Analysis of 24-Hour Blood Pressure Profile and Antihypertensive Therapy in Male Heart Transplant Patients
Abstract
1. Introduction
2. Materials and Methods
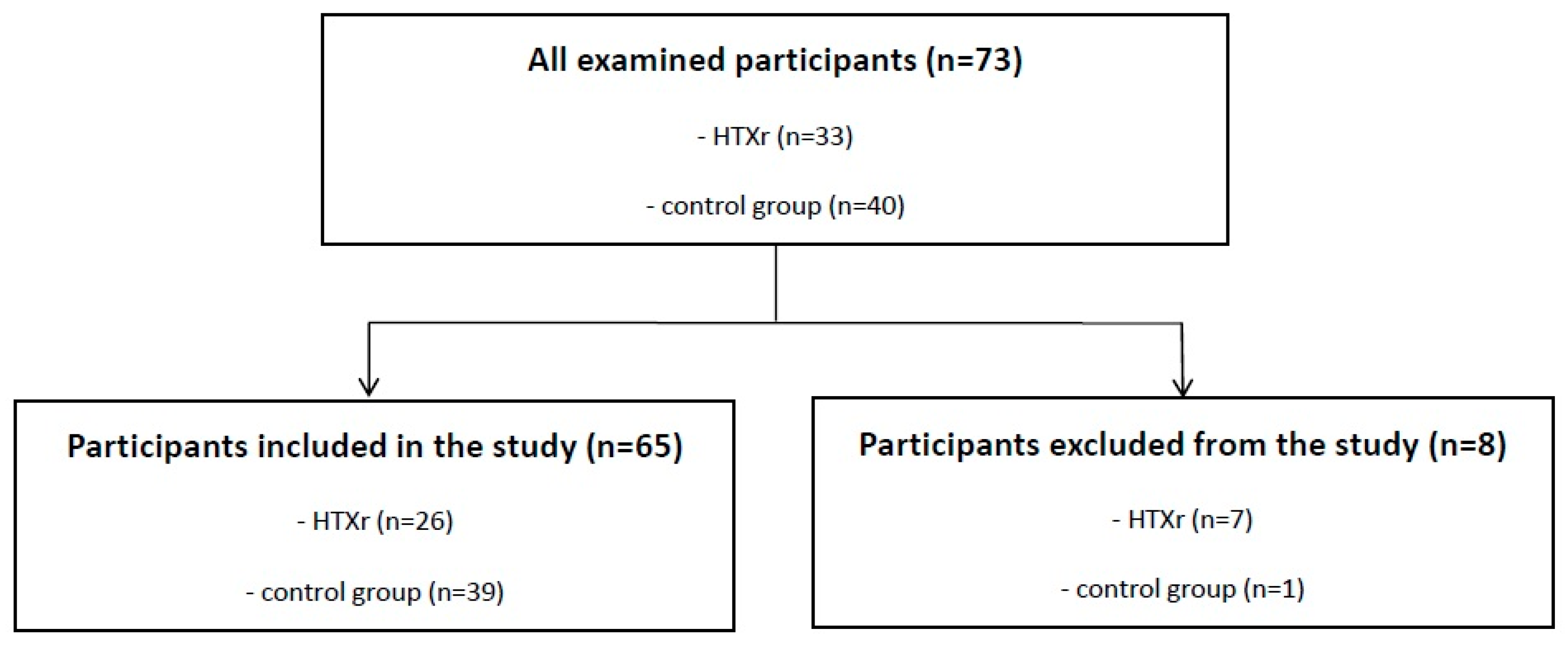
2.1. Design and Participants
2.2. Blood Pressure Recordings
2.3. Statistics
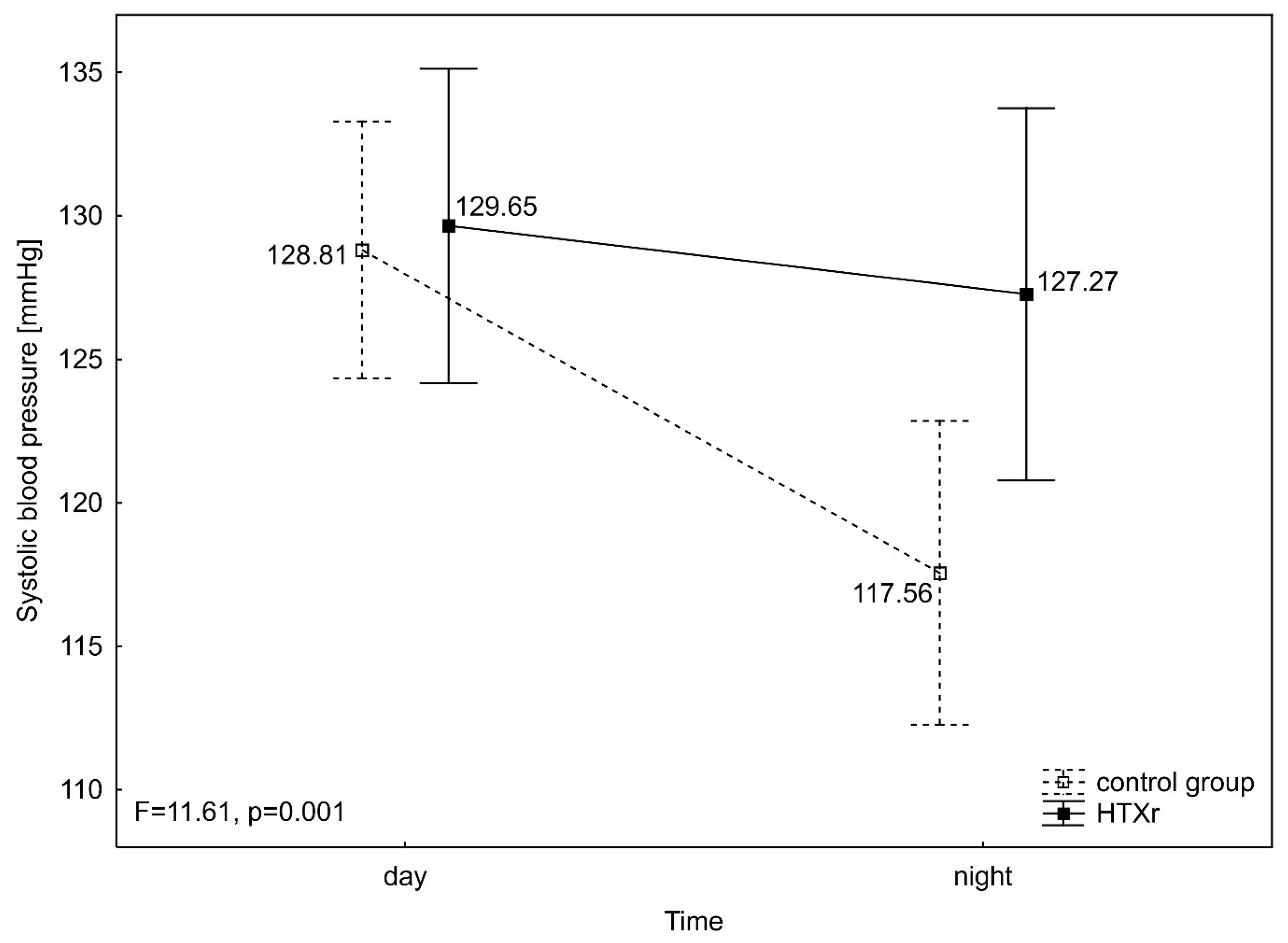
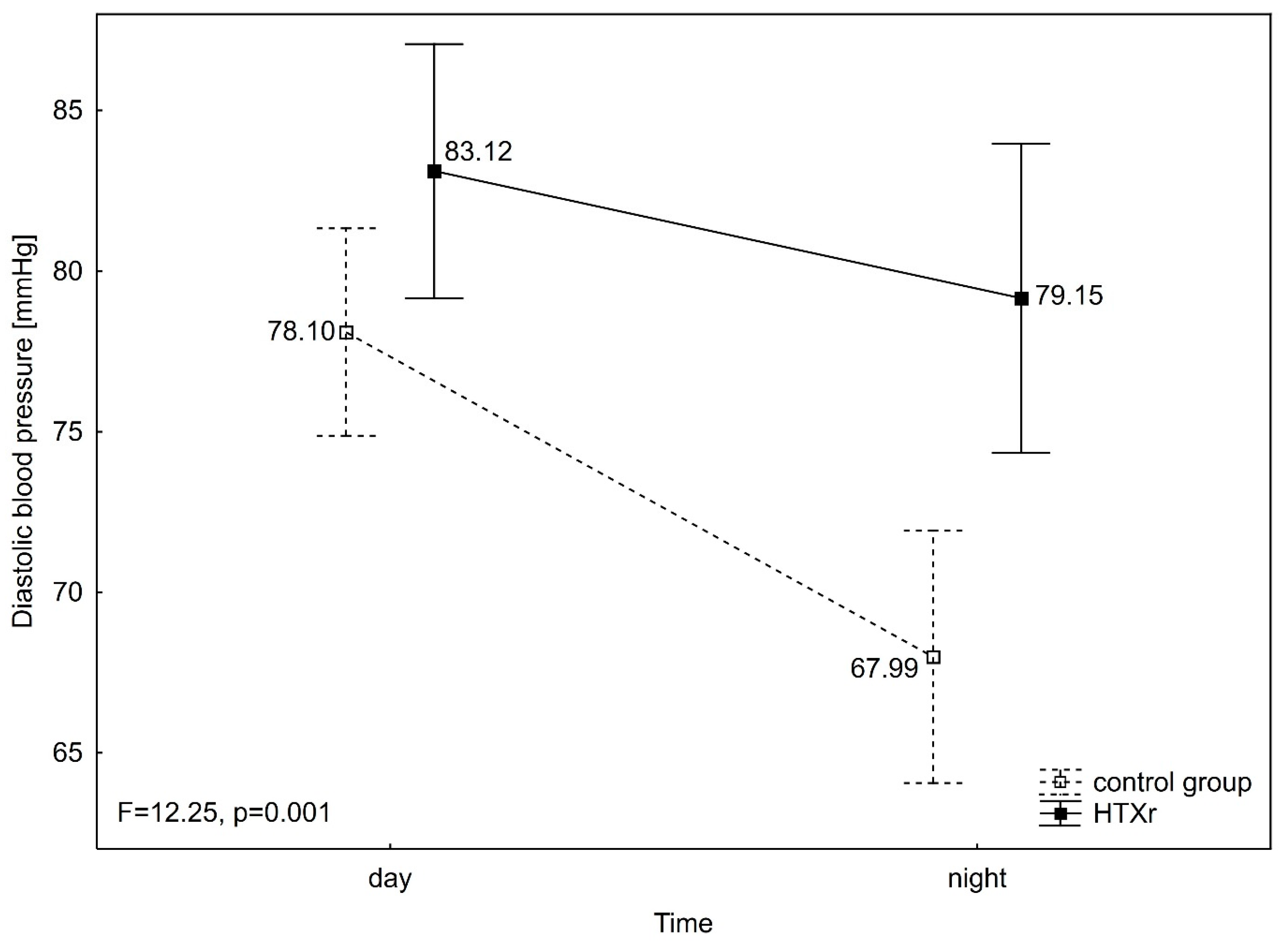
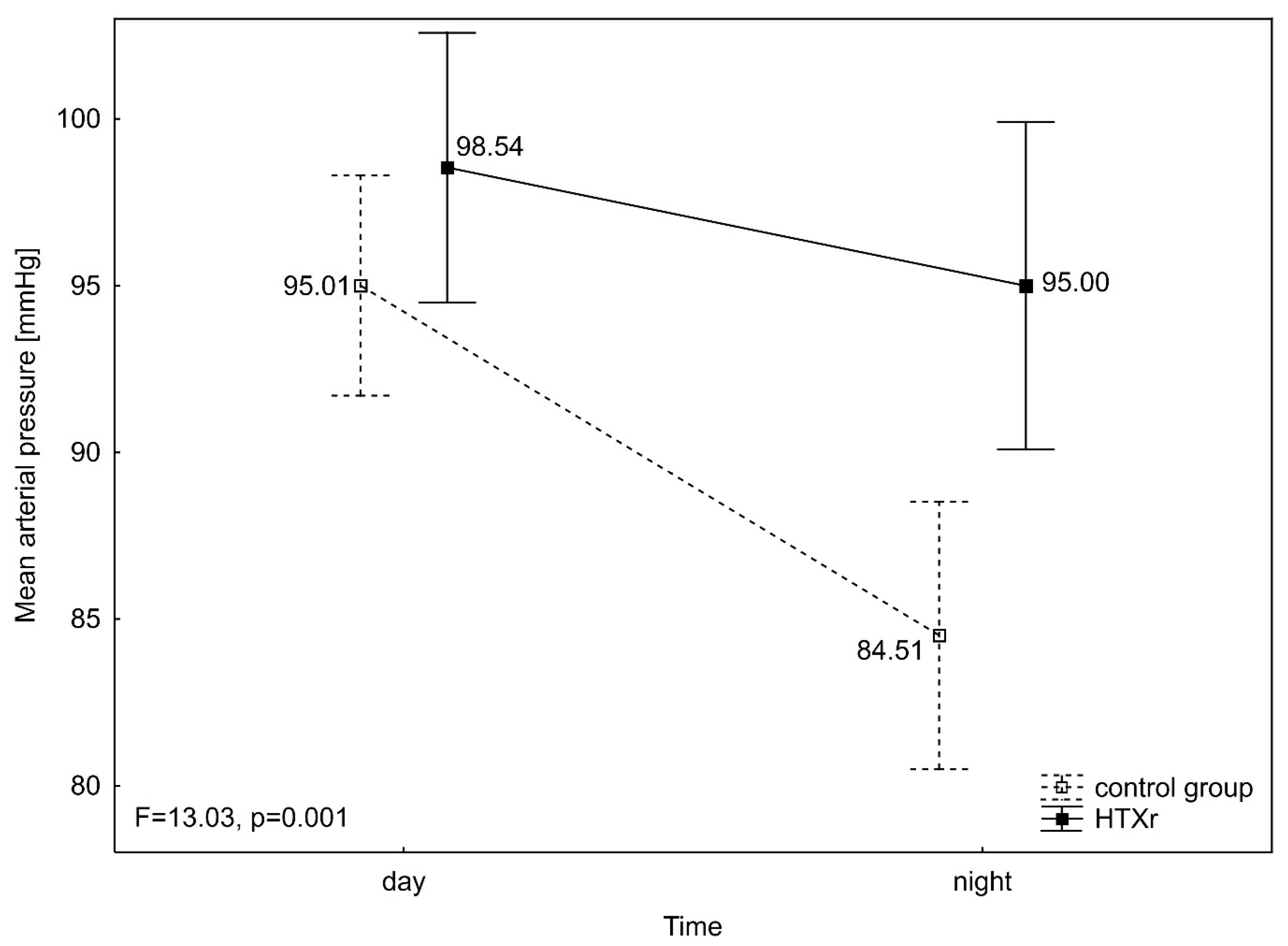
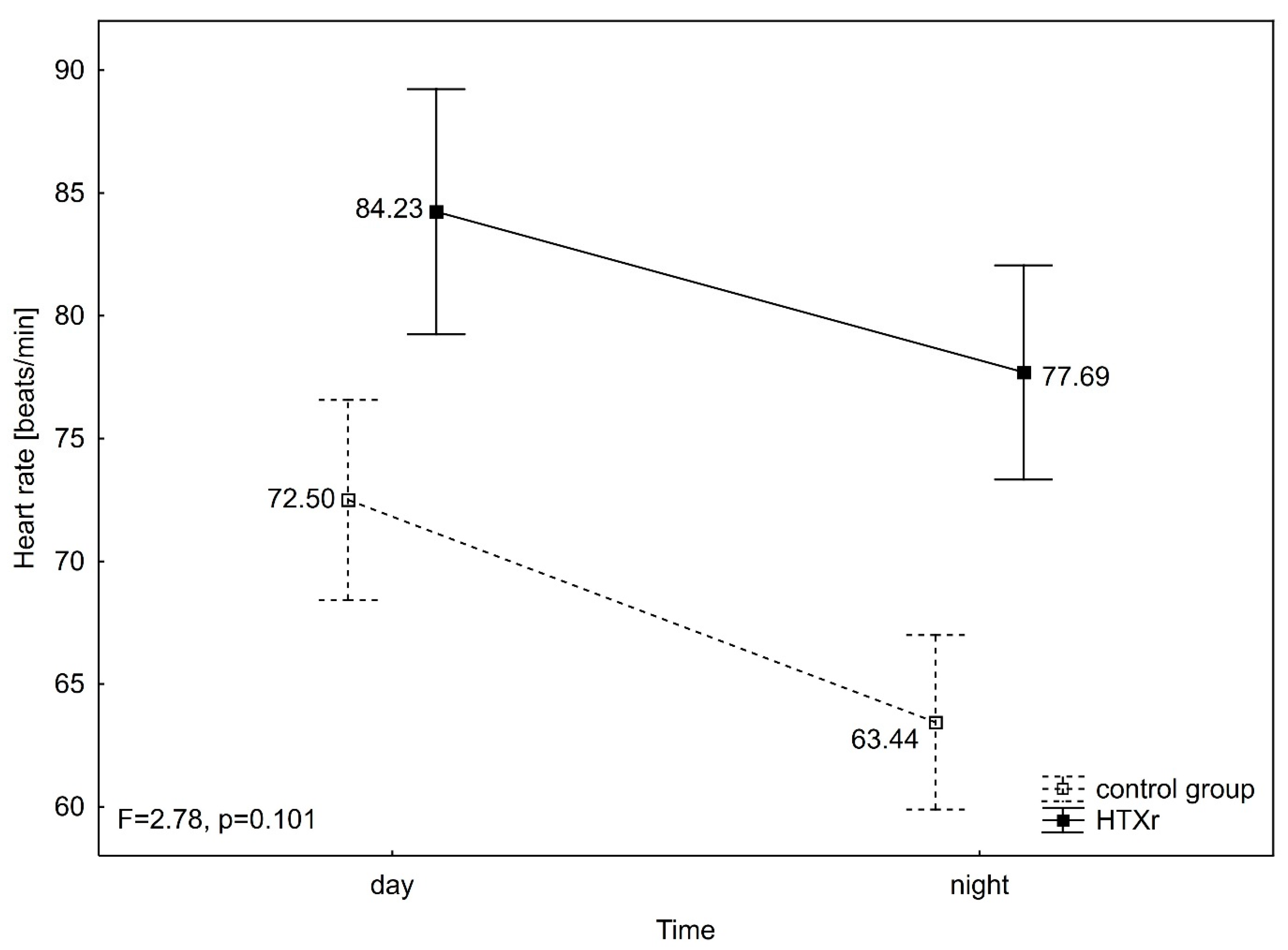
3. Results
4. Discussion
4.1. Association with Drugs
4.2. Association with ABPM Variability
4.3. New Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, A.L.; Ventura, H.O. Hypertension in patients with cardiac transplantation. Med. Clin. N. Am. 2017, 101, 53–64. [Google Scholar] [CrossRef]
- Idema, R.N.; van den Meiracker, A.H.; Balk, A.H.; Bos, E.; A Schalekamp, M.; Veld, A.J.M.I. Abnormal diurnal variation of blood pressure, cardiac output, and vascular resistance in cardiac transplant recipients. Circulation 1994, 90, 2797–2803. [Google Scholar] [CrossRef]
- Taylor, D.O.; Edwards, L.B.; Mohacsi, P.J.; Boucek, M.M.; Trulock, E.P.; Keck, B.M.; I Hertz, M. The registry of the International Society for Heart and Lung Transplantation: Twentieth official adult heart transplant report—2003. J. Heart Lung Transplant. 2003, 22, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.G.; Thomas, G.D.; Marban, E.; O’Rourke, B. Presynaptic modulation of cortical syn-aptic activity by calcineurin. Proc. Natl. Acad. Sci. USA 1995, 92, 6269–6273. [Google Scholar] [CrossRef] [PubMed]
- Lyson, T.; Ermel, L.D.; Belshaw, P.J.; Victor, R.G. Cyclosporine- and FK506-induced sympa- thetic activation correlates with calcineurin-mediated inhibition of T-cell signaling. Circ. Res. 1993, 73, 596–602. [Google Scholar] [CrossRef]
- Karabesheh, S.; Verma, D.R.; Jain, M.; Stoddard, G.; Brunisholz, K.; Stehlik, J.; Kfoury, A.; Gilbert, E.; Bader, F. Clinical and hemodynamic effects of renin-angiotensin system blockade in cardiac transplant recipients. Am. J. Cardiol. 2011, 108, 1836–1839. [Google Scholar] [CrossRef]
- El-Gowelli, H.M.; El-Mas, M.M. Central modulation of cyclosporine-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 351–361. [Google Scholar] [CrossRef]
- Yard, A.C.; Kadowitz, P.J. Studies on the mechanism of hydrocortisone potentiation of vasoconstrictor responses to epinephrine in the anesthetized animal. Eur. J. Pharmacol. 1972, 20, 1–9. [Google Scholar] [CrossRef]
- Besse, J.C.; Bass, A.D. Potentiation by hydrocortisone of responses to catechol- amines in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1966, 154, 224–238. [Google Scholar] [CrossRef]
- Luke, R.G.; Curtis, J.J. Biology and treatment of transplant hypertension. In Hypertension: Pathophysiology, Diagnosis and Management, 2nd ed.; Laragh, J.H., Brenner, B.M., Eds.; Raven Press: New York, NY, USA, 1995; pp. 2471–2481. [Google Scholar]
- Sanders, M.; Victor, R.G. Hypertension after cardiac transplantation: Pathophysiology and management. Curr. Opin. Nephrol. Hypertens. 1995, 4, 443–451. [Google Scholar] [CrossRef]
- Ventura, H.O.; Lavie, C.J.; Messerli, F.H.; Valentino, V.; Smart, F.W.; Stapleton, D.D.; Ochsner, J.L. Cardiovascular adaptation to cyclosporine-induced hypertension. J. Hum. Hypertens. 1994, 8, 233–237. [Google Scholar] [PubMed]
- Whitworth, J.A.; Schyvens, C.G.; Zhang, Y.; Andrews, M.C.; Mangos, G.J.; Kelly, J.J. The nitric oxide system in glucocorticoid-induced hypertension. J. Hypertens. 2002, 20, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Diez, R.; González-Guerrero, C.; Ocaña-Salceda, C.; Rodrigues-Diez, R.R.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M.; Ramos, A.M. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci. Rep. 2016, 6, 27915. [Google Scholar] [CrossRef]
- Ambrosi, P.; Kreitmann, B.; Habib, G. Does heart rate predict allograft vasculopathy in heart transplant recipients? Int. J. Cardiol. 2010, 145, 256–257. [Google Scholar] [CrossRef]
- Murphy, D.A.; Thompson, G.W.; Ardell, J.L.; McCraty, R.; Stevenson, R.S.; Sangalang, V.E.; Cardinal, R.; Wilkinson, M.; Craig, S.; Smith, F.M.; et al. The heart reinnervates after trans-plantation. Ann. Thorac. Surg. 2000, 69, 1769–1781. [Google Scholar] [CrossRef]
- Arrowood, J.A.; Minisi, A.J.; Goudreau, E.; Davis, A.B.; King, A.L. Absence of parasympathetic control of heart rate after human orthotopic cardiac transplantation. Circulation 1997, 96, 3492–3498. [Google Scholar] [CrossRef]
- Palatini, P.; Casiglia, E.; Pauletto, P.; Staessen, J.; Kaciroti, N.; Julius, S. Relationship of tachycardia with high blood pressure and metabolic abnormalities: A study with mixture analysis in three populations. Hypertension 1997, 30, 1267–1273. [Google Scholar] [CrossRef]
- Kobashigawa, J.K. Physiology of the transplanted heart. In Primer on Transplantation, 2nd ed.; Norma, D.J., Turka, L.A., Eds.; American Society of Transplant Physicians: Thorofare, NJ, USA, 2001; pp. 358–362. [Google Scholar]
- Scott, C.D.; McComb, J.M.; Dark, J.H. Heart rate and late mortality in cardiac trans- plant recipients. Eur. Heart J. 1993, 14, 530–533. [Google Scholar] [CrossRef]
- Christensen, A.H.; Nygaard, S.; Rolid, K.; Nytrøen, K.; Gullestad, L.; Fiane, A.; Thaulow, E.; Døhlen, G.; Saul, J.P.; Wyller, V.B. Early signs of sinoatrial reinnervation in the transplanted heart. Transplantation 2021, 105, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P. Prognostic value of ambulatory blood pressure: Current evidence and clinical implications. Hypertension 2000, 35, 844–851. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Molnar, M.Z.; Ho, B.T.; Reddy, U.G.; Dafoe, D.C.; Ichii, H.; Ferrey, A.J.; Hanna, R.M.; Kalantar-Zadeh, K.; Amin, A. Approach and management of hypertension after kidney transplantation. Front. Med. 2020, 7, 229. [Google Scholar] [CrossRef]
- Masarone, D.; Tedford, R.J.; Melillo, E.; Petraio, A.; Pacileo, G. Angiotensin-converting enzyme inhibitor therapy after heart transplant: From molecular basis to clinical effects. Clin. Transplant. 2022, 36, e14696. [Google Scholar] [CrossRef]
- Brozena, S.C.; Johnson, M.R.; Ventura, H.; Hobbs, R.; Miller, L.; Olivari, M.T.; Clemson, B.; Bourge, R.; Quigg, R.; Mills, R.M.; et al. Effectiveness and safety of diltiazem or lisinopril in treatment of hypertension after heart transplantation. Results of a prospective, randomized multicenter trail. J. Am. Coll. Cardiol. 1996, 27, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Ghotra, A.S.; Angus, C.; Price, J.; Hussain, Z.; McCants, K.; Slaughter, M.S.; Cheng, A.; Lenneman, A.; Birks, E.J. Safety and long- term outcomes of using beta blockers after heart transplantation. J. Card. Fail. 2015, 2, S37–S38. [Google Scholar] [CrossRef]
- Castel, M.Á.; Roig, E.; Rios, J.; Tomas, C.; Mirabet, S.; Cardona, M.; Brossa, V.; López, L.; Vargas, L.; Sionis, A. Long-term prognostic value of elevated heart rate one year after heart transplantation. Int. J. Cardiol. 2013, 168, 2003–2007. [Google Scholar] [CrossRef]
- Shah, A.B.; Patel, J.K.; Rafiei, M.; Morrissey, R.P.; Kittleson, M.M.; Kobashigawa, J.A. Select search result to email or save The impact of mean first-year heart rate on outcomes after heart transplantation: Does it make a difference? Transplant 2013, 27, 659–665. [Google Scholar]
- Nash, D.T. Alpha-adrenergic blockers: Mechanism of action, blood pressure control, and effects of lipoprotein metabolism. Clin. Cardiol. 1990, 13, 764. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Sponga, S.; Vendramin, I.; Ferrara, V.; Marinoni, M.; Valdi, G.; Di Nora, C.; Nalli, C.; Benedetti, G.; Piani, D.; Lechiancole, A.; et al. Metabolic syndrome and heart transplantation: An underestimated risk factor? Transpl. Int. 2024, 37, 11075. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol. Int. 2010, 27, 1629–1651. [Google Scholar] [CrossRef]
- Kario, K.; Schwartz, J.E.; Pickering, T.G. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alfa-adrenergic blocker, doxazosin: Results from the HALT study. Hypertension 2000, 35, 787–794. [Google Scholar] [CrossRef]
- Kario, K.; Nariyama, J.; Kido, H.; Ando, S.; Takiuchi, S.; Eguchi, K.; Niijima, Y.; Ando, T.; Noda, M. Effect of a novel calcium channel blocker on abnormal nocturnal blood pressure in hypertensive patients. J. Clin. Hypertens 2013, 15, 465–472. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Okada, K.; Kato, M.; Nishizawa, M.; Yoshida, T.; Asano, T.; Uchiyama, K.; Niijima, Y.; Katsuya, T.; Urata, H. 24-h blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hyper-tension: Results from the randomized, placebo-controlled SACRA study. Circulation 2019, 139, 2089–2097. [Google Scholar] [CrossRef]
- Muir, C.A.; Greenfield, J.R.; MacDonald, P.S. Empagliflozin in the management of diabetes mellitus after cardiac transplantation Research Correspondence retain. J. Heart Lung Transplant. 2017, 36, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Cehic, M.G.; Muir, C.A.; Greenfield, J.R.; Hayward, C.; Jabbour, A.; Keogh, A.; Kotlyar, E.; Muthiah, K.; Macdonald, P.S. Efficacy and safety of empagliflozin in the management of diabetes mellitus in heart transplant recipients. Transplant. Direct 2019, 5, e450. [Google Scholar] [CrossRef]
| Variables | HTXr (n = 26) | Cx (n = 39) | Test Results | p |
|---|---|---|---|---|
| Mean ± SD | ||||
| Age [years] | 54.46 ± 10.51 | 57.61 ± 7.12 | −1.34 b | 0.189 |
| Height [m] | 1.75 ± 0.08 | 1.73 ± 0.05 | 1.19 b | 0.243 |
| Body weight [kg] | 81.50 ± 12.15 | 82.51 ± 9.87 | −0.36 a | 0.719 |
| BMI [kg/m2] | 26.54 ± 3.42 | 27.55 ± 2.97 | −1.24 a | 0.222 |
| Tacrolimus level [ug/L] | 9.33 ± 3.65 | - | - | - |
| Time since transplantation [years] | 4.90 ± 5.07 | - | - | - |
| Variables | HTXr (n = 26) | Control Group (n = 39) | Test Results | p |
|---|---|---|---|---|
| % (n) | ||||
| Current smoking | 8% (2) | 5% (2) | 0.00 c | 0.947 |
| History of smoking | 60% (15) | 69% (27) | 0.57 b | 0.452 |
| History of cardiovascular diseases | 42% (10) | 82% (32) | 10.73 b | 0.001 |
| History of diabetes | 58% (15) | 26% (10) | 6.77 a | 0.009 |
| Percentage of patients taking statins | 92% (24) | 87% (34) | 0.06 c | 0.806 |
| Antihypertensive medications: | 4.67 d | 0.197 | ||
| does not take medications | 4% (1) | 18% (7) | ||
| 1 medication | 19% (5) | 21% (8) | ||
| 2 medications | 54% (14) | 33% (13) | ||
| ≥3 medications | 23% (6) | 28% (11) | ||
| Beta-blocker | 73% (19) | 64% (25) | 0.57 b | 0.452 |
| α1-receptor blocker | 8% (2) | 0% (0) | 1.05 c | 0.305 |
| Calcium channel blocker | 42% (11) | 28% (11) | 1.36 b | 0.243 |
| ACE inhibitors | 58% (15) | 62% (24) | 0.10 a | 0.757 |
| Mineralocorticoid receptor antagonist (MRA) | 0% (0) | 5% (2) | 0.19 d | 0.660 |
| Sartans (ARBs) | 19% (5) | 5% (2) | 1.93 d | 0.165 |
| Loop diuretics | 62% (16) | 3% (1) | 27.66 b | <0.001 |
| Thiazide diuretics | 19% (5) | 15% (6) | 0.00 c | 0.946 |
| Variables | HTXr (n = 26) | Control Group (n = 39) | Test Results | p |
|---|---|---|---|---|
| Mean ± SD | ||||
| Creatinine [mg/dL] | 1.42 ± 0.53 | 1.09 ± 0.17 | 2.22 b | 0.027 |
| Glucose [mg/dL] | 112.12 ± 22.85 | 111.41 ± 25.46 | 0.52 b | 0.601 |
| TC [mg/dL] | 184.19 ± 66.14 | 196.49 ± 49.26 | −1.47 b | 0.141 |
| LDL-C [mg/dL] | 103.08 ± 53.99 | 115.17 ± 38.47 | −1.77 b | 0.077 |
| HDL-C [mg/dL] | 45.92 ± 16.70 | 58.98 ± 16.12 | −3.40 b | 0.001 |
| TG [mg/dL] | 175.35 ± 99.06 | 113.59 ± 59.02 | 3.21 b | 0.001 |
| HbA1c [%] | 6.05 ± 0.97 | 6.02 ± 0.93 | −0.08 b | 0.938 |
| Fibrinogen [G/L] | 4.14 ± 1.40 | 3.73 ± 1.10 | 0.81 a | 0.421 |
| CRP [mg/L] | 5.04 ± 5.56 | 4.43 ± 7.25 | 1.27 b | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raczyńska, W.; Radtke-Łysek, A.; Bohdan, M.; Frankiewicz, A.; Sobiczewski, W.; Gruchała, M. Analysis of 24-Hour Blood Pressure Profile and Antihypertensive Therapy in Male Heart Transplant Patients. J. Clin. Med. 2025, 14, 6590. https://doi.org/10.3390/jcm14186590
Raczyńska W, Radtke-Łysek A, Bohdan M, Frankiewicz A, Sobiczewski W, Gruchała M. Analysis of 24-Hour Blood Pressure Profile and Antihypertensive Therapy in Male Heart Transplant Patients. Journal of Clinical Medicine. 2025; 14(18):6590. https://doi.org/10.3390/jcm14186590
Chicago/Turabian StyleRaczyńska, Wioletta, Alicja Radtke-Łysek, Michał Bohdan, Anna Frankiewicz, Wojciech Sobiczewski, and Marcin Gruchała. 2025. "Analysis of 24-Hour Blood Pressure Profile and Antihypertensive Therapy in Male Heart Transplant Patients" Journal of Clinical Medicine 14, no. 18: 6590. https://doi.org/10.3390/jcm14186590
APA StyleRaczyńska, W., Radtke-Łysek, A., Bohdan, M., Frankiewicz, A., Sobiczewski, W., & Gruchała, M. (2025). Analysis of 24-Hour Blood Pressure Profile and Antihypertensive Therapy in Male Heart Transplant Patients. Journal of Clinical Medicine, 14(18), 6590. https://doi.org/10.3390/jcm14186590






