Evolution and Comparative Analysis of Clinical Trials on Psilocybin in the Treatment of Psychopathologies: Trends in the EU and the US
Abstract
1. Introduction
- How do clinical trial trends involving psilocybin differ between the US and the EU?
- How do regulatory policies address psilocybin’s potential medical use?
- What are the observed differences in public and medical perceptions across the two regions?
2. Materials and Methods
2.1. Quantitative Methods
2.2. Qualitative Methods
3. Psilocybin and Its Therapeutic Potential in Mental Disorders
3.1. Mental Disorders Investigated in Psilocybin Studies
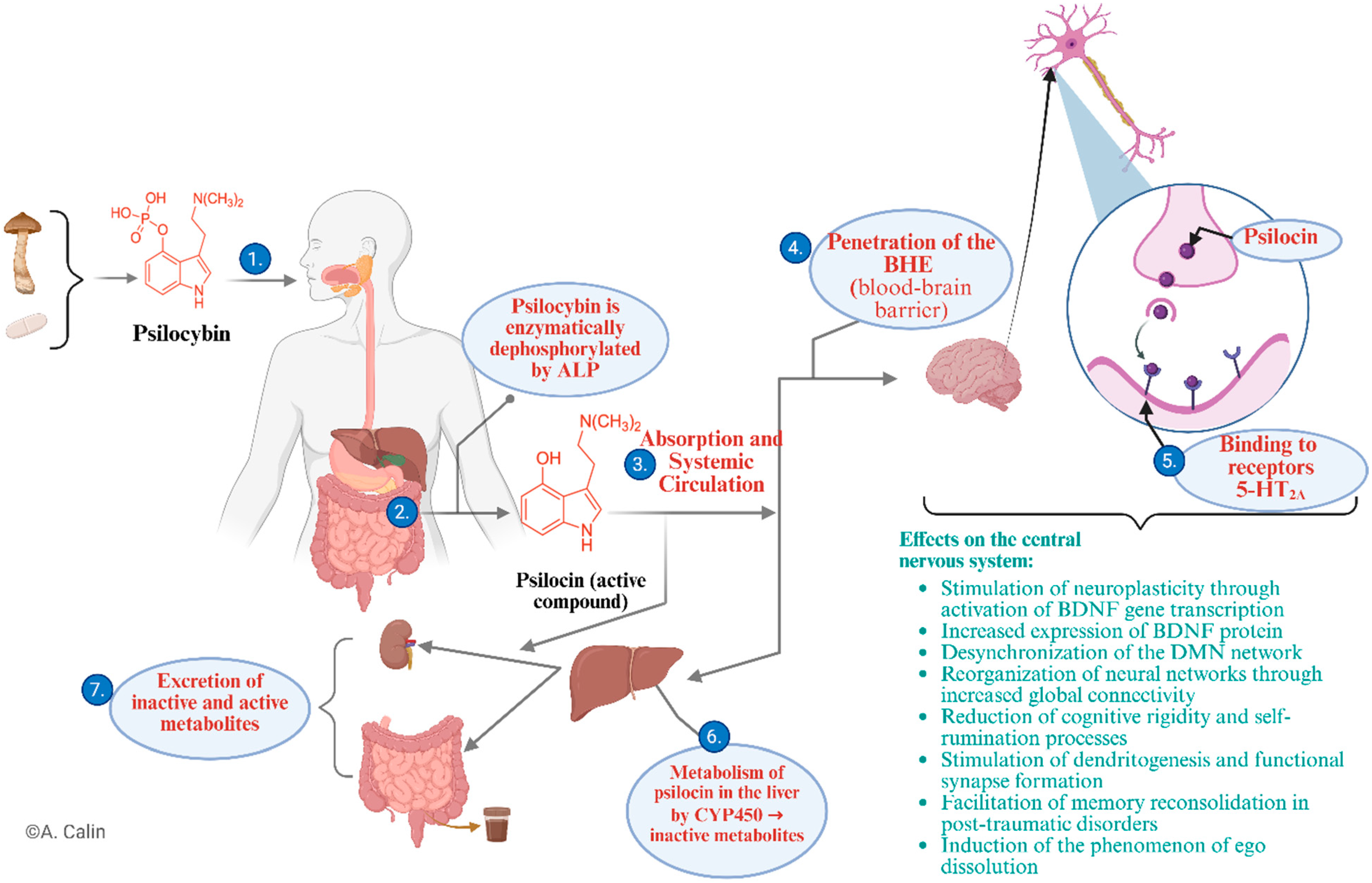
3.2. Psilocybin—Origin, Mechanisms of Action, Effects
- (1)
- After ingestion in natural or synthetic form, psilocybin is transported to the gastrointestinal tract;
- (2)
- In the small intestine, under the action of alkaline phosphatase, psilocybin is enzymatically dephosphorylated, resulting in the active metabolite psilocin, which is pharmacologically responsible for the effects on the central nervous system (CNS) [8];
- (3)
- Psilocin is absorbed at the enteric level and enters the systemic circulation, reaching therapeutically effective concentrations [37];
- (4)
- Due to its lipophilic structure, psilocin easily crosses the BBB, reaching the CNS [34];
- (5)
- At the synaptic level, it acts as a partial agonist of serotonergic 5-HT2A receptors, triggering profound changes [36];
- (6)
- At the same time, psilocin is metabolised in the liver, mainly by cytochrome P450 (CYP450) enzymes into inactive metabolites [8];
- (7)
- Excretion occurs via the kidneys and bile, in the form of active and inactive metabolites [31].
- Stimulation of neuroplasticity through activation of neurotrophic gene transcription and increased expression of BDNF (brain-derived neurotrophic factor) protein [16];
- Desynchronization of the Default Mode Network (DMN), associated with reduced rumination and negative self-referentiality [38];
- Reorganisation of functional neural connectivity, which supports better cognitive flexibility and emotional integration [39];
- Stimulation of dendritogenesis and the formation of functional synapses [40];
- Facilitation of memory reconsolidation, an important process in post-traumatic disorder [19];
- Induction of the phenomenon of ego dissolution, a phenomenon associated with profound restructuring of self-perception [20].
4. Quantitative Analysis of Clinical Studies on Psilocybin
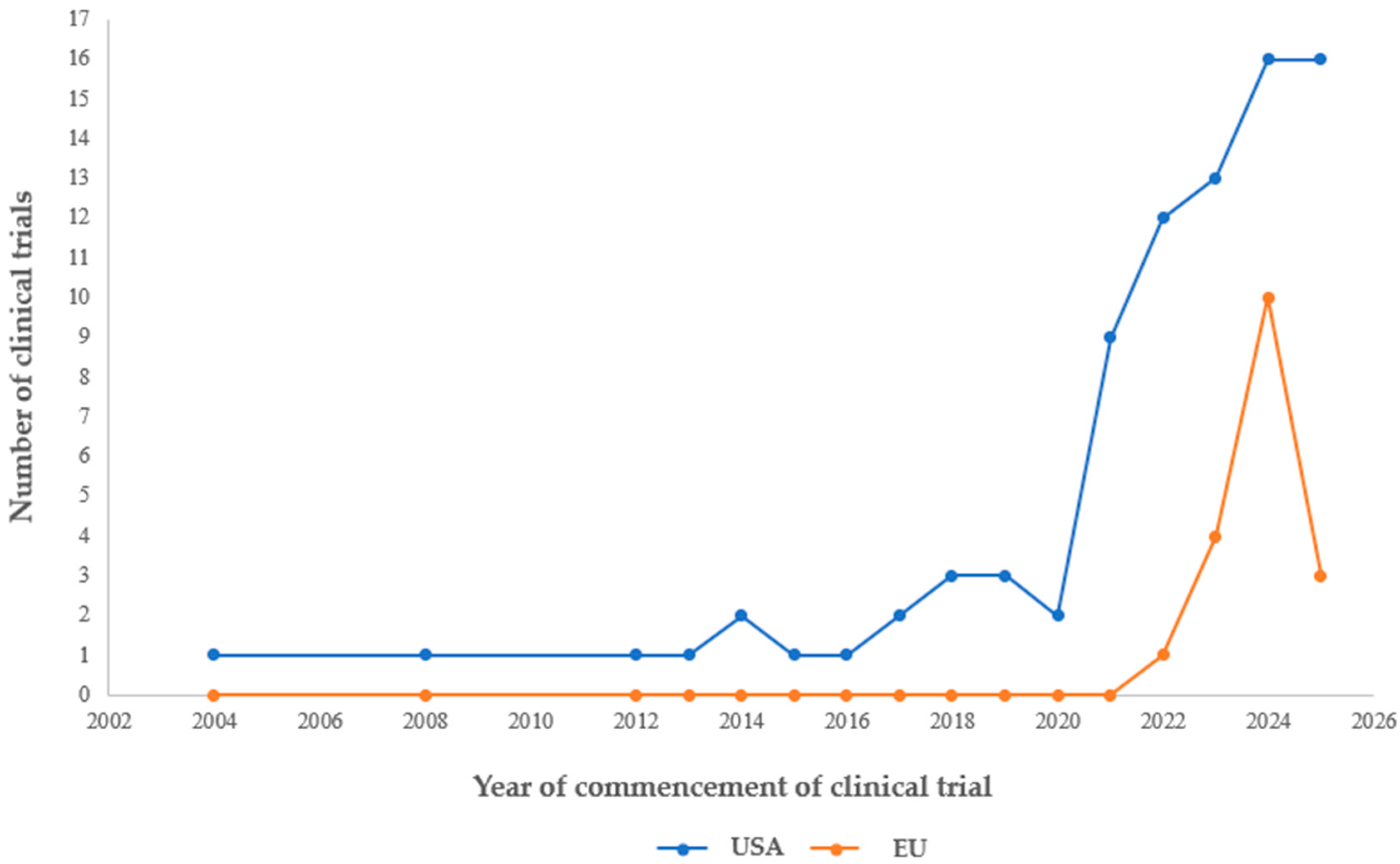
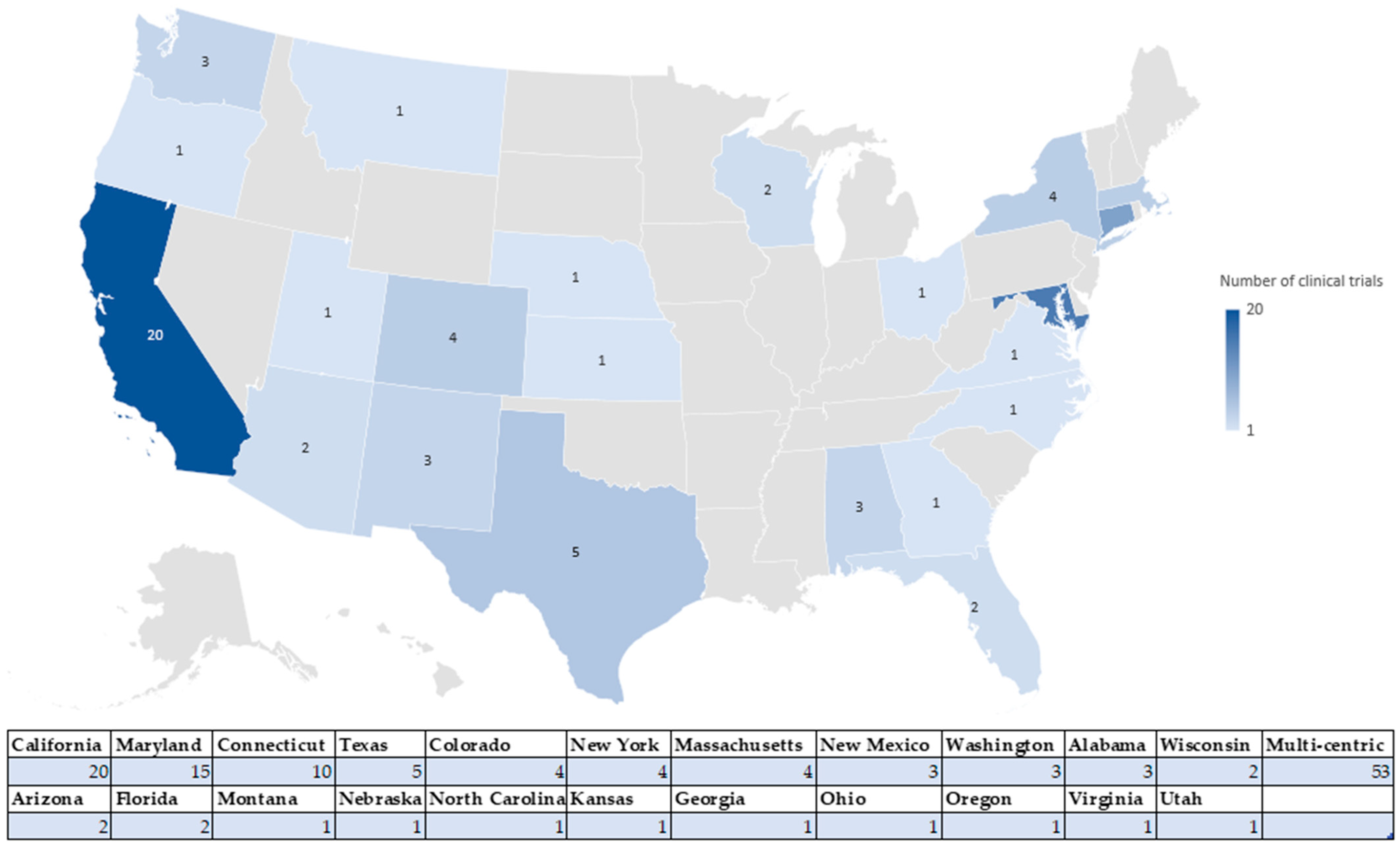
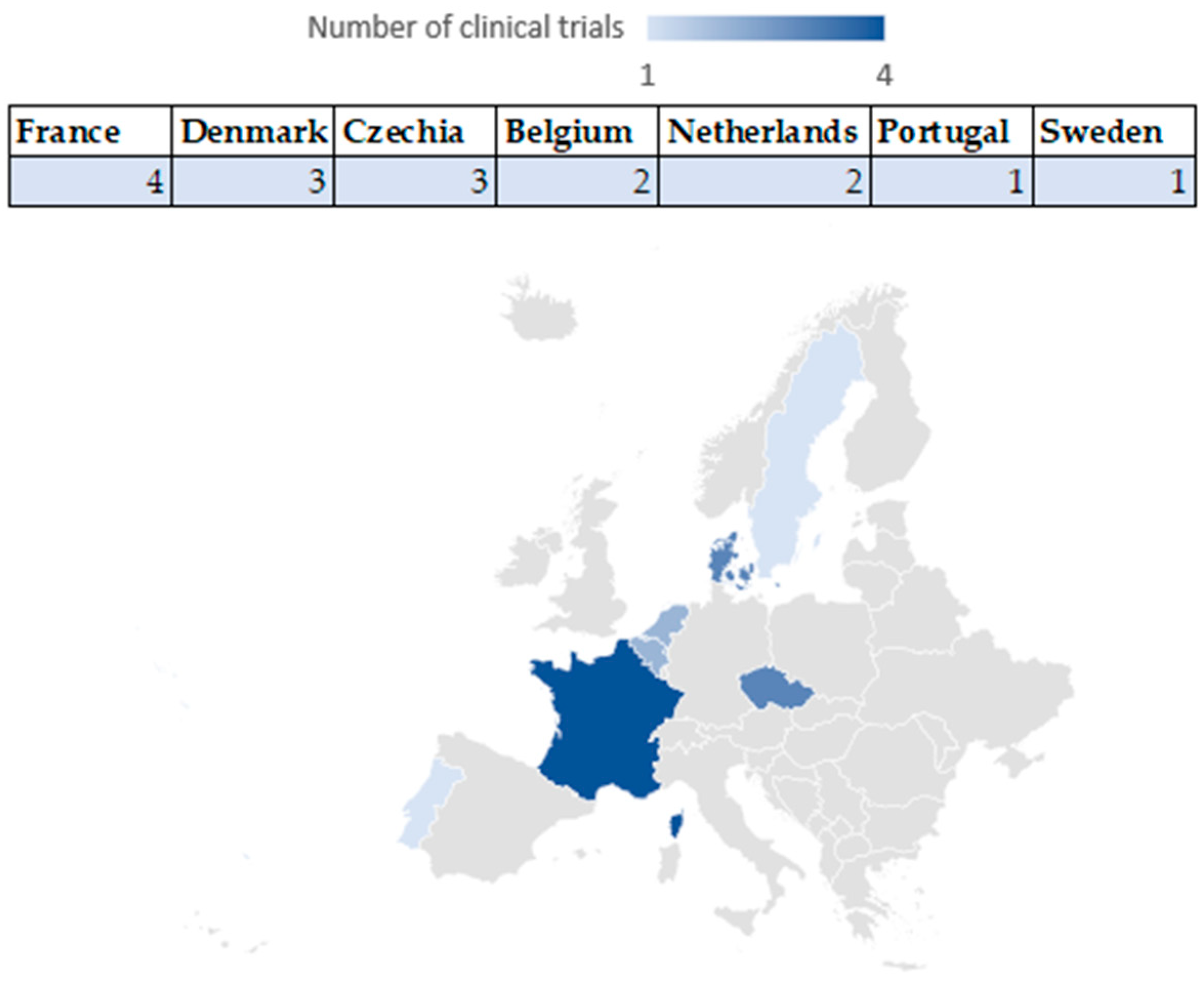
4.1. Number of Clinical Studies Registered in the US and EU: Geographical Distribution
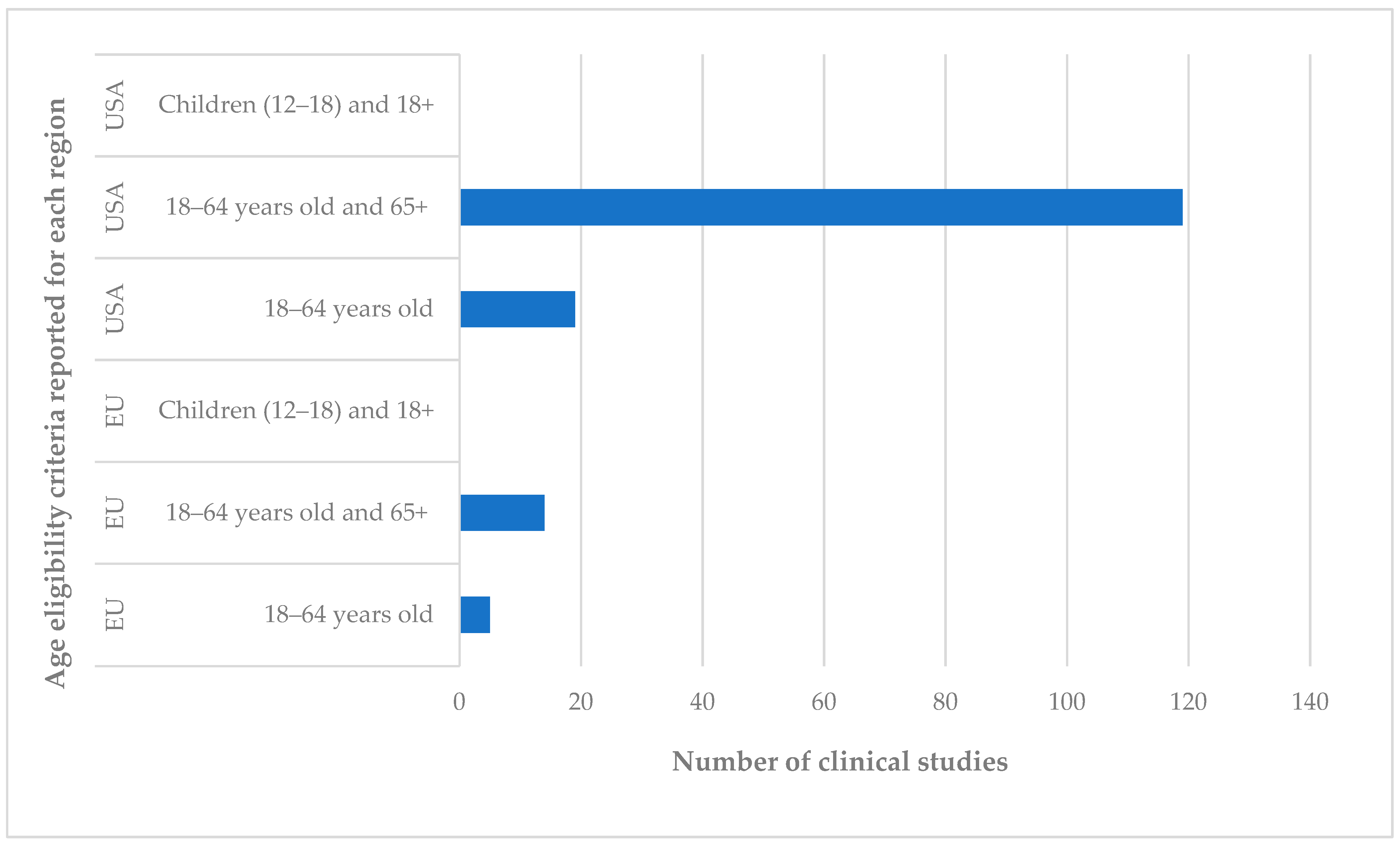
4.1.1. Total Number of Studies and Participants: Age of Participants
4.1.2. Geographic Distribution
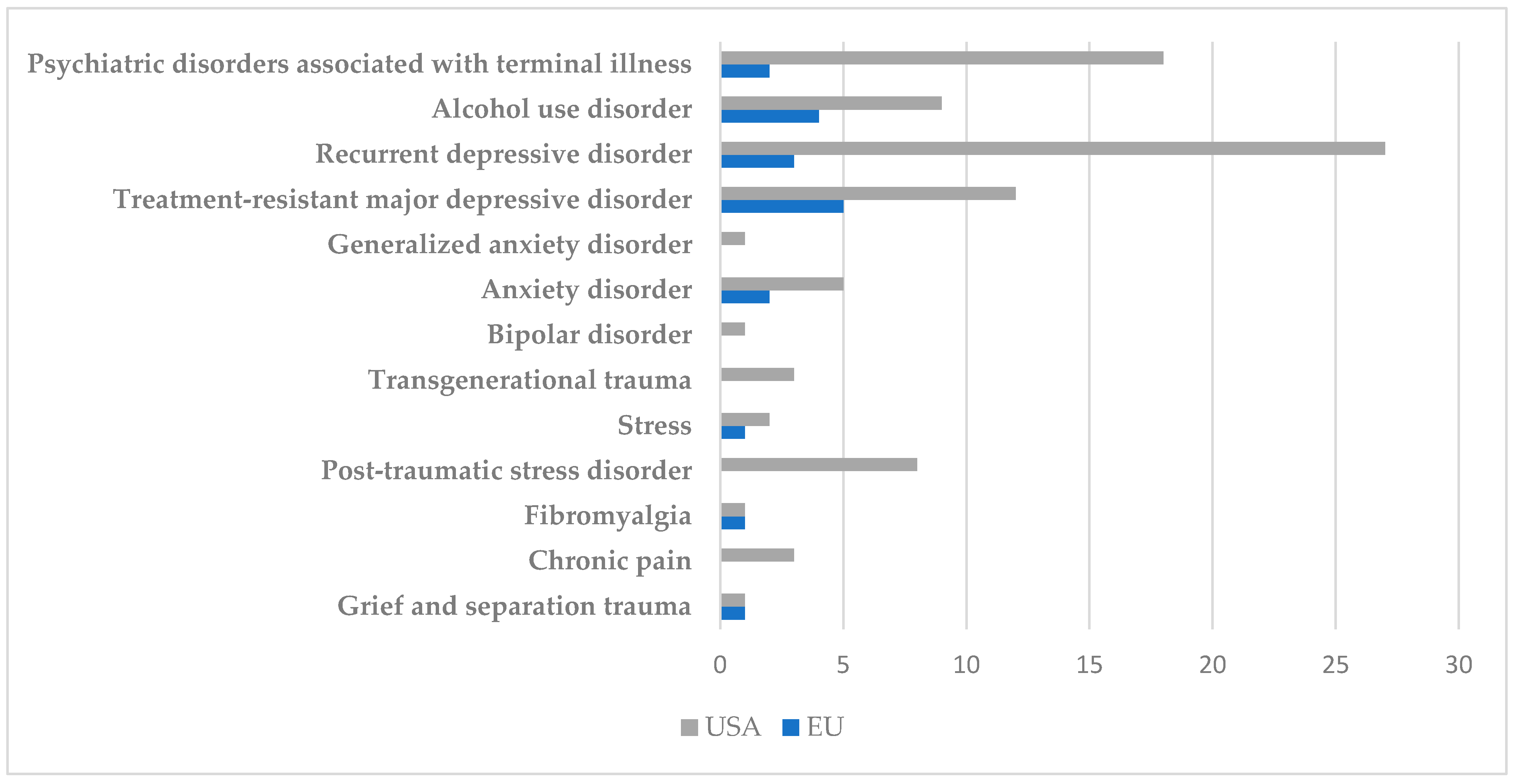
4.2. Types of Conditions Targeted in Identified Clinical Trials
4.2.1. Affective Disorders
4.2.2. PTSD, Trauma, and Stress
4.2.3. Addictions and Eating Disorders
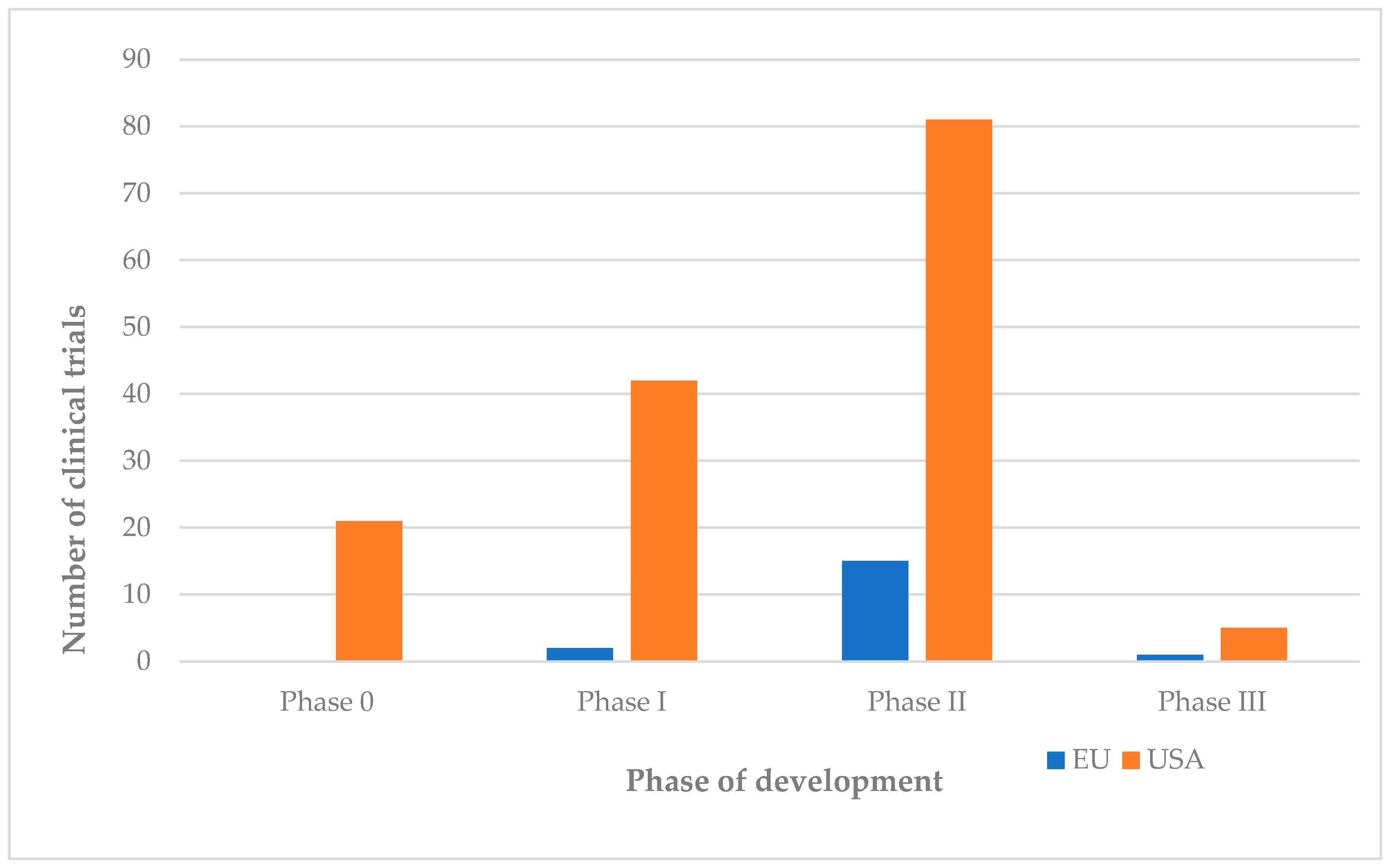
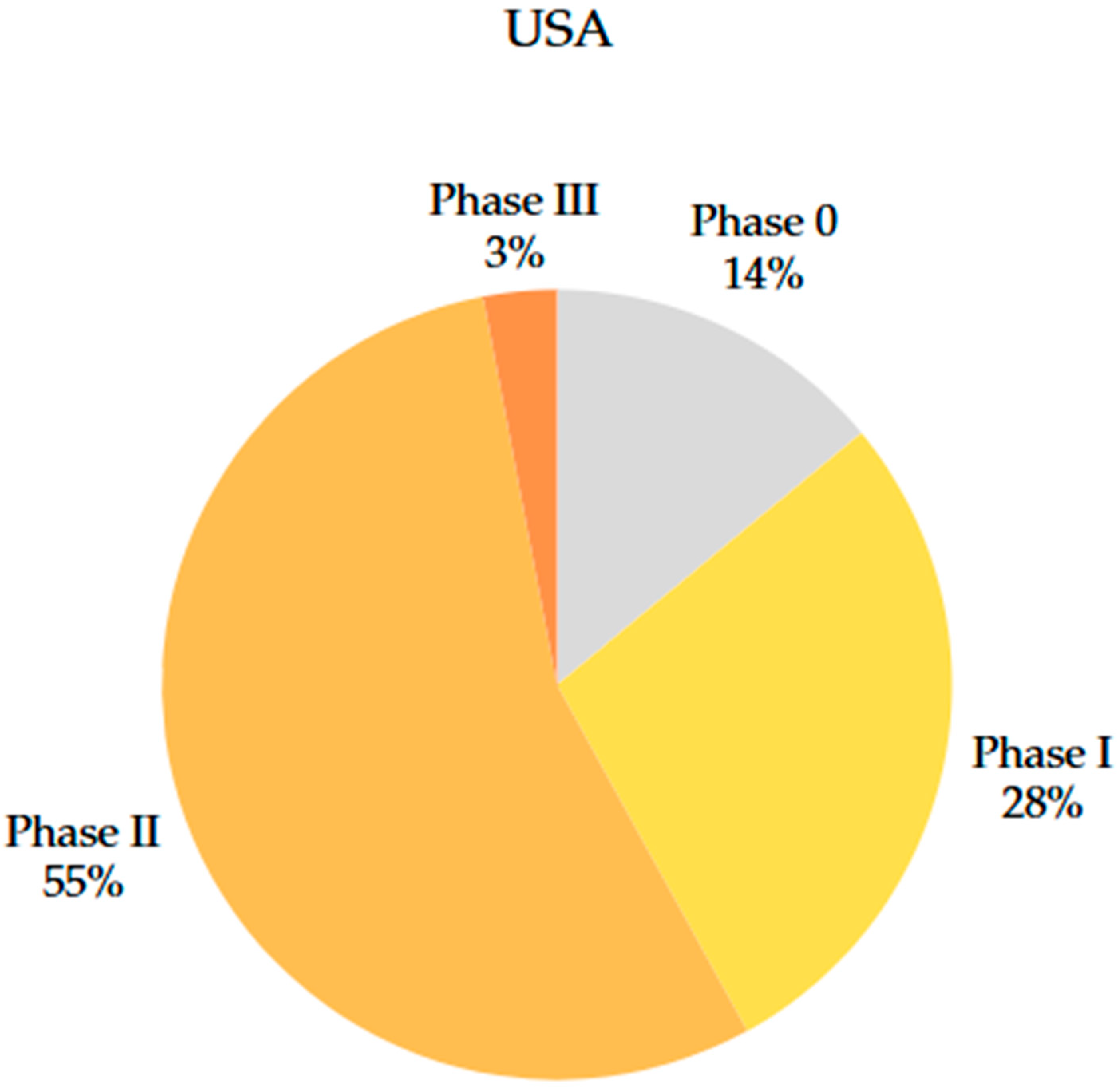
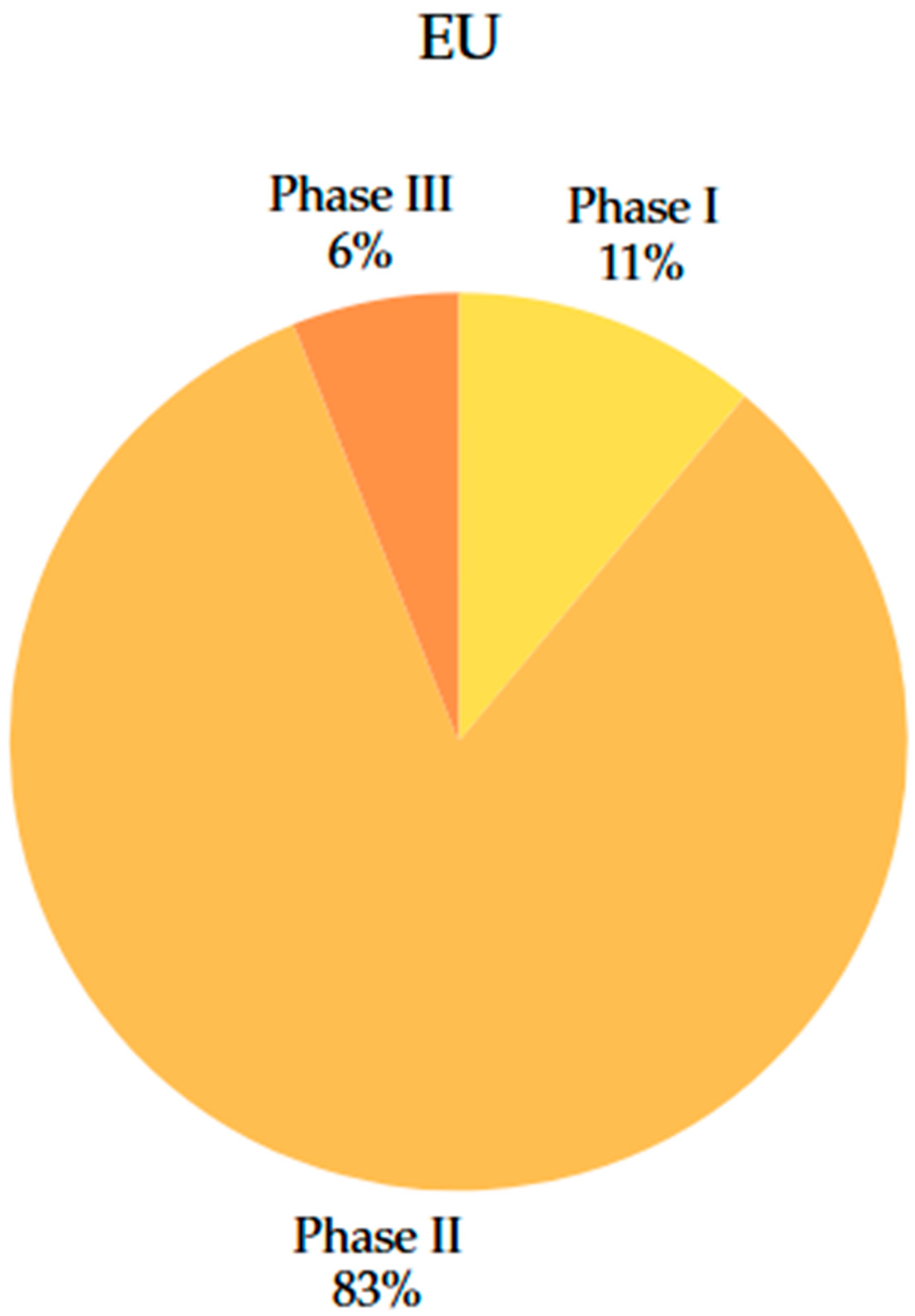
4.3. Comparison of Clinical Trial Phases
5. Qualitative Analysis of Clinical Studies on Psilocybin
5.1. Clinical Trial Phases: Characteristics, Conduct, and Examples
5.2. Legislative and Regulatory Framework: US vs. EU
- −
- application for IND;
- −
- protocol review by the IRB;
- −
- supervision of phases 0–IV, as appropriate, with a focus on safety and efficacy;
- −
- the possibility of Fast Track or Breakthrough Therapy designation for innovative therapies, which facilitates the documentation process and access to funding.
- −
- submission of a Clinical Trial Application (CTA) to the national authority (ANMDMR in Romania) and the bioethics committee;
- −
- evaluation of the CTA by the bioethics committee and the competent authority;
- −
- registration in the EU-CTR database;
- −
- ensuring compliance with EU Regulation 536/2014 on clinical trials.
5.3. Comparative Study: Relevant Clinical Cases: US vs. EU
- -
- Design: phase II, double-blind study, three patient groups (25 mg psilocybin, 10 mg psilocybin, 1 mg psilocybin); psilocybin was administered in a single dose, and the total number of participants was 233; the evaluation was conducted over six weeks, using the standardised MADRS scale to compare symptoms;
- -
- Sponsor: COMPASS Pathways, USA;
- -
- Design features: adaptive, sample size (from 180 to 233) and maximum concentration dose (from 20 mg to 25 mg) were increased with FDA approval after analysis of interim results; the study also included participant training sessions, integration, and psychological support, following FDA protocols.
- -
- Target population: patients with TRD who have previously failed at least two antidepressant treatments;
- -
- Centres: US, Canada, UK;
- -
- Results: significant reduction in depressive symptoms in the 25 mg group compared to the control group at 3 weeks post-single dose;
- -
- Status: Phase II completed, actively recruiting for Phase III.
- -
- Design: phase II, randomised, double-blind, active placebo-controlled (niacin) study with parallel groups; psilocybin was administered in a single dose of 25 mg and, for comparison, a control dose of 250 mg niacin; assessment was conducted over 4 weeks after the single dose, using the standardised MADRS scale to compare depressive symptoms;
- -
- Sponsor: Central Institute of Mental Health, Germany;
- -
- Design features: use of an active placebo, strict supervision by German regulatory authorities, BfArM and European authorities, EMA;
- -
- Target population: adult patients (18–64 years) suffering from treatment-resistant depression who had a history of at least two previous failed pharmacological treatments;
- -
- Centre: single centre, conducted exclusively in Germany, Mannheim;
- -
- Results: final results have not yet been published;
- -
- Status: study completed, with no reports of withdrawals, suspensions, or significant events during the study.
5.4. Factors Determining Differences Between the US and the EU in the Field of Clinical Trials with Psilocybin
5.4.1. Government Support and Public Health Policies
- −
- Oregon legalised the therapeutic use of psilocybin under regulated conditions, such as those included in clinical trials, in 2020 by adopting Measure 109; this led to the creation of a licensing system for the production and use of psilocybin [55];
- −
- Colorado—followed a similar system to Oregon, adopting Proposition 122 in 2022, which allowed the use of psilocybin in clinical trials and for therapeutic use [56];
- −
- Other states, such as California and Washington, developed pilot projects in 2023 to facilitate legislative changes to allow the development of Phase III and IV clinical trials for psilocybin [57].
5.4.2. Public Attitude and Social Acceptance
5.4.3. Involvement of the Pharmaceutical Industry and Private Investors
6. Challenges in Psilocybin Clinical Trials
6.1. Legislative and Institutional Barriers and Infrastructure
6.2. Ethical Challenges and Social Acceptability
6.3. Financial and Methodological Barriers
6.4. Patient Safety Risks: Challenges in Scaling Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | anxiety disorders |
| BBB | blood–brain barrier |
| BDNF CTIS | brain-derived neurotrophic factor Clinical Trials Information System |
| CTA | Clinical Trial Application |
| DMN | Default Mode Network |
| DMT | N,N-Dimethyltryptamine |
| EMA | European Medicines Agency |
| EU | European Union |
| EU CTR | EU Clinical Trials Register |
| EUDA | European Union Drugs Agency |
| FDA | Food and Drug Administration |
| IRB | Institutional Review Board |
| LSD | lysergic acid diethylamide |
| MAPS | Multidisciplinary Association for Psychedelic Studies |
| MD | major depression |
| MDMA | 3,4-methylenedioxy-N-methylamphetamine |
| OCD | obsessive-compulsive disorders |
| PAREA | Psychedelics Access and Research European Alliance |
| PTSD | post-traumatic stress disorder |
| RDD | recurrent depressive disorder |
| TRD | treatment-resistant major depression |
| USA/US | United States of America |
| WHO | World Health Organisation |
References
- Schenberg, E.E. Psychedelic-Assisted Psychotherapy: A Paradigm Shift in Psychiatric Research and Development. Front. Pharmacol. 2018, 9, 733. [Google Scholar] [CrossRef]
- Pepe, M.; Hesami, M.; de la Cerda, K.A.; Perreault, M.L.; Hsiang, T.; Jones, A.M.P. A Journey with Psychedelic Mushrooms: From Historical Relevance to Biology, Cultivation, Medicinal Uses, Biotechnology, and Beyond. Biotechnol. Adv. 2023, 69, 108247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K. The Magic of Mushrooms: Turning “Public Enemy Number One” Into an Ally to Help Put an End to the War on Drugs. Available online: https://uclawreview.org/2023/02/14/the-magic-of-mushrooms/ (accessed on 20 July 2025).
- Carbonaro, T.M.; Bradstreet, M.P.; Barrett, F.S.; MacLean, K.A.; Jesse, R.; Johnson, M.W.; Griffiths, R.R. Survey Study of Challenging Experiences after Ingesting Psilocybin Mushrooms: Acute and Enduring Positive and Negative Consequences. J. Psychopharmacol. 2016, 30, 1268–1278. [Google Scholar] [CrossRef]
- Fahrenkopf, L. Substance and Protocol: Federalist Regulation of Psychedelic-Assisted Therapy. Food Drug Law J. 2024, 79, 125–161. Available online: https://www.fdli.org/wp-content/uploads/2024/09/Fahrenkopf-Substance-and-Protocol-FDLJ-79-1.pdf (accessed on 21 July 2025).
- American Psychological Association Psychedelics as Medicine. Available online: https://www.apa.org/monitor/2024/06/psychedelics-as-medicine/ (accessed on 21 July 2025).
- Mithoefer, M.C.; Grob, C.S.; Brewerton, T.D. Novel Psychopharmacological Therapies for Psychiatric Disorders: Psilocybin and MDMA. Lancet Psychiatry 2016, 3, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, P.S.; Johnson, M.W.; Griffiths, R.R. Psilocybin, Psychological Distress, and Suicidality. J. Psychopharmacol. 2015, 29, 1041–1043. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 20 May 2025).
- Smausz, R.; Neill, J.; Gigg, J. Neural Mechanisms Underlying Psilocybin’s Therapeutic Potential—The Need for Preclinical in Vivo Electrophysiology. J. Psychopharmacol. 2022, 36, 781–793. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Stenbæk, D.S.; Kristiansen, S.; Burmester, D.; Lehel, S.; Páleníček, T.; Kuchař, M.; Svarer, C.; Ozenne, B.; et al. A Single Psilocybin Dose Is Associated with Long-Term Increased Mindfulness, Preceded by a Proportional Change in Neocortical 5-HT2A Receptor Binding. Eur. Neuropsychopharmacol. 2020, 33, 71–80. [Google Scholar] [CrossRef]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder. JAMA Psychiatry 2021, 78, 481. [Google Scholar] [CrossRef]
- World Health Organisation Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders/ (accessed on 21 July 2025).
- de Vos, C.M.H.; Mason, N.L.; Kuypers, K.P.C. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 724606. [Google Scholar] [CrossRef]
- National Research Council (US) and Institute of Medicine (US) Committee on Depression, Parenting Practices, and the Healthy Development of Children. Depression in Parents, Parenting, and Children; England, M., Sim, L., Eds.; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-12178-1. [Google Scholar]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic Plasticity and Depression: New Insights from Stress and Rapid-Acting Antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- von Rotz, R.; Schindowski, E.M.; Jungwirth, J.; Schuldt, A.; Rieser, N.M.; Zahoranszky, K.; Seifritz, E.; Nowak, A.; Nowak, P.; Jäncke, L.; et al. Single-dose psilocybin-assisted therapy in major depressive disorder: A placebo-controlled, double-blind, randomised clinical trial. EClinicalMedicine 2022, 56, 101809. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. Traumatic stress: Effects on the brain. Dialogues Clin. Neurosci. 2006, 8, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-Traumatic Stress Disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344. [Google Scholar] [CrossRef]
- Etkin, A.; Wager, T.D. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Kaye, W.H.; Wierenga, C.E.; Bailer, U.F.; Simmons, A.N.; Bischoff-Grethe, A. Nothing Tastes as Good as Skinny Feels: The Neurobiology of Anorexia Nervosa. Trends Neurosci. 2013, 36, 110–120. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of Addiction: A Neurocircuitry Analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.; Strassman, R.J. Psilocybin-Assisted Treatment for Alcohol Dependence: A Proof-of-Concept Study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Cosimano, M.P.; Griffiths, R.R. Pilot Study of the 5-HT 2A R Agonist Psilocybin in the Treatment of Tobacco Addiction. J. Psychopharmacol. 2014, 28, 983–992. [Google Scholar] [CrossRef]
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocybin—Summary of Knowledge and New Perspectives. Eur. Neuropsychopharmacol. 2013, 24, 342–356. [Google Scholar] [CrossRef]
- European Medicines Agency Guideline on clinical investigation of medicinal products in the treatment of depression. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-depression-revision-3_en.pdf/ (accessed on 21 July 2025).
- Ministerul Justiției, Legea nr. 143 din 26 iulie 2000 (republicată). Available online: https://legislatie.just.ro/Public/DetaliiDocument/23629/ (accessed on 21 July 2025).
- Ministerul Justiției, Legea nr. 339 din 29 Noiembrie 2005 Privind Regimul Juridic al Plantelor, Substanțelor și Preparatelor Stupefiante și Psihotrope. Available online: https://legislatie.just.ro/public/detaliidocument/66456/ (accessed on 21 July 2025).
- Vollenweider, F.X.; Kometer, M. The Neurobiology of Psychedelic Drugs: Implications for the Treatment of Mood Disorders. Nat. Rev. Neurosci. 2010, 11, 642–651. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Nutt, D. Serotonin and Brain Function: A Tale of Two Receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Stenbæk, D.S.; Arvidsson, A.; Armand, S.; Marstrand-Joergensen, M.R.; Johansen, S.S.; Linnet, K.; Ozenne, B.; Knudsen, G.M.; Fisher, P.M. Psilocybin-Induced Changes in Brain Network Integrity and Segregation Correlate with Plasma Psilocin Level and Psychedelic Experience. Eur. Neuropsychopharmacol. 2021, 50, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The Entropic Brain: A Theory of Conscious States Informed by Neuroimaging Research with Psychedelic Drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.V.; Lövdén, M.; Rosenthal, G.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. Finding the Self by Losing the Self: Neural Correlates of Ego-Dissolution under Psilocybin. Hum. Brain Mapp. 2015, 36, 3137–3153. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Johnson, M.; Richards, W.; Griffiths, R. Human Hallucinogen Research: Guidelines for Safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research Psychedelic Drugs: Considerations for Clinical Investigations. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/psychedelic-drugs-considerations-clinical-investigations/ (accessed on 21 July 2025).
- Norring, S.; Spigarelli, M. The Promise of Therapeutic Psilocybin: An Evaluation of the 134 Clinical Trials, 54 Potential Indications, and 0 Marketing Approvals on ClinicalTrials.Gov. Drug Des. Dev. Ther. 2024, 18, 1143–1151. [Google Scholar] [CrossRef]
- Multidisciplinary Association for Psychedelic Studies (MAPS) Psychedelic Policy Reform Tracker. Available online: https://maps.org/ (accessed on 21 July 2025).
- Kaelen, M.; Roseman, L.; Kahan, J.; Santos-Ribeiro, A.; Orban, C.; Lorenz, R.; Barrett, F.S.; Bolstridge, M.; Williams, T.; Williams, L.; et al. LSD Modulates Music-Induced Imagery via Changes in Parahippocampal Connectivity. Eur. Neuropsychopharmacol. 2016, 26, 1099–1109. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with Psychological Support for Treatment-Resistant Depression: An Open-Label Feasibility Study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef]
- Compass Pathways Successfully Achieves Primary Endpoint in First Phase 3 Trial Evaluating COMP360 Psilocybin for Treatment-Resistant Depression. Available online: https://ir.compasspathways.com/News--Events-/news/news-details/2025/Compass-Pathways-Successfully-Achieves-Primary-Endpoint-in-First-Phase-3-Trial-Evaluating-COMP360-Psilocybin-for-Treatment-Resistant-Depression/default.aspx (accessed on 21 July 2025).
- Cave, A.; Kurz, X.; Arlett, P. Real-World Data for Regulatory Decision Making: Challenges and Possible Solutions for Europe. Clin. Pharmacol. Ther. 2019, 106, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; Erritzoe, D.; Carhart-Harris, R. Psychedelic Psychiatry’s Brave New World. Cell 2020, 181, 24–28. [Google Scholar] [CrossRef]
- Butlen-Ducuing, F.; Silva, F.; Silva, I.; Balabanov, P.; Thirstrup, S. Applying the EU Regulatory Framework for the Clinical Use of Psychedelics. Lancet Psychiatry 2025, 12, 7–9. [Google Scholar] [CrossRef]
- Tim Ferriss Psychedelic Research Fundraising Campaign Attracts $30 Million in Donations in 6 Months, Prepares MDMA-Assisted Therapy for FDA Approval. Available online: https://maps.org/news/media/press-release-psychedelic-research-fundraising-campaign-attracts-30-million-in-donations-in-6-months-prepares-mdma-assisted-psychotherapy-for-fda-approval/ (accessed on 21 July 2025).
- University Medical Centre Groningen European Union Funds Groundbreaking Research into Psychedelic Therapy. Available online: https://umcgresearch.org/w/european-union-funds-groundbreaking-research-into-psychedelic-therapy/ (accessed on 21 July 2025).
- Gravanis, I.; Berntgen, M.; Vamvakas, S.; Demolis, P.; Foggi, P. Challenges and Ongoing Initiatives towards Better Integrated EU Scientific Advice. Front. Med. 2025, 12, 1473346. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Nowakowska, A.; Atli, M.; Dunlop, B.W.; Feifel, D.; Hellerstein, D.J.; Marwood, L.; Shabir, Z.; Mistry, S.; Stansfield, S.C.; et al. Results from a Long-Term Observational Follow-Up Study of a Single Dose of Psilocybin for a Treatment-Resistant Episode of Major Depressive Disorder. J. Clin. Psychiatry 2025, 86, 24m15449. [Google Scholar] [CrossRef]
- Oregon Health Authority. Oregon Psilocybin Services—2023 Rulemaking. Available online: https://www.oregon.gov/oha/ph/preventionwellness/pages/psilocybin-2023-rulemaking.aspx/ (accessed on 21 July 2025).
- Toomer, L. Colorado Can Now Issue Licenses to Psychedelic Mushroom Therapy Facilitators. Available online: https://stateline.org/2025/01/06/colorado-can-now-issue-licenses-to-psychedelic-mushroom-therapy-facilitators/ (accessed on 21 July 2025).
- Ballotpedia California Psilocybin Legalization Initiative (#21-0005). Available online: https://ballotpedia.org/California_Psilocybin_Legalization_Initiative_(2022) (accessed on 21 July 2025).
- Psychedelic Alpha Psychedelic Funding & Public Markets in 2023. Available online: https://psychedelicalpha.com/news/psychedelic-funding-public-markets-in-2023 (accessed on 21 July 2025).
- Dlestikova, T. The Legal Perspective on Psilocybin for Medical Use in Czechia: A Key Milestone and the Case for Broader Consideration Beyond the Clinical Setting. Psychoactives 2025, 4, 34. [Google Scholar] [CrossRef]
- European College of Neuropsychopharmacology. Challenges and Funding Gaps in European Psychedelic Research. Available online: https://www.ecnp.eu/ (accessed on 21 July 2025).
- Gregory McCarriston. Majority of Americans Ready to Embrace Psychedelic Therapy. Available online: https://today.yougov.com/society/articles/18466-americans-ready-embrace-psychedelic-therapy/ (accessed on 12 September 2025).
- UC Berkeley Center for the Science of Psychedelics. New National Poll: More Than 60 Percent of U.S. Voters Support Legalizing Psychedelic Therapy. Available online: https://psychedelics.berkeley.edu/berkeley-psychedelics-survey-2023 (accessed on 12 September 2025).
- Kilmer, B.; Priest, M.; Ramchand, R.; Rogers, R.C.; Senator, B.; Palmer, K. Considering Alternatives to Psychedelic Drug Prohibition; RAND: Santa Monica, CA, USA, 2024. [Google Scholar]
- EUDA. Other Drugs—The Current Situation in Europe (European Drug Report 2025). Available online: https://www.euda.europa.eu/publications/european-drug-report/2025/other-drugs_en/ (accessed on 12 September 2025).
- Žuljević, M.F.; Hren, D.; Storman, D.; Kaliterna, M.; Duplančić, D. Attitudes of European Psychiatrists on Psychedelics: A Cross-Sectional Survey Study. Sci. Rep. 2024, 14, 18716. [Google Scholar] [CrossRef]
- Psychedelic Alpha. PsychedeliCare Launches First European Citizens’ Initiative Demanding Political Action on Psychedelic-Assisted Therapies. Available online: https://psychedelicalpha.com/news/psychedelicare-launches-first-european-citizens-initiative-demanding-political-action-on-psychedelic-assisted-therapies/ (accessed on 12 September 2025).
- Compass Pathways Investor Updates and Clinical Pipeline. Available online: https://compasspathways.com/our-work/pipeline-overview/ (accessed on 21 July 2025).
- Atai Life Sciences Corporate Reports and Investor Relations. Available online: https://atai.life (accessed on 21 July 2025).
- Drug Discovery. Trends MindMed to Lead First LSD Phase 3 Trials for Anxiety Treatment. Available online: https://www.drugdiscoverytrends.com/lsd-phase-3-trials-anxiety-treatment-mindmed/ (accessed on 21 July 2025).
- European Medicines Agency Clinical Trials Regulation (EU) 536/2014. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trials-regulation (accessed on 21 July 2025).
- Grob, C. MDMA Research: Preliminary Investigations with Human Subjects. Int. J. Drug Policy 1998, 9, 119–124. [Google Scholar] [CrossRef]
- Singh, B. MDMA-Assisted Therapy for Post-Traumatic Stress Disorder: Regulatory Challenges and a Path Forward. CNS Drugs 2025, 39, 339–343. [Google Scholar] [CrossRef]
- Imperial Centre for Psychedelic Research Pioneering Research. Available online: https://www.imperial.ac.uk/psychedelic-research-centre/research (accessed on 12 September 2025).
- White, E.; Kennedy, T.; Ruffell, S.; Perkins, D.; Sarris, J. Ayahuasca and Dimethyltryptamine Adverse Events and Toxicity Analysis: A Systematic Thematic Review. Int. J. Toxicol. 2024, 43, 327–339. [Google Scholar] [CrossRef]
- Köck, P.; Froelich, K.; Walter, M.; Lang, U.; Dürsteler, K.M. A Systematic Literature Review of Clinical Trials and Therapeutic Applications of Ibogaine. J. Subst. Abus. Treat. 2022, 138, 108717. [Google Scholar] [CrossRef]
- Homewood Research Institute Research Reports. Available online: https://hriresearch.com/exploratory-research/research-reports/ (accessed on 12 September 2025).
- Destoop, M.; Mohr, P.; Butlen, F.; Kéri, P.; Samochowiec, J.; De Picker, L.; Fiorillo, A.; Kuypers, K.P.C.; Dom, G. Use of Psychedelic Treatments in Psychiatric Clinical Practice: An EPA Policy Paper. Eur. Psychiatry 2025, 68, e3. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration. Controlled Substances Act. Washington. Available online: https://www.dea.gov/drug-information/csa (accessed on 21 July 2025).
- Johnson, M.W.; Hendricks, P.S.; Barrett, F.S.; Griffiths, R.R. Classic Psychedelics: An Integrative Review of Epidemiology, Therapeutics, Mystical Experience, and Brain Network Function. Pharmacol. Ther. 2019, 197, 83–102. [Google Scholar] [CrossRef]
- Borissova, A.; Rucker, J.J. The Development of Psilocybin Therapy for Treatment-Resistant Depression: An Update. BJPsych Bull. 2024, 48, 38–44. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calin, A.; Burlec, A.F.; Mircea, C.; Macovei, I.; Hancianu, M.; Corciova, A. Evolution and Comparative Analysis of Clinical Trials on Psilocybin in the Treatment of Psychopathologies: Trends in the EU and the US. J. Clin. Med. 2025, 14, 6613. https://doi.org/10.3390/jcm14186613
Calin A, Burlec AF, Mircea C, Macovei I, Hancianu M, Corciova A. Evolution and Comparative Analysis of Clinical Trials on Psilocybin in the Treatment of Psychopathologies: Trends in the EU and the US. Journal of Clinical Medicine. 2025; 14(18):6613. https://doi.org/10.3390/jcm14186613
Chicago/Turabian StyleCalin, Anastasia, Ana Flavia Burlec, Cornelia Mircea, Irina Macovei, Monica Hancianu, and Andreia Corciova. 2025. "Evolution and Comparative Analysis of Clinical Trials on Psilocybin in the Treatment of Psychopathologies: Trends in the EU and the US" Journal of Clinical Medicine 14, no. 18: 6613. https://doi.org/10.3390/jcm14186613
APA StyleCalin, A., Burlec, A. F., Mircea, C., Macovei, I., Hancianu, M., & Corciova, A. (2025). Evolution and Comparative Analysis of Clinical Trials on Psilocybin in the Treatment of Psychopathologies: Trends in the EU and the US. Journal of Clinical Medicine, 14(18), 6613. https://doi.org/10.3390/jcm14186613





