Impact of Discordant Antibiotics on Outcomes After Percutaneous Cholecystostomy for Acute Cholecystitis: A Retrospective Analysis of 184 PCC Patients
Abstract
1. Introduction
2. Materials and Methods
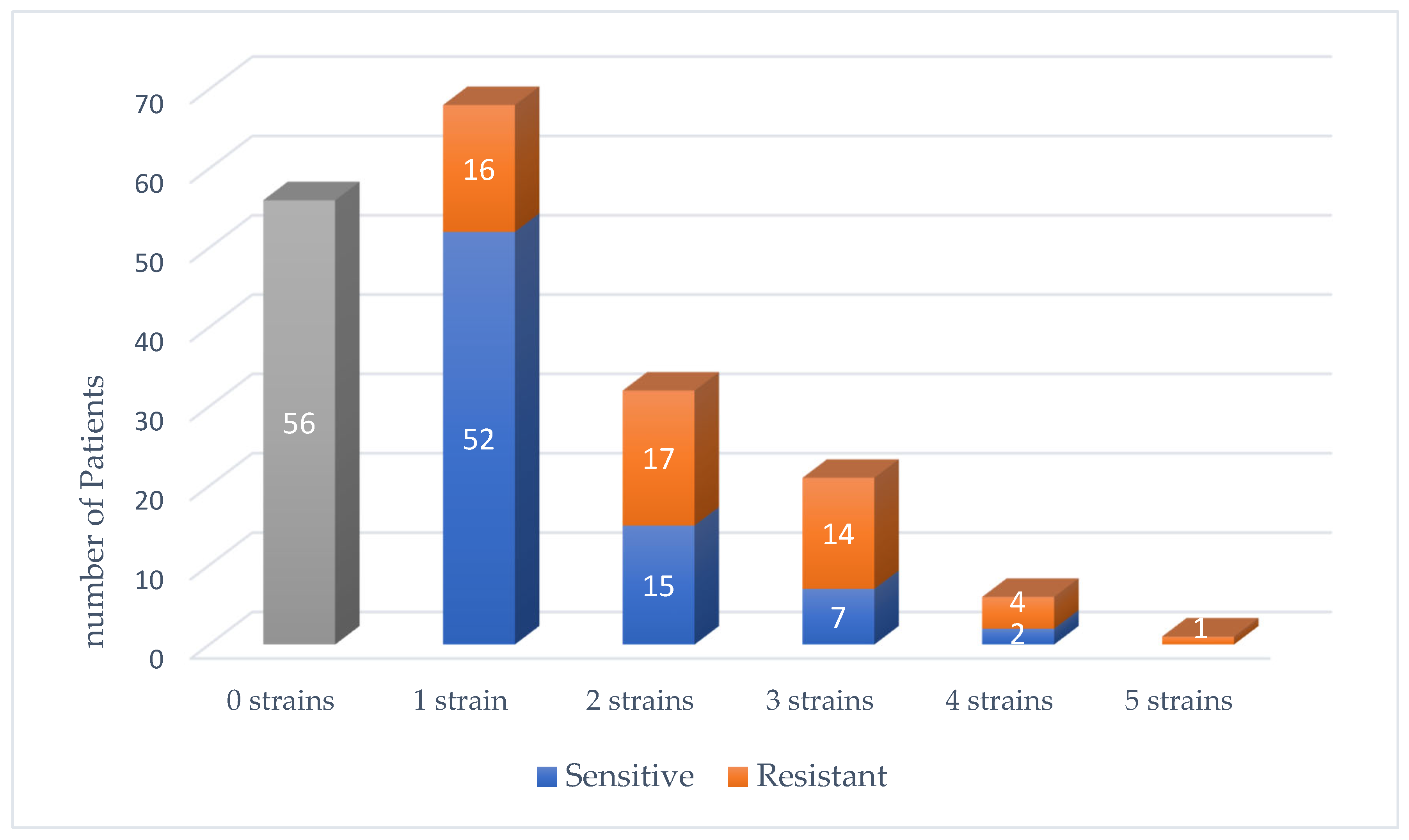
3. Results
3.1. Outcomes and Follow-Up
3.2. Subanalysis of Late Concordant Patients
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisano, M.; Allievi, N.; Gurusamy, K.; Borzellino, G.; Cimbanassi, S.; Boerna, D.; Coccolini, F.; Tufo, A.; Di Martino, M.; Leung, J.; et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J. Emerg. Surg. 2020, 15, 61. [Google Scholar] [CrossRef]
- Endo, I.; Takada, T.; Hwang, T.; Akazawa, K.; Mori, R.; Miura, F.; Yokoe, M.; Itoi, T.; Gomi, H.; Chen, M.; et al. Optimal treatment strategy for acute cholecystitis based on predictive factors: Japan-Taiwan multicenter cohort study. J. Hepato-Biliary-Pancreatic Sci. 2017, 24, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Pesce, A.M.; Ramírez-Giraldo, C.; Arkoudis, N.-A.; Ramsay, G.; Popivanov, G.; Gurusamy, K.; Bejarano, N.; Bellini, M.I.; Allegritti, M.; Tesei, J.; et al. Management of high-surgical-risk patients with acute cholecystitis following percutaneous cholecystostomy: Results of an international Delphi consensus study. Int. J. Surg. 2025, 111, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Binda, C.; Crinò, S.F.; Lisotti, A.; Spadaccini, M.; Amato, A.; Carrozza, L.; Catena, F.; Cobianchi, L.; Coluccio, C.; et al. The i-EUS consensus on EUS-guided gallbladder drainage: A 3-step modified Delphi approach. Endosc. Ultrasound 2025, 14, 106–119. [Google Scholar] [CrossRef]
- Mori, Y.; Itoi, T.; Baron, T.H.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Ukai, T.; Shikata, S.; Noguchi, Y.; Teoh, A.Y.B.; et al. Tokyo Guidelines 2018: Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J. Hepatobiliary Pancreat Sci. 2018, 25, 87–95. [Google Scholar] [CrossRef]
- Hernandez, M.; Murphy, B.; Aho, J.M.; Haddad, N.N.; Saleem, H.; Zeb, M.; Morris, D.S.; Jenkins, D.H.; Zielinski, M. Validation of the AAST EGS acute cholecystitis grade and comparison with the Tokyo guide-lines. Surgery 2018, 163, 739–746. [Google Scholar] [CrossRef]
- Okamoto, K.; Suzuki, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Iwashita, Y.; Hibi, T.; Pitt, H.A.; Umezawa, A.; et al. Tokyo Guidelines 2018: Flowchart for the management of acute cholecystitis. J. Hepatobiliary Pancreatic Sci. 2018, 25, 55–72. [Google Scholar] [CrossRef]
- Gomi, H.; Solomkin, J.S.; Schlossberg, D.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Ukai, T.; Endo, I.; Iwashita, Y.; Hibi, T.; et al. Tokyo Guidelines 2018: Antimicrobial therapy for acute cholangitis and cholecystitis. J. Hepatobiliary Pancreatic Sci. 2018, 25, 3–16. [Google Scholar] [CrossRef]
- Rossi, F.; Baquero, F.; Hsueh, P.-R.; Paterson, D.L.; Bochicchio, G.V.; Snyder, T.A.; Satishchandran, V.; McCarroll, K.; DiNubile, M.J.; Chow, J.W. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J. Antimicrob. Chemother. 2006, 58, 205–210. [Google Scholar] [CrossRef]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.; Baron, E.J.; O’NEill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg. Infect. (Larchmt) 2010, 11, 79–109. [Google Scholar] [CrossRef]
- Kaplan, U.; Handler, C.; Chazan, B.; Weiner, N.; Hatoum, O.A.; Yanovskay, A.; Kopelman, D. The Bacteriology of Acute Cholecystitis: Comparison of Bile Cultures and Clinical Outcomes in Diabetic and Non-Diabetic Patients. World J. Surg. 2021, 45, 2426–2431. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef] [PubMed]
- Buckman, S.A.; Mazuski, J.E. Review of the Tokyo guidelines 2018: Antimicrobial therapy for acute cholangitis and cholecystitis. JAMA Surg. 2019, 154, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’nEill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N. Engl. J. Med. 2015, 372, 1996–2005. [Google Scholar] [CrossRef]
- Nitzan, O.; Brodsky, Y.; Edelstein, H.; Hershko, D.; Saliba, W.; Keness, Y.; Peretz, A.; Chazan, B. Microbiologic data in acute cholecystitis: Ten years’ experience from bile cultures obtained during percutaneous cholecystostomy. Surg. Infect. 2017, 18, 345–349. [Google Scholar] [CrossRef]
- Joseph, T.; Unver, K.; Hwang, G.L.; Rosenberg, J.; Sze, D.Y.; Hashimi, S.; Kothary, N.; Louie, J.D.; Kuo, W.T.; Hofmann, L.V.; et al. Percutaneous cholecystostomy for acute cholecystitis: Ten-Year experience. J. Vasc. Interv. Radiol. 2012, 23, 83–88. [Google Scholar] [CrossRef]
- Kim, J.; Ihm, C. Usefulness of bile cultures and predictive factors for bacteriobilia in percutaneous cholecystostomy in patients with acute cholecystitis. Ann. Lab. Med. 2007, 27, 281–285. [Google Scholar] [CrossRef][Green Version]
- Thompson, J.E.; Bennion, R.S.; Doty, J.E.; Muller, E.L.; Pitt, H.A. Predictive factors for bactibilia in acute cholecystitis. Arch. Surg. 1990, 125, 261–264. [Google Scholar] [CrossRef]
- Csendes, A.; Burdiles, P.; Maluenda, F.; Diaz, J.C.; Csendes, P.; Mitru, N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch. Surg. 1996, 131, 389–394. [Google Scholar] [CrossRef]
- Kaval, S.; Tewari, S. Bacteriological profile of bile in cholecystectomy patients in tertiary care center. World J. Surg. Infect. 2022, 1, 3–6. [Google Scholar] [CrossRef]
- Yun, S.P.; Seo, H.-I. Clinical aspects of bile culture in patients undergoing laparoscopic cholecystectomy. Medicine 2018, 97, e11326. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, G.; Favara, G.; Maugeri, A.; Barchitta, M.; Agodi, A.; Basile, G. Comparing percutaneous treatment and cholecystectomy outcomes in acute cholecystitis patients: A systematic review and meta-analysis. World J. Emerg. Surg. 2025, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Loozen, C.S.; van Santvoort, H.C.; van Duijvendijk, P.; Besselink, M.G.; Gouma, D.J.; AP Nieuwenhuijzen, G.; Kelder, J.C.; Donkervoort, S.C.; van Geloven, A.A.; Kruyt, P.M.; et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ 2018, 363, k3965. [Google Scholar] [CrossRef]
- de Miguel-Palacio, M.; González-Castillo, A.M.; Membrilla-Fernández, E.; Pons-Fragero, M.J.; Pelegrina-Manzano, A.; Grande-Posa, L.; Morera-Casaponsa, R.; Sancho-Insenser, J.J. Impact of empiric antibiotic therapy on the clinical outcome of acute calculous cholecystitis. Langenbeck’s Arch. Surg. 2023, 408, 345. [Google Scholar] [CrossRef]
- Wu, P.-S.; Chou, C.-K.; Hsieh, Y.-C.; Chen, C.-K.; Lin, Y.-T.; Huang, Y.-H.; Hou, M.-C.; Lin, H.-C.; Lee, K.-C. Antibiotic use in patients with acute cholecystitis after percutaneous cholecystostomy. J. Chin. Med. Assoc. 2019, 83, 134–140. [Google Scholar] [CrossRef]

| Variables | Concordant n = 106 | Discordant n = 78 | p Value |
|---|---|---|---|
| Age, (years) mean ± SD | 72.64 ± 13.91 | 77.29 ± 12.91 | 0.02 |
| Male, n (%) | 60 (56.6) | 45 (57.7) | 0.88 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 44 (41.5) | 33 (42.3) | 0.91 |
| Hypertension | 67 (63.2) | 57 (73.1) | 0.15 |
| Dyslipidemia | 51 (48.1) | 43 (55.1) | 0.34 |
| Congestive Heart Failure | 16 (15.1) | 13 (16.7) | 0.77 |
| Ischemic Heart Disease | 21 (19.8) | 19 (24.4) | 0.46 |
| Chronic kidney disease | 9 (8.5) | 8 (10.3) | 0.68 |
| Signs and symptoms, n (%) | |||
| RUQ pain | 93 (87.7) | 64 (82.1) | 0.28 |
| RUQ tenderness | 84 (79.2) | 60 (76.9) | 0.70 |
| Fever > 37.9 °C | 23 (21.7) | 22 (28.2) | 0.31 |
| Tachycardia > 99 BPM | 25 (23.6) | 18 (23.1) | 0.93 |
| Laboratory tests, mean ± SD | |||
| WBC count (×109/L) | 15.7 ± 6 | 17.2 ± 7.7 | 0.25 |
| Bilirubin (mg/dL) | 1.4 ± 1. | 1.7 ± 2.2 | 0.52 |
| ALK-P (U/L) | 165 ± 1245 | 169 ± 153 | 0.56 |
| GGT (U/L) | 165 ± 217 | 169 ± 232 | 0.52 |
| ALT (U/L) | 87 ± 156 | 86 ± 143 | 0.45 |
| AST (U/L) | 100 ± 186 | 107 ± 190 | 0.49 |
| CRP (mg/dL) | 18.4 ± 11.6 | 18.5 ± 12.8 | 0.98 |
| Ultrasound exam findings—n (%) | |||
| GB Distention | 98 (92.5) | 77 (98.7) | 0.08 |
| GB wall thickened | 96 (90.6) | 76 (97.4) | 0.06 |
| GB stones | 87 (82.1) | 69 (88.5) | 0.23 |
| Bile duct enlarged | 19 (17.9) | 21 (26.9) | 0.14 |
| GB infiltration | 77 (72.6) | 47 (60.3) | 0.07 |
| Peri-Cholecystic Findings | 41 (38.7) | 17 (21.8) | 0.01 |
| Positive blood cultures, n (%) | 18 (17) | 20 (25.6) | 0.34 |
| PCC Procedure length (min), mean ± SD | 45 ± 38 | 53 ± 97 | 0.85 |
| Hours from admission to drainage, mean ± SD | 63.7 ± 89.2 | 50.5 ± 73.3 | 0.04 |
| Empiric Antibiotic regimen, n (%) | |||
| Cefuroxime | 26 (24.5) | 26 (33.3) | 0.39 |
| Cefuroxime + Metronidazole | 71 (67.0) | 50 (64.1) | |
| Amoxicillin Clavulanate | 1 (0.9) | 0 (0.0) | |
| Cefuroxime + Metronidazole + Ampicillin | 1 (0.9) | 0 (0.0) | |
| Gentamicin + Metronidazole + Ampicillin | 4 (3.8) | 2 (2.6) | |
| Piperacillin + Tazobactam | 3 (2.8) | 0 (0.0) |
| Clinical Outcomes | Concordant n = 106 | Discordant n = 78 | p Value |
|---|---|---|---|
| Post-PCC insertion Complications | 48 (45.3) | 42 (53.8) | 0.25 |
| Clavien–Dindo grade ≥ 3 | 13 (12.3) | 13 (16.6) | 0.40 |
| Length of hospitalization (days) | 7.3 ± 6.3 | 8.6 ± 12.6 | 0.68 |
| 90 days Readmission rate | 50 (47.2) | 50 (64.1) | 0.02 |
| Interval between admissions (days) | 27.7 ± 24.9 | 28.4 ± 24.1 | 0.90 |
| Urgent readmission rate, n (% of readmissions) | 38 (76.0) | 41 (82.0) | 0.46 |
| Readmission cause, n (%) | |||
| Elective for fluoroscopy | 9 (18.0) | 8 (15.7) | 0.75 |
| Acute biliary disease | 25 (50) | 30 (58.8) | |
| Difficulty with drain | 4 (8.0) | 2 (3.9) | |
| Other Internal Medicine cause | 12 (24.0) | 11 (21.6) | |
| Elective Cholecystectomy | 36 (34) | 17 (21.8) | 0.19 |
| Urgent Cholecystectomy | 10 (9.4) | 9 (11.5) | |
| Interval from drainage to surgery (days) | 113 ± 117 | 113 ± 124 | 0.65 |
| Length of follow-up (month), mean | 44.2 | 41.3 | 0.36 |
| Mortality during follow-up period, n (%) | 25 (23.6) | 23 (29.5) | 0.36 |
| Variables | Concordant n = 106 | Late Concordant n = 34 | p Value |
|---|---|---|---|
| Age (years), mean ± SD | 72.6 ± 13.9 | 80 ± 11.2 | <0.01 |
| Male, n (%) | 60 (56.6) | 21 (61.8) | 0.59 |
| Pre interventional variables | |||
| Diabetes mellitus | 44 (41.5) | 16 (47.1) | 0.57 |
| Hypertension | 67 (63.2) | 26 (76.5) | 0.15 |
| RUQ tenderness | 84 (79.2) | 26 (76.5) | 0.73 |
| Fever > 37.9 °C | 23 (21.7) | 13 (39.4) | 0.04 |
| WBC count (×109/L) | 15.7 ± 5.9 | 18.4 ± 8.1 | 0.08 |
| CRP (mg/dL) | 18.4 ± 11.6 | 19.4 ± 11.5 | 0.64 |
| Hours from admission to drainage | 63.7 ± 89.2 | 59.7 ± 106.2 | 0.04 |
| Post-Interventional Outcomes | |||
| Clavien–Dindo grade ≥ 3 | 13 (12.3) | 8 (23.5) | 0.109 |
| Length of hospitalization (days) | 7.4 ± 6.3 | 9.4 ± 8.6 | 0.02 |
| Patients with LOH > 7 days | 39 (36.8) | 21 (61.8) | 0.01 |
| 90 days Readmission rate | 50 (47.2) | 25 (73.5) | <0.01 |
| Urgent readmission rate—n (% of readmission) | 38 (76) | 22 (88) | 0.22 |
| Readmission cause, n (%) | |||
| Elective for fluoroscopy | 9 (18) | 3 (12) | 0.78 |
| Acute biliary disease | 25 (50) | 15 (60) | |
| Difficulty with drain | 4 (8) | 1 (4) | |
| Other Internal Medicine cause | 12 (24) | 6 (24) | |
| Urgent Cholecystectomy | 10 (9.4) | 6 (17.6) | 0.03 |
| Mortality during the follow-up period, n(%) | 25 (23.6) | 11 (32.4) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahav, L.; Goldberg, N.; Jiryis, T.; Cristo, H.; Soback, H.; Avital, S.; Rudnicki, Y. Impact of Discordant Antibiotics on Outcomes After Percutaneous Cholecystostomy for Acute Cholecystitis: A Retrospective Analysis of 184 PCC Patients. J. Clin. Med. 2025, 14, 6589. https://doi.org/10.3390/jcm14186589
Lahav L, Goldberg N, Jiryis T, Cristo H, Soback H, Avital S, Rudnicki Y. Impact of Discordant Antibiotics on Outcomes After Percutaneous Cholecystostomy for Acute Cholecystitis: A Retrospective Analysis of 184 PCC Patients. Journal of Clinical Medicine. 2025; 14(18):6589. https://doi.org/10.3390/jcm14186589
Chicago/Turabian StyleLahav, Lauren, Nitzan Goldberg, Tamara Jiryis, Hadasa Cristo, Hagai Soback, Shmuel Avital, and Yaron Rudnicki. 2025. "Impact of Discordant Antibiotics on Outcomes After Percutaneous Cholecystostomy for Acute Cholecystitis: A Retrospective Analysis of 184 PCC Patients" Journal of Clinical Medicine 14, no. 18: 6589. https://doi.org/10.3390/jcm14186589
APA StyleLahav, L., Goldberg, N., Jiryis, T., Cristo, H., Soback, H., Avital, S., & Rudnicki, Y. (2025). Impact of Discordant Antibiotics on Outcomes After Percutaneous Cholecystostomy for Acute Cholecystitis: A Retrospective Analysis of 184 PCC Patients. Journal of Clinical Medicine, 14(18), 6589. https://doi.org/10.3390/jcm14186589








