Cross-Linked Carboxymethyl Cellulose and Silk Proteins in Corneal Re-Epithelialization: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Surgical Procedures
2.3. Treatment
2.4. Tomography
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian. J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, N.A.; Jalil, N.A.A.; Kamarudin, T.A. Wound healing of the corneal epithelium: A review. Asian. Biomed. (Res. Rev. News) 2021, 15, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Giovannetti, F. Main Complications of Photorefractive Keratectomy and their Management. Clin. Ophthalmol. 2019, 13, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Abbouda, A.; Valle, D.D.; Del Castillo, J.M.; Fernandez, J.A. Corneal cross linking and infectious keratitis: A systematic review with a meta-analysis of reported cases. J. Ophthalmic. Inflamm. Infect. 2013, 3, 47. [Google Scholar] [CrossRef]
- Bonzano, C.; Cutolo, C.A.; Musetti, D.; Di Mola, I.; Pizzorno, C.; Scotto, R.; Traverso, C.E. Delayed Re-epithelialization After Epithelium-Off Crosslinking: Predictors and Impact on Keratoconus Progression. Front. Med. 2021, 8, 657993. [Google Scholar] [CrossRef]
- Durrie, D.S.; Wolsey, D.; Thompson, V.; Assang, C.; Mann, B.; Wirostko, B. Ability of a new crosslinked polymer ocular bandage gel to accelerate reepithelialization after photorefractive keratectomy. J. Cataract. Refract. Surg. 2018, 44, 369–375. [Google Scholar] [CrossRef]
- Serrao, S.; Lombardo, M. Corneal epithelial healing after photorefractive keratectomy: Analytical study. J. Cataract. Refract. Surg. 2005, 31, 930–937. [Google Scholar] [CrossRef]
- Eliacik, M.; Bayramlar, H.; Erdur, S.K.; Karabela, Y.; Demirci, G.; Gulkilik, I.G.; Ozsutcu, M. Anterior segment optical coherence tomography evaluation of corneal epithelium healing time after 2 different surface ablation methods. Saudi Med. J. 2015, 36, 67–72. [Google Scholar] [CrossRef]
- Barrientez, B.; Nicholas, S.E.; Whelchel, A.; Sharif, R.; Hjortdal, J.; Karamichos, D. Corneal injury: Clinical and molecular aspects. Exp. Eye Res. 2019, 186, 107709. [Google Scholar] [CrossRef]
- Kourenkov, V.V.; Mytiagina, O.N.; Kasparov, A.A.; Pavluk, A.G. Stimulating re-epithelialization after photorefractive keratectomy. J. Refract. Surg. 1999, 15, S234–S237. [Google Scholar] [CrossRef]
- Detorakis, E.T.; Siganos, D.S.; Kozobolis, V.P.; Pallikaris, I.G. Corneal epithelial wound healing after excimer laser photorefractive and photoastigmatic keratectomy (PRK and PARK). Cornea 1999, 18, 25–28. [Google Scholar] [CrossRef]
- Rajan, M.S.; Watters, W.; Patmore, A.; Marshall, J. In vitro human corneal model to investigate stromal epithelial interactions following refractive surgery. J. Cataract. Refract. Surg. 2005, 31, 1789–1801. [Google Scholar] [CrossRef]
- Gyenes, A.; Szentmary, N.; Toth, G.; Kiss, H.; Szekrenyesi, C.; Langenbucher, A.; Nagy, Z.Z. Impact of crosslinking on corneal epithelial healing. Orv. Hetil. 2017, 158, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zheng, S.; Qi, B.; Guo, R.; Hou, G. Decellularized Human Stromal Lenticules Combine with Corneal Epithelial-Like Cells: A New Resource for Corneal Tissue Engineering. Stem Cells Int. 2019, 2019, 4252514. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.S.; Friend, J.; Thoft, R.A. Corneal re-epithelialization from the conjunctiva. Investig. Ophthalmol. Vis. Sci. 1981, 21, 135–142. [Google Scholar]
- Lazzarotto, M.; Tomasello, E.M.; Caporossi, A. Clinical evaluation of corneal epithelialization after photorefractive keratectomy in a patient treated with Polydeoxyribonucleotide (PDRN) eye drops: A randomized, double-blind, placebo-controlled trial. Eur. J. Ophthalmol. 2004, 14, 284–289. [Google Scholar] [CrossRef]
- Jullienne, R.; Garcin, T.; Crouzet, E.; He, Z.; Renault, D.; Thuret, G.; Gain, P. Evaluation of corneal epithelial wound healing after penetrating keratoplasty in patients receiving a new matrix therapy agent (regenerating agent). Eur. J. Ophthalmol. 2020, 30, 119–124. [Google Scholar] [CrossRef]
- Tomas-Juan, J.; Murueta-Goyena Larranaga, A.; Hanneken, L. Corneal Regeneration After Photorefractive Keratectomy: A Review. J. Optom. 2015, 8, 149–169. [Google Scholar] [CrossRef]
- Lim, L.; Lim, E.W.L. Therapeutic Contact Lenses in the Treatment of Corneal and Ocular Surface Diseases-A Review. Asia Pac. J. Ophthalmol. 2020, 9, 524–532. [Google Scholar] [CrossRef]
- Ozek, D.; Kemer, O.E. Effect of the bioprotectant agent trehalose on corneal epithelial healing after corneal cross-linking for keratoconus. Arq. Bras. Oftalmol. 2018, 81, 505–509. [Google Scholar] [CrossRef]
- Wolsey, D.; Slade, S.; Wirostko, B.M.; Brandano, L.A.; Mann, B.K.; Durrie, D.S.; Thompson, V. Novel Cross-Linked Ocular Bandage Gel Improves Reepithelialization After Photorefractive Keratectomy: A Randomized, Masked Prospective Study. J. Ocul. Pharmacol. Ther. 2020, 36, 602–608. [Google Scholar] [CrossRef]
- Bata, A.M.; Witkowska, K.J.; Wozniak, P.A.; Fondi, K.; Schmidinger, G.; Pircher, N.; Szegedi, S.; Aranha Dos Santos, V.; Pantalon, A.; Werkmeister, R.M.; et al. Effect of a Matrix Therapy Agent on Corneal Epithelial Healing After Standard Collagen Cross-linking in Patients with Keratoconus: A Randomized Clinical Trial. JAMA Ophthalmol. 2016, 134, 1169–1176. [Google Scholar] [CrossRef]
- Garrett, Q.; Simmons, P.A.; Xu, S.; Vehige, J.; Zhao, Z.; Ehrmann, K.; Willcox, M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Semp, D.A.; Beeson, D.; Sheppard, A.L.; Dutta, D.; Wolffsohn, J.S. Artificial Tears: A Systematic Review. Clin. Optom. 2023, 15, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.S.; Chay, E.Y.; Healey, J.; Sullenberger, R.; Klarlund, J.K. Activation of the epidermal growth factor receptor by hydrogels in artificial tears. Exp. Eye Res. 2008, 86, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, W.; Cole, B.; Liu, A.; Liu, J.; Wan, P.; Schreiner, R.; Infanger, D.W.; Paulson, N.B.; Lawrence, B.D.; Rosenblatt, M.I. Treatment with solubilized Silk-Derived Protein (SDP) enhances rabbit corneal epithelial wound healing. PLoS ONE 2017, 12, e0188154. [Google Scholar] [CrossRef]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in rat debrided corneal epithelium. Biol. Pharm. Bull. 2009, 32, 933–936. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Karpecki, P.M.; Infanger, D.W.; Levy, B. Silk-Derived Protein-4 Versus Vehicle Control in Treating Patients with Moderate to Severe Dry Eye Disease: A Randomized Clinical Trial. Am. J. Ophthalmol. 2025, 269, 315–326. [Google Scholar] [CrossRef]
- Manoochehrabadi, T.; Solouki, A.; Majidi, J.; Khosravimelal, S.; Lotfi, E.; Lin, K.; Daryabari, S.H.; Gholipourmalekabadi, M. Silk biomaterials for corneal tissue engineering: From research approaches to therapeutic potentials; A review. Int. J. Biol. Macromol. 2025, 305, 141039. [Google Scholar] [CrossRef]
- Kundu, J.; Mohapatra, R.; Kundu, S.C. Silk fibroin/sodium carboxymethylcellulose blended films for biotechnological applications. J. Biomater. Sci. Polym. Ed. 2011, 22, 519–539. [Google Scholar] [CrossRef]
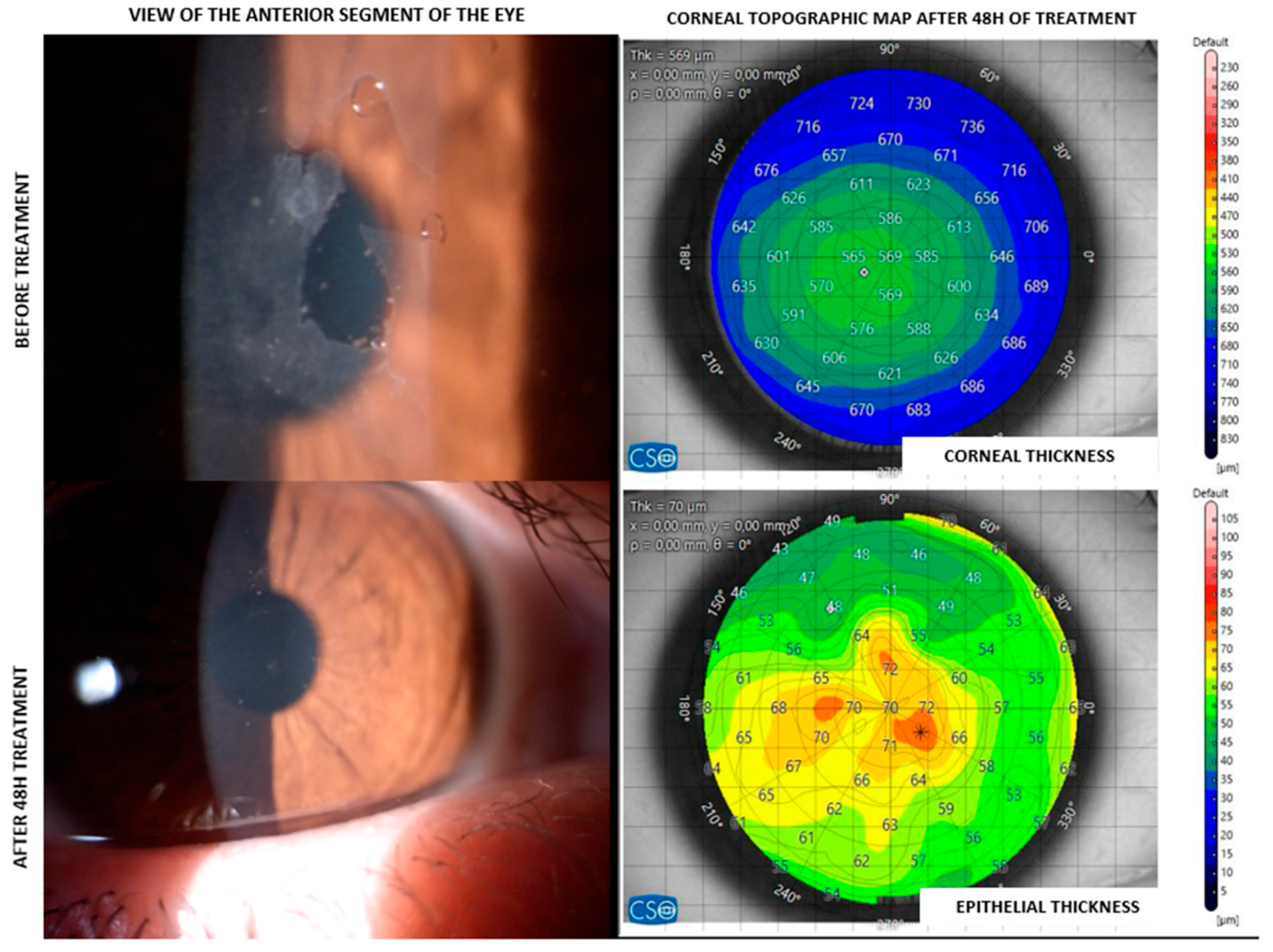
| ID | Age | Sex | Clinical History | Epithelial Thickness at 48 h (µm) |
|---|---|---|---|---|
| #001 | 30 | F | Corneal abrasion | 67.0 |
| #002 | 29 | M | CXL | 74.2 |
| #003 | 16 | M | CXL | 77.0 |
| #004 | 29 | F | CXL | 62.8 |
| #005 | 26 | M | CXL | 68.4 |
| #006 | 25 | F | PRK | 75.8 |
| #007 | 19 | M | CXL | 86.4 |
| #008 | 35 | F | PTK | 54.0 |
| #009 | 57 | M | PTK | 66.6 |
| #010 | 32 | M | PRK | 73.6 |
| #011 | 28 | M | CXL | 74.0 |
| #012 | 46 | M | PRK | 82.3 |
| #013 | 25 | F | PRK | 68.4 |
| #014 | 15 | M | CXL | 76.1 |
| #015 | 16 | F | Corneal abrasion | 82.9 |
| Patient ID | Kavg (D) | Cyl (D @ Axis) | Kmax (D/mm) | ThkMIN (µm) |
|---|---|---|---|---|
| #001 | 44.22 | −1.49 D @ 43° | 48.28 D (6.99 mm) | 589 |
| #002 | 44.49 | −1.91 D @ 178° | 48.53 D (6.95 mm) | 510 |
| #003 | 45.55 | −2.59 D @ 12° | 50.85 D (6.64 mm) | 534 |
| #004 | 43.40 | −3.82 D @ 11° | 51.45 D (6.56 mm) | 434 |
| #005 | 46.26 | −2.42 D @ 16° | 60.74 D (5.56 mm) | 446 |
| #006 | 38.40 | −1.23 D @ 177° | 47.52 D (7.10 mm) | 407 |
| #007 | 43.24 | −4.16 D @ 101° | 58.52 D (5.77 mm) | 530 |
| #008 | 39.71 | −1.10 D @ 2° | 45.94 D (7.35 mm) | 433 |
| #009 | 38.28 | −1.13 D @ 174° | 47.10 D (7.16 mm) | 489 |
| #010 | 38.64 | −1.21 D @ 23° | 46.89 D (7.20 mm) | 500 |
| #011 | 49.79 | −4.72 D @ 164° | 66.44 D (5.08 mm) | 405 |
| #012 | 43.75 | −2.74 D @ 9° | 52.11 D (6.49 mm) | 445 |
| #013 | 40.26 | −1.24 D @ 176° | 46.72 D (7.21 mm) | 498 |
| #014 | 46.36 | −3.31 D @ 15° | 59.15 D (5.71 mm) | 421 |
| #015 | 42.47 | −1.29 D @ 25° | 49.03 D (6.88 mm) | 512 |
| Preoperative | 48 h | 1-Month | |
|---|---|---|---|
| ΔET | 0.7 ± 6.4 µm | −1.7 ± 6.5 µm | 3.2 ± 7.5 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boselli, F.; Scarinci, F.; Fasciani, R. Cross-Linked Carboxymethyl Cellulose and Silk Proteins in Corneal Re-Epithelialization: A Case Series. J. Clin. Med. 2025, 14, 6600. https://doi.org/10.3390/jcm14186600
Boselli F, Scarinci F, Fasciani R. Cross-Linked Carboxymethyl Cellulose and Silk Proteins in Corneal Re-Epithelialization: A Case Series. Journal of Clinical Medicine. 2025; 14(18):6600. https://doi.org/10.3390/jcm14186600
Chicago/Turabian StyleBoselli, Francesco, Fabio Scarinci, and Romina Fasciani. 2025. "Cross-Linked Carboxymethyl Cellulose and Silk Proteins in Corneal Re-Epithelialization: A Case Series" Journal of Clinical Medicine 14, no. 18: 6600. https://doi.org/10.3390/jcm14186600
APA StyleBoselli, F., Scarinci, F., & Fasciani, R. (2025). Cross-Linked Carboxymethyl Cellulose and Silk Proteins in Corneal Re-Epithelialization: A Case Series. Journal of Clinical Medicine, 14(18), 6600. https://doi.org/10.3390/jcm14186600






