the United States: 1988–2010. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Tiisanoja, A.; Syrjala, A.H.; Kullaa, A.; Ylostalo, P. Anticholinergic Burden and Dry Mouth in Middle-Aged People. JDR Clin. Trans. Res. 2020, 5, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Murray Thomson, W. Epidemiology of oral health conditions in older people. Gerodontology 2014, 31 (Suppl. 1), 9–16. [Google Scholar] [CrossRef]
- Fox, P.C.; Busch, K.A.; Baum, B.J. Subjective reports of xerostomia and objective measures of salivary gland performance. J. Am. Dent. Assoc. 1987, 115, 581–584. [Google Scholar] [CrossRef]
- Navazesh, M.; Kumar, S.K. Measuring salivary flow: Challenges and opportunities. J. Am. Dent. Assoc. 2008, 139, 35S–40S. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, M.; Caetano de Souza Valentim, E.; Barmak, A.B.; Arany, S. Potential association of anticholinergic medication intake and caries experience in young adults with xerostomia. J. Dent. Sci. 2023, 18, 1693–1698. [Google Scholar] [CrossRef]
- Proctor, G.B.; Shaalan, A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. J. Dent. Res. 2021, 100, 1201–1209. [Google Scholar] [CrossRef]
- Galvao-Moreira, L.V.; de Andrade, C.M.; de Oliveira, J.F.F.; Bomfim, M.R.Q.; Figueiredo, P.M.S.; Branco-de-Almeida, L.S. Sex Differences in Salivary Parameters of Caries Susceptibility in Healthy Individuals. Oral. Health Prev. Dent. 2018, 16, 71–77. [Google Scholar] [CrossRef]
- Canfora, F.; Calabria, E.; Spagnuolo, G.; Coppola, N.; Armogida, N.G.; Mazzaccara, C.; Solari, D.; D’Aniello, L.; Aria, M.; Pecoraro, G.; et al. Salivary Complaints in Burning Mouth Syndrome: A Cross Sectional Study on 500 Patients. J. Clin. Med. 2023, 12, 5561. [Google Scholar] [CrossRef]
- Lee, Y.H.; Won, J.H.; Auh, Q.S.; Noh, Y.K.; Lee, S.W. Prediction of xerostomia in elderly based on clinical characteristics and salivary flow rate with machine learning. Sci. Rep. 2024, 14, 3423. [Google Scholar] [CrossRef]
- Lalla, R.V.; Helgeson, E.S.; Virk, K.; Lu, H.; Treister, N.S.; Sollecito, T.P.; Schmidt, B.L.; Patton, L.L.; Lin, A.; Brennan, M.T. Females have lower salivary flow than males, before and after radiation therapy for head/neck cancer. Oral. Dis. 2025, 31, 857–864. [Google Scholar] [CrossRef]
- Sanchez Garrido, I.; Ramirez, L.; Munoz Corcuera, M.; Garrido, E.; Sanchez, L.; Martinez Acitores, M.L.; Hernandez, G.; Lopez-Pintor, R.M. Xerostomia and Salivary Dysfunction in Patients With Diabetes Mellitus. A Cross-Sectional Study. J. Oral. Pathol. Med. 2024, 53, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Kohli, D.; Madhu, N.; Korczeniewska, O.A.; Eliav, T.; Arany, S. Association between medication-induced xerostomia and orofacial pain: A systematic review. Quintessence Int. 2023, 54, 658–670. [Google Scholar] [CrossRef]
- Miranda-Rius, J.; Brunet-Llobet, L.; Lahor-Soler, E.; Farre, M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int. J. Med. Sci. 2015, 12, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhu, H.; Wu, M. Disproportionality analysis of drug-induced dry mouth using data from the United States food and drug administration adverse event reporting system database. Heliyon 2024, 10, e38561. [Google Scholar] [CrossRef]
- da Silva, L.A.; Teixeira, M.J.; de Siqueira, J.T.; de Siqueira, S.R. Xerostomia and salivary flow in patients with orofacial pain compared with controls. Arch. Oral. Biol. 2011, 56, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.L.; Mulsant, B.H.; Pollock, B.G.; Lehman, M.E.; Greenspan, A.; Mahmoud, R.A.; Kirshner, M.A.; Sorisio, D.A.; Bies, R.R.; Gharabawi, G. Anticholinergic activity of 107 medications commonly used by older adults. J. Am. Geriatr. Soc. 2008, 56, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Kersten, H.; Molden, E.; Willumsen, T.; Engedal, K.; Bruun Wyller, T. Higher anticholinergic drug scale (ADS) scores are associated with peripheral but not cognitive markers of cholinergic blockade. Cross sectional data from 21 Norwegian nursing homes. Br. J. Clin. Pharmacol. 2013, 75, 842–849. [Google Scholar] [CrossRef]
- Michail, A.; Almirza, M.; Alwaely, F.; Arany, S. Anticholinergic burden of medications is associated with dry mouth and reflected in minor labial gland secretion. Arch. Oral. Biol. 2023, 156, 105824. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Tune, L.E.; Damlouji, N.F.; Holland, A.; Gardner, T.J.; Folstein, M.F.; Coyle, J.T. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet 1981, 2, 651–653. [Google Scholar] [CrossRef]
- Navazesh, M.; Christensen, C.M. A comparison of whole mouth resting and stimulated salivary measurement procedures. J. Dent. Res. 1982, 61, 1158–1162. [Google Scholar] [CrossRef]
- Thomson, W.M.; Williams, S.M. Further testing of the xerostomia inventory. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2000, 89, 46–50. [Google Scholar] [CrossRef]
- Kiss, A.F.; Vasko, D.; Deri, M.T.; Toth, K.; Monostory, K. Combination of CYP2C19 genotype with non-genetic factors evoking phenoconversion improves phenotype prediction. Pharmacol. Rep. 2018, 70, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Luyk, N.H.; Beck, F.M.; Weaver, J.M. A visual analogue scale in the assessment of dental anxiety. Anesth. Prog. 1988, 35, 121–123. [Google Scholar]
- Slade, G.D. Derivation and validation of a short-form oral health impact profile. Community Dent. Oral. Epidemiol. 1997, 25, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A. Diagnosing, managing, and preventing salivary gland disorders. Oral. Dis. 2002, 8, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sorensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekstrom, J. Salivary secretion in health and disease. J. Oral. Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Grushka, M. Burning mouth syndrome: Pain disorder remains difficult to treat. Ont. Dent. 1987, 64, 26–30. [Google Scholar]
- Carlson, C.R.; Miller, C.S.; Reid, K.I. Psychosocial profiles of patients with burning mouth syndrome. J. Orofac. Pain. 2000, 14, 59–64. [Google Scholar]
- Lopez-Pintor, R.M.; Casanas, E.; Gonzalez-Serrano, J.; Serrano, J.; Ramirez, L.; de Arriba, L.; Hernandez, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef] [PubMed]
- Zieba, S.; Zalewska, A.; Zukowski, P.; Maciejczyk, M. Can smoking alter salivary homeostasis? A systematic review on the effects of traditional and electronic cigarettes on qualitative and quantitative saliva parameters. Dent. Med. Probl. 2024, 61, 129–144. [Google Scholar] [CrossRef] [PubMed]
| Total | Female | Male | |
|---|---|---|---|
| Mean ± S.D. (Min, Max), N | Mean ± S.D. (Min, Max), N | Mean ± S.D. (Min, Max), N | |
| Age | 55.86 ± 5.73 (45, 64), 147 | 55.81 ± 5.72 (45, 64), 104 | 56.00 ± 5.80 (45, 64), 43 |
| Race (N) | Asian (1) | Asian (1) | Asian (0) |
| Black (25) | Black (17) | Black (8) | |
| Native American (10) | Native American (4) | Native American (6) | |
| Other (6) | Other (4) | Other (2) | |
| Prefer not to answer (2) | Prefer not to answer (2) | Prefer not to answer (0) | |
| White (103) | White (76) | White (27) | |
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | |
| Xerostomia Score | 36.29 ± 8.17 | 37.36 ± 7.70 | 33.70 ± 8.78 |
| Painful Aching in the Mouth (N) | Never (55) | Never (39) | Never (16) |
| Hardly Ever (47) | Hardly Ever (28) | Hardly Ever (19) | |
| Occasionally (29) | Occasionally (22) | Occasionally (7) | |
| Fairly Often (10) | Fairly Often (10) | Fairly Often (0) | |
| Very Often (6) | Very Often (5) | Very Often (1) | |
| Smoking (N) | Yes, current (28) | Yes, current (18) | Yes, current (10) |
| Former (52) | Former (36) | Former (16) | |
| Never (67) | Never (50) | Never (17) | |
| Diabetes (N) | Yes (38) | Yes (26) | Yes (12) |
| No (109) | No (78) | No (31) | |
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Minor Saliva Flow (MSF) (ml/min) | 6 (3.5, 8) | 5 (3.5, 8.0) | 7 (5, 10) |
| Unstimulated Whole Saliva Flow (UWS) (ml/min) | 0.19 (0.07, 0.32) | 0.16 (0.06, 0.28) | 0.25 (0.14, 0.60) |
| Anxiety | 3 (0.75, 6) | 4 (2, 6) | 3 (0, 4) |
| Predictor Variable | Estimate | Std Error | F Ratio | t Ratio | Prob > |t| | Lower 95% | Upper 95% | VIF |
|---|---|---|---|---|---|---|---|---|
| UWS | −8.50 | 2.73 | 9.66 | −3.11 | 0.0023 | −13.90 | −3.09 | 1.04 |
| Anxiety | 0.42 | 0.22 | 3.48 | 1.86 | 0.0643 | −0.02 | 0.86 | 1.11 |
| Painful Aching in the Mouth | 1.83 | 0.61 | 9.12 | 3.02 | 0.0030 | 0.63 | 3.03 | 1.11 |
| Diabetes [No] | −1.93 | 0.74 | 6.88 | −2.62 | 0.0097 | −3.38 | −0.47 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korczeniewska, O.A.; Eliav, E.; Arany, S. Medication-Induced Xerostomia: Cross-Sectional Analysis of Salivary Flow, Intraoral Aching, and Anxiety. J. Clin. Med. 2025, 14, 6624. https://doi.org/10.3390/jcm14186624
Korczeniewska OA, Eliav E, Arany S. Medication-Induced Xerostomia: Cross-Sectional Analysis of Salivary Flow, Intraoral Aching, and Anxiety. Journal of Clinical Medicine. 2025; 14(18):6624. https://doi.org/10.3390/jcm14186624
Chicago/Turabian StyleKorczeniewska, Olga A., Eli Eliav, and Szilvia Arany. 2025. "Medication-Induced Xerostomia: Cross-Sectional Analysis of Salivary Flow, Intraoral Aching, and Anxiety" Journal of Clinical Medicine 14, no. 18: 6624. https://doi.org/10.3390/jcm14186624
APA StyleKorczeniewska, O. A., Eliav, E., & Arany, S. (2025). Medication-Induced Xerostomia: Cross-Sectional Analysis of Salivary Flow, Intraoral Aching, and Anxiety. Journal of Clinical Medicine, 14(18), 6624. https://doi.org/10.3390/jcm14186624






