Innovations in Intraocular Lens Power Calculation—A Review
Abstract
1. Introduction
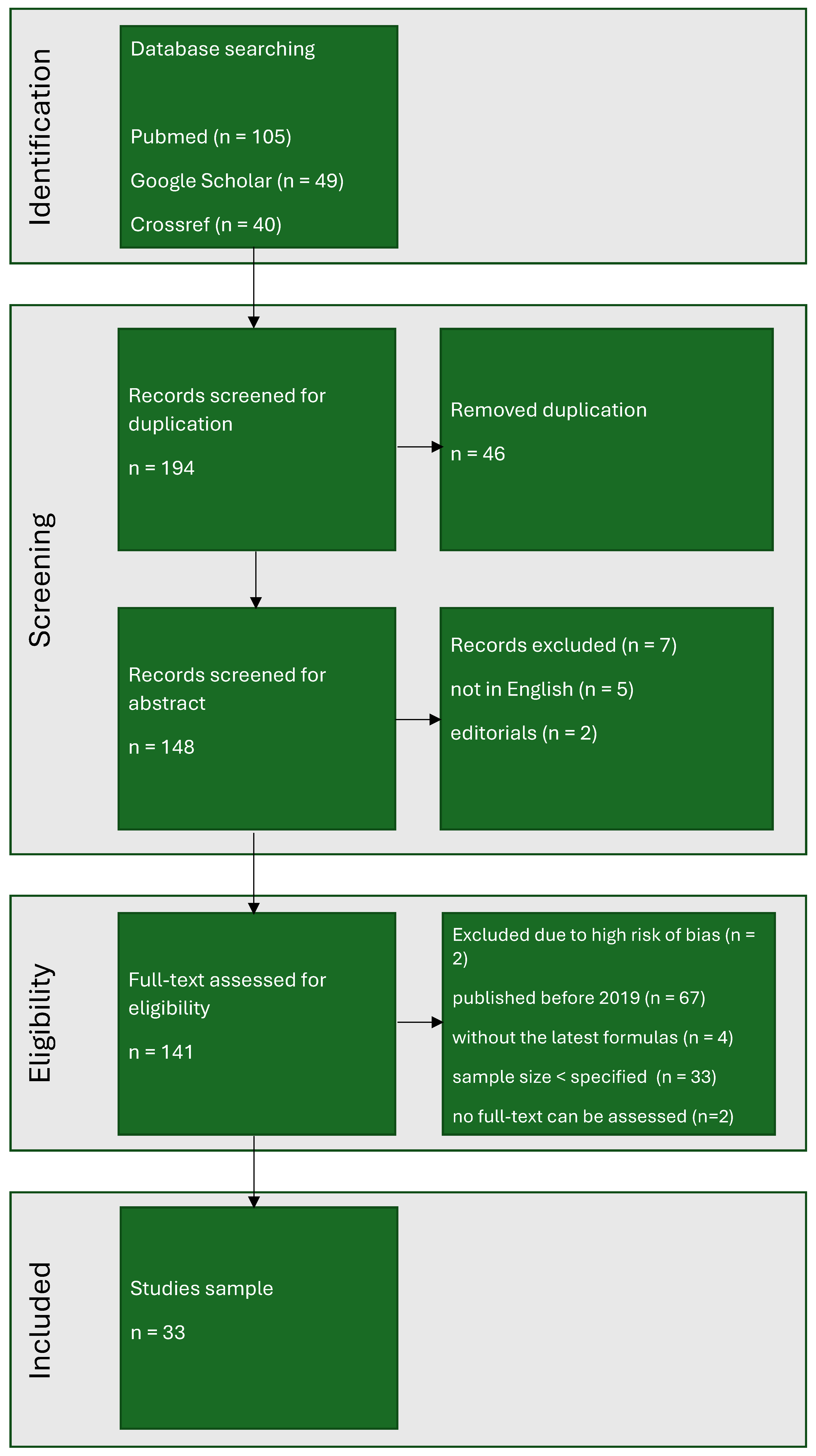
2. Methods
3. Ethics
4. Results
5. The Latest IOL Power Calculation Formulas
5.1. AI-Based Formulas
5.1.1. The Karmona Formula—Solely an AI-Driven Model
5.1.2. The Hoffer QST Formula—A Machine Learning–Enhanced Evolution of a Classic Vergence-Based Model
5.1.3. The Nallasamy Formula—A Two-Layer Ensemble Machine Learning Model
5.1.4. The Zhu-Lu Formula—A Machine Learning–Based Ensemble Model for Highly Myopic Eyes
5.1.5. The Zeiss-AI Formula—A Hybrid Model Integrating AI and Paraxial Ray Tracing
5.2. Vergence Formulas
5.2.1. The Emmetropia Verifying Optical (EVO) Formula—A Thick-Lens IOL Power Model Based on Emmetropization Theory
5.2.2. The Naeser Formulas—Thick- and Thin-Lens Models
5.2.3. The Voytsekhivskyy Regression Function–Gender (VRF-G) Formula—A Hybrid Optical and Regression-Based Model
5.2.4. The Castrop Formula—A Paraxial Vergence-Based Model Incorporating Detailed Ocular Geometry
- C, analogous to the Olsen formula’s constant;
- H, which compensates for systematic shifts in the IOL plane depending on the IOL’s optic and haptic design; and
- R, a refraction offset constant that corrects for systematic prediction errors in postoperative refraction.
5.3. Ray-Tracing Formulas
The O Formula—A Novel OCT-Based Approach
6. Discussion
- m—slope of the correlation between the arithmetic error and AL;
- n—the percentage of eyes with an absolute error within 0.5 D.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leffler, C.T.; Spalton, D.; Schwartz, S.G.; Grzybowski, A.; Maloney, R.K. Sir Harold Ridley (1906–2001) and His Cure for Aphakia: New Historical Insights Into the Invention of the Intraocular Lens. Am. J. Ophthalmol. 2025, 273, 167–175. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kolinko, A.I. A method of calculating the optical power of the intraocular lens. Vestn. Oftalmol. 1967, 80, 27–31. Available online: https://pubmed.ncbi.nlm.nih.gov/5609244/ (accessed on 10 July 2025). (In Russian).
- Stopyra, W. Effectiveness, Sensitivity and Specificity of Intraocular Lens Power Calculation Formulas for Short Eyes. Turk. J. Ophthalmol. 2022, 52, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J. The Hoffer Q formula: A comparison of theoretic and regression formulas. J. Cataract Refract. Surg. 1993, 19, 700–712. [Google Scholar] [CrossRef]
- Retzlaff, J.A.; Sanders, D.R.; Kraff, M.C. Development of the SRK/T intraocular lens implant power calculation formula. J. Cataract Refract. Surg. 1990, 16, 333–340. [Google Scholar] [CrossRef]
- Chung, J.; Bu, J.J.; Afshari, N.A. Advancements in intraocular lens power calculation formulas. Curr. Opin. Ophthalmol. 2022, 33, 35–40. [Google Scholar] [CrossRef]
- Haigis, W.; Lege, B.; Miller, N.; Schneider, B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 765–773. [Google Scholar] [CrossRef]
- Sheard, R.M.; Smith, G.T.; Cooke, D.L. Improving the prediction accuracy of the SRK/T formula: The T2 formula. J. Cataract Refract. Surg. 2010, 36, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J.; Savini, G. IOL Power Calculation in Short and Long Eyes. Asia Pac. J. Ophthalmol. 2017, 6, 330–331. [Google Scholar] [CrossRef]
- Ladas, J.G.; Siddiqui, A.A.; Devgan, U.; Jun, A. A 3-D “Super Surface” Combining Intraocular Lens Formulas to Generate a “Super Formula” and Maximize Accuracy. JAMA Ophthalmol. 2015, 133, 1431–1436. [Google Scholar] [CrossRef]
- Næser, K.; Savini, G. Accuracy of thick-lens intraocular lens power calculation based on cutting-card or calculated data for lens architecture. J. Cataract Refract. Surg. 2019, 45, 1422–1429. [Google Scholar] [CrossRef]
- Debellemanière, G.; Dubois, M.; Gauvin, M.; Wallerstein, A.; Brenner, L.; Rampat, R.; Saad, A.; Gatinel, D. The PEARL-DGS Formula: The Development of an Open-source Machine Learning-based Thick IOL Calculation Formula. Am. J. Ophthalmol. 2021, 232, 58–69. [Google Scholar] [CrossRef]
- Voytsekhivskyy, O.V. Development and clinical accuracy of a new intraocular lens power formula (VRF) compared to other formulas. Am. J. Ophthalmol. 2018, 185, 56–67. [Google Scholar] [CrossRef]
- Kane, J.X.; Van Heerden, A.; Atik, A.; Petsoglou, C. Accuracy of 3 new method for intraocular lens power selection. J. Cataract. Refract. Surg. 2017, 43, 333–339. [Google Scholar] [CrossRef]
- Stopyra, W. Analysis of accuracy of twelve intraocular lens power calculation formulas for eyes with axial myopia. Taiwan J. Ophthalmol. 2022, 13, 225–230. [Google Scholar] [CrossRef]
- Darcy, K.; Gunn, D.; Tavassoli, S.; Sparrow, J.; Kane, J.X. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10930 eyes from the UK National Health Service. J. Cataract. Refract. Surg. 2020, 46, 2–7. [Google Scholar]
- Stopyra, W.; Voytsekhivskyy, O.; Grzybowski, A. Accuracy of 7 artificial intelligence based intraocular lens power calculation formulas in extremely long Caucasian eyes. Am. J. Ophthalmol. 2025, 271, 337–346. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, R.; Liu, Z.; Young, C.A.; Wu, Y.; Zheng, D.; Zhang, X.; Jin, G. Network Meta-analysis of Intraocular Lens Power Calculation Formula Accuracy in 1016 Eyes With Long Axial Length. Am. J. Ophthalmol. 2024, 257, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Sun, Y.; Xiao, B.; Ainiwear, M.; Ji, Y. A Bayesian network meta-analysis on comparisons of intraocular lens power calculation methods for paediatric cataract eyes. Eye 2023, 37, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Stopyra, W.; Grzybowski, A. Intraocular Lens Power Calculation Formulas in Children—A Systematic Review. J. Clin. Med. 2024, 13, 4400. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of Intraocular Lens calculation Formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef]
- Kane, J.X.; Chang, D.F. Intraocular Lens Power Formulas, Biometry, and Intraoperative Aberrometry. A Review. Ophthalmology 2021, 128, e94–e114. [Google Scholar] [CrossRef]
- Olsen, T. Calculation of intraocular lens power: A review. Acta Ophthalmol. Scand. 2007, 85, 472–485. [Google Scholar] [CrossRef]
- Koch, D.D.; Hill, W.; Abulafia, A.; Wang, L. Pursuing perfection in intraocular lens calculations: I. Logical approach for classifying IOL calculation formulas. J. Cataract Refract. Surg. 2017, 43, 717–718. [Google Scholar] [CrossRef]
- Carmona-González, D.; Palomino-Bautista, C. Accuracy of a new intraocular lens power calculation method based on artificial intelligence. Eye 2021, 35, 517–522. [Google Scholar] [CrossRef]
- Taroni, L.; Hoffer, K.J.; Pellegrini, M.; Lupardi, E.; Savini, G. Comparison of the New Hoffer QST with 4 Modern Accurate Formulas. J. Cataract Refract. Surg. 2023, 49, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; He, W.; Wei, L.; Song, Y.; Qi, J.; Yao, Y.; Chen, X.; Huang, J.; Lu, Y.; Zhu, X. The Zhu-Lu formula: A machine learning-based intraocular lens power calculation formula for highly myopic eyes. Eye Vis. 2023, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Stein, J.; Nallasamy, N. Evaluation of the Nallasamy formula: A stacking ensemble machine learning method for refraction prediction in cataract surgery. Br. J. Ophthalmol. 2023, 107, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.I.; Kozhaya, K.; Truong, P.; Weikert, M.P.; Hill, W.E.; Koch, D.D. Efficacy of segmented axial length and artificial intelligence approaches to intraocular lens power calculation in short eyes. J. Cataract Refract. Surg. 2023, 49, 697–703. [Google Scholar] [CrossRef]
- Savini, G.; Hoffer, K.J.; Kohnen, T. IOL power formulas classifications. J. Cataract Refract. Surg. 2024, 50, 105–107. [Google Scholar] [CrossRef]
- Wang, L.; Koch, D.D.; Hill, W.; Abulafia, A. Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J. Cataract Refract. Surg. 2017, 43, 999–1002. [Google Scholar] [CrossRef]
- Holladay, J.T.; Wilcox, R.R.; Koch, D.D.; Wang, L. Review and recommendations for univariate statistical analysis of spherical equivalent prediction error for IOL power calculations. J. Cataract Refract. Surg. 2021, 47, 65–77. [Google Scholar] [CrossRef]
- Moshirfar, M.; Sperry, R.A.; Altaf, A.W.; Stoakes, I.M.; Hoopes, P.C. Predictability of Existing IOL Formulas After Cataract Surgery in Patients with a Previous History of Radial Keratotomy: A Retrospective Cohort Study and Literature Review. Ophthalmol. Ther. 2024, 13, 1703–1722. [Google Scholar] [CrossRef]
- Moore, J.E.; McNeely, R.N.; Moutari, S. Cataract Surgery in the Small Adult Eye: A Review. Clin. Exp. Ophthalmol. 2025, 53, 558–569. [Google Scholar] [CrossRef]
- Wójcik-Niklewska, B.; Nocoń-Bratek, M.; Szala, K. Intraocular lens power calculation in pediatric cataract surgery: A narrative review. Medicine 2025, 104, e42072. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, M.; Findl, O. Update on intraocular lens formulas. Curr. Opin. Ophthalmol. 2025, 36, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Taroni, L.; Hoffer, K.J. Recent developments in intraocular lens power calculation methods-update 2020. Ann. Transl. Med. 2020, 8, 1553. [Google Scholar] [CrossRef]
- Naeser, K.; Nielsen, R. Accuracy of thick and thin intraocular lens power formulas using paraxial vergence calculation. J. Cataract Refract. Surg. 2025, 51, 182–187. [Google Scholar] [CrossRef]
- Hipólito-Fernandes, D.; Luís, M.E.; Gil, P.; Maduro, V.; Feijão, J.; Yeo, T.K.; Voytsekhivskyy, O.; Alves, N. VRF-G, a New Intraocular Lens Power Calculation Formula: A 13-Formulas Comparison Study. Clin. Ophthalmol. 2020, 14, 4395–4402. [Google Scholar] [CrossRef]
- Langenbucher, A.; Szentmáry, N.; Cayless, A.; Weisensee, J.; Fabian, E.; Wendelstein, J.; Hoffmann, P. Considerations on the Castrop formula for calculation of intraocular lens power. PLoS ONE 2021, 16, e0252102. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Maeda, N.; Ohnuma, K.; Lawu, T.; Kawasaki, R.; Koh, S.; Nishida, K.; Noda, T. Preliminary demonstration of a novel intraocular lens power calculation:the O formula. J. Cataract Refract. Surg. 2022, 48, 1305–1311. [Google Scholar] [CrossRef]
- Holladay, J.T.; Wilcox, R.R.; Koch, D.D.; Wang, L. Statistics of prediction error for dependent and independent datasets. J. Cataract Refract. Surg. 2023, 49, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Gökce, S.E.; Zeiter, J.H.; Weikert, M.P.; Koch, D.D.; Hill, W.; Wang, L. Intraocular lens power calculations in short eyes using 7 formulas. J. Cataract Refract. Surg. 2017, 43, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J.; Savini, G. Update on Intraocular Lens Power Calculation Study Protocols: The Better Way to Design and Report Clinical Trials. Ophthalmology 2021, 128, e115–e120. [Google Scholar] [CrossRef]
- Redden, L.D.; Graubauer, B.; Hoffmann, P.; Langenbucher, A.; Riaz, K.M.; Gatinel, D.; Wagner, H.; Wendelstein, J.A. Intraocular Lens Power calculation–Comparing Big Data Approaches to Established Formulas. Am. J. Ophthalmol. 2025, 273, 141–150. [Google Scholar] [CrossRef]
- Simpson, M.J.; Charman, W.N. The effect of testing distance on intraocular lens power calculation. J. Refract. Surg. 2014, 30, 726. [Google Scholar] [CrossRef]
- Stopyra, W.; Voytsekhivskyy, O.; Grzybowski, A. Prediction of Seven Artificial Intelligence-Based Intraocular Lens Power Calculation Formulas in Medium-Long Caucasian Eyes. Life 2025, 15, 45. [Google Scholar] [CrossRef]
- Shammas, H.J.; Taroni, L.; Pellegrini, M.; Shammas, M.C.; Jivrajka, R.V. Accuracy of never IOL power formulas in short and long eyes using sum-of-segment biometry. J. Cataract Refract. Surg. 2022, 48, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Voytsekhivskyy, O.V.; Hoffer, K.J.; Savini, G.; Tutchenko, L.P.; Hipólito-Fernandes, D. Clinical Accuracy of 18 IOL Power Formulas in 241 Short Eyes. Curr. Eye Res. 2021, 46, 1832–1843. [Google Scholar] [CrossRef]
- Voytsekhivskyy, O.V.; Hoffer, K.J.; Tutchenko, L.; Cooke, D.L.; Savini, G. Accuracy of 24 IOL Power Calculation Methods. J. Refract. Surg. 2023, 39, 249–256. [Google Scholar] [CrossRef]
- Stopyra, W.; Voytsekhivskyy, O.; Grzybowski, A. Accuracy of 20 Intraocular Lens Power Calculation Formulas in Medium-Long Eyes. Ophthalmol. Ther. 2024, 13, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Stopyra, W.; Voytsekhivskyy, O.; Grzybowski, A. Accuracy of 7 artificial intelligence-base intraocular lens power calculation formulas in medium-long eyes: 2-center study. Can. J. Ophthalmol. 2025, 60, 200–207. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Cione, F.; Capasso, L.; Coppola, A.; Rosa, N. A formula to improve the reliability of optical axial length measurement in IOL power calculation. Sci. Rep. 2022, 12, 18845. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.X.; Melles, R.B. Intraocular lens formula comparison in axial hyperopia with a high-power intraocular lens of 30 or more diopters. J. Cataract Refract. Surg. 2020, 46, 1236–1239. [Google Scholar] [CrossRef]
- Nemeth, G.; Modis, L., Jr. Accuracy of the Hill-radial basis function method and the Barrett Universal II formula. Eur. J. Ophthalmol. 2021, 31, 566–571. [Google Scholar] [CrossRef]
| Group | Subgroup | Formula |
|---|---|---|
| Vergence | Thin lens | Holladay 1 |
| Hoffer Q | ||
| SRK/T | ||
| Haigis | ||
| Holladay 2 | ||
| Castrop | ||
| Cooke K6 | ||
| Panacea | ||
| T2 | ||
| VRF | ||
| VRF-G | ||
| Thick lens | EVO 2.0 | |
| Naeser 2 | ||
| Artificial intelligence (AI) | Only AI | Hill-RBF 2.0 |
| Karmona | ||
| Hill-RBF 3.0 | ||
| Nallasamy | ||
| AI + thin lens | Ladas SF AI | |
| 3C Calculator | ||
| Kane | ||
| Hoffer QST | ||
| Zhu-Lu | ||
| AI + thick lens | Pearl-DGS | |
| AI + paraxial | Zeiss AI | |
| Ray tracing | Paraxial | Olsen |
| Barrett Universal II | ||
| O | ||
| Exact | Okulix |
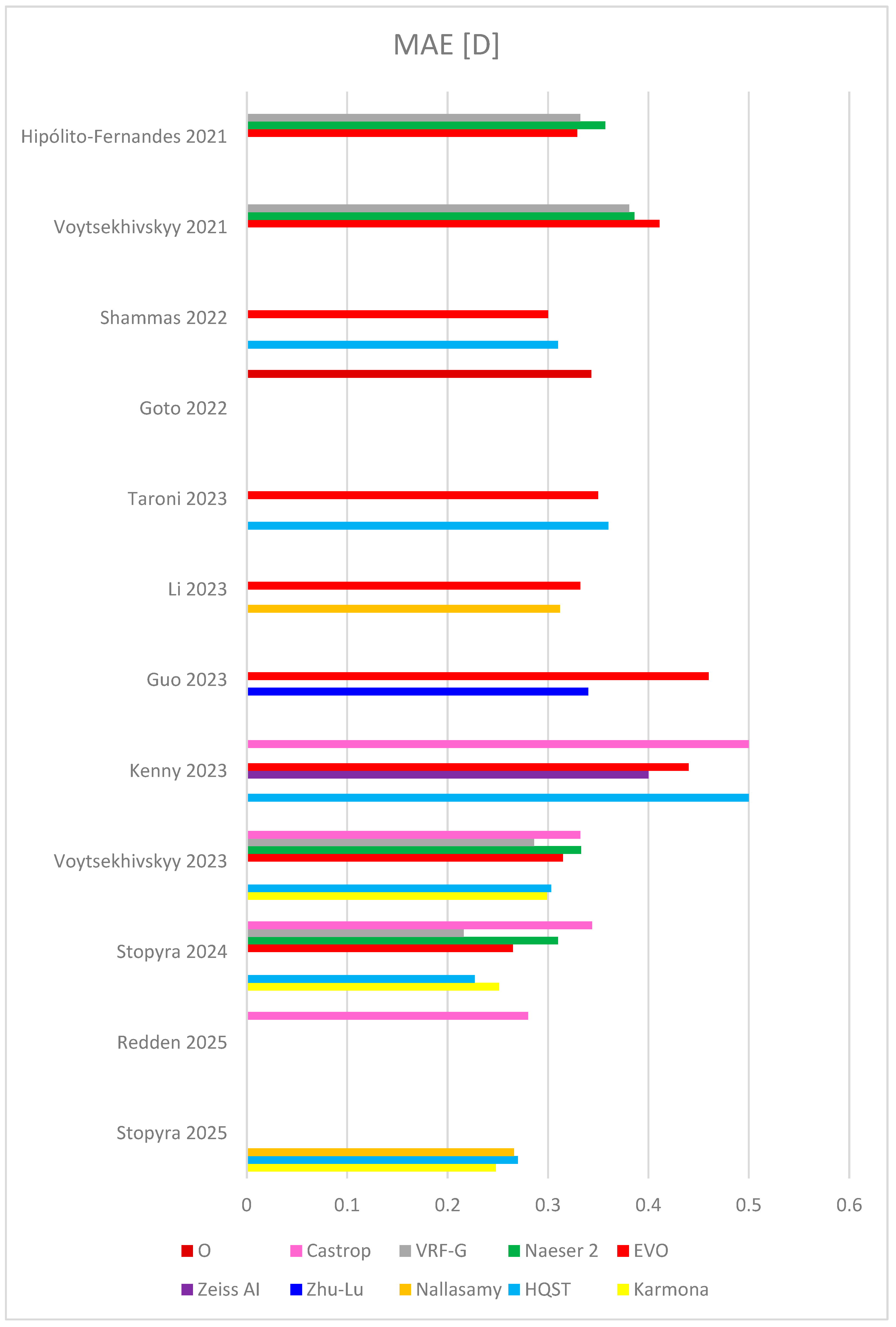
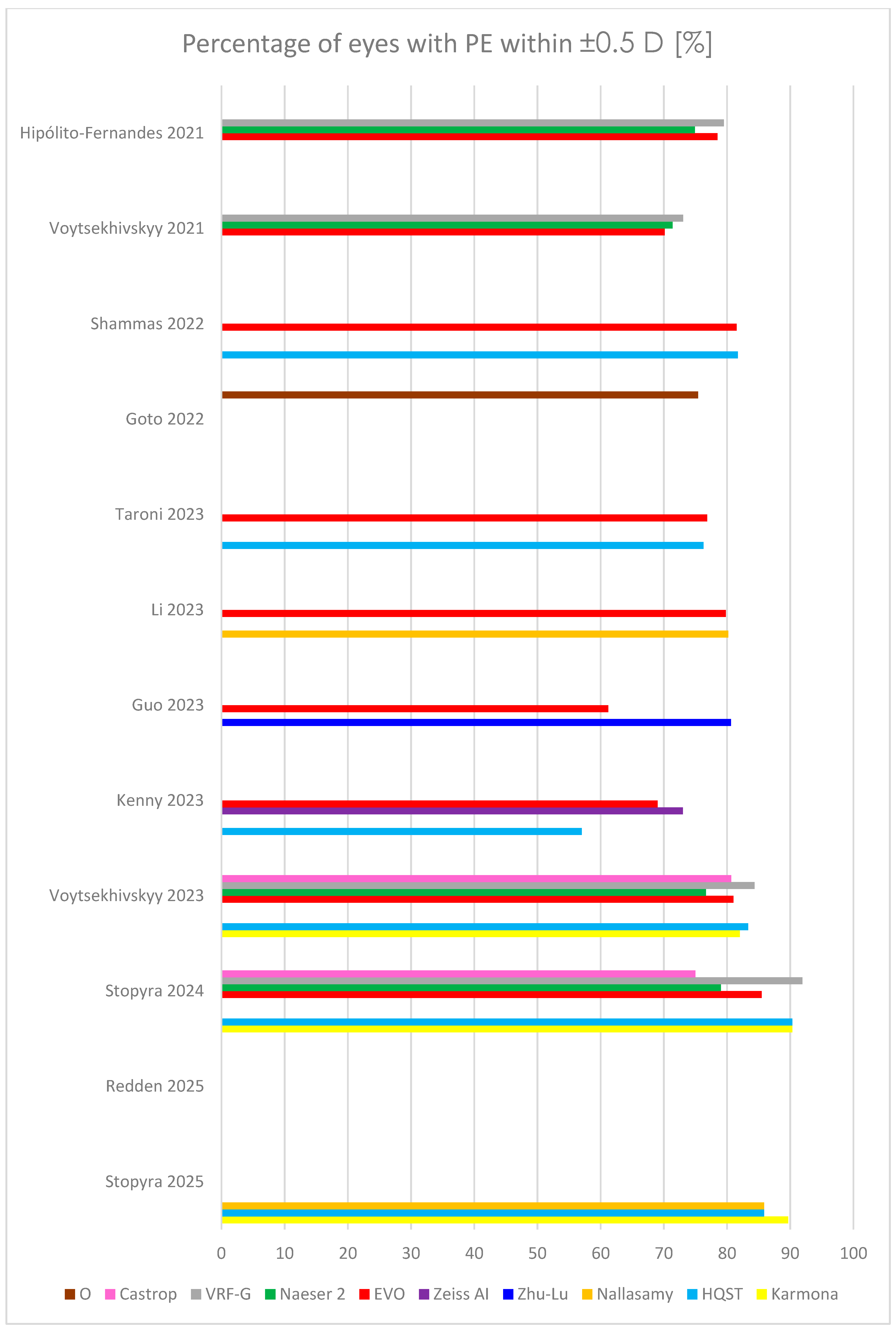
| Study | Karmona | HQST | Nallasamy | Zhu-Lu | Zeiss AI | EVO | Naeser 2 | VRF-G | Castrop | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hipólito-Fernandes 2021 (828 eyes) [39] | MAE | 0.329 | 0.357 | 0.332 | |||||||
| MedAE | |||||||||||
| ±0.5 D | 78.5 | 74.9 | 79.5 | ||||||||
| RMSAE | |||||||||||
| Voytsekhivskyy 2021 (241 eyes) [49] | MAE | 0.411 | 0.386 | 0.381 | |||||||
| MedAE | 0.325 | 0.277 | 0.276 | ||||||||
| ±0.5 D | 70.12 | 71.37 | 73.03 | ||||||||
| RMSAE | |||||||||||
| Shammas 2022 (595 eyes) [48] | MAE | 0.31 | 0.30 | ||||||||
| MedAE | 0.28 | 0.27 | |||||||||
| ±0.5 D | 81.7 | 81.5 | |||||||||
| RMSAE | |||||||||||
| Goto 2022 (423 eyes) [41] | MAE | 0.343 | |||||||||
| MedAE | 0.290 | ||||||||||
| ±0.5 D | 75.4 | ||||||||||
| RMSAE | |||||||||||
| Taroni 2023 (1259 eyes) [26] | MAE | 0.36 | 0.35 | ||||||||
| MedAE | 0.29 | 0.30 | |||||||||
| ±0.5 D | 76.29 | 76.87 | |||||||||
| RMSAE | 0.47 | 0.46 | |||||||||
| Li 2023 (6893 eyes) [28] | MAE | 0.312 | 0.322 | ||||||||
| MedAE | 0.242 | 0.251 | |||||||||
| ±0.5 D | 80.2 | 79.8 | |||||||||
| RMSAE | |||||||||||
| Guo 2023 (1828 eyes) [27] | MAE | 0.34 | 0.46 | ||||||||
| MedAE | 0.26 | 0.40 | |||||||||
| ±0.5 D | 80.61 | 61.22 | |||||||||
| RMSAE | |||||||||||
| Kenny 2023 (278 eyes) [29] | MAE | 0.50 | 0.40 | 0.44 | 0.50 | ||||||
| MedAE | |||||||||||
| ±0.5 D | 57.0 | 69.0 | |||||||||
| RMSAE | 0.56 | 0.55 | 0.60 | 0.66 | |||||||
| Voytsekhivskyy 2023 (300 eyes) [50] | MAE | 0.299 | 0.303 | 0.315 | 0.333 | 0.286 | 0.334 | ||||
| MedAE | 0.231 | 0.227 | 0.244 | 0.255 | 0.209 | 0.250 | |||||
| ±0.5 D | 82.00 | 83.33 | 81.00 | 76.67 | 84.33 | 80.67 | |||||
| RMSAE | |||||||||||
| Stopyra 2024 (124 eyes) [51] | MAE | 0.251 | 0.227 | 0.265 | 0.310 | 0.216 | 0.344 | ||||
| MedAE | 0.229 | 0.169 | 0.206 | 0.228 | 0.169 | 0.261 | |||||
| ±0.5 D | 90.32 | 90.32 | 85.48 | 79.03 | 91.94 | 75.00 | |||||
| RMSAE | 0.313 | 0.298 | 0.341 | 0.400 | 0.286 | 0.442 | |||||
| Redden 2025 (886 eyes) [45] | MAE | 0.280 | |||||||||
| MedAE | |||||||||||
| ±0.5 D | |||||||||||
| RMSAE | 0.355 | ||||||||||
| Stopyra 2025 (184 eyes) [52] | MAE | 0.248 | 0.270 | 0.266 | |||||||
| MedAE | 0.200 | 0.220 | 0.222 | ||||||||
| ±0.5 D | 89.67 | 85.87 | 85.87 | ||||||||
| RMSAE | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stopyra, W.; Grzybowski, A. Innovations in Intraocular Lens Power Calculation—A Review. J. Clin. Med. 2025, 14, 6585. https://doi.org/10.3390/jcm14186585
Stopyra W, Grzybowski A. Innovations in Intraocular Lens Power Calculation—A Review. Journal of Clinical Medicine. 2025; 14(18):6585. https://doi.org/10.3390/jcm14186585
Chicago/Turabian StyleStopyra, Wiktor, and Andrzej Grzybowski. 2025. "Innovations in Intraocular Lens Power Calculation—A Review" Journal of Clinical Medicine 14, no. 18: 6585. https://doi.org/10.3390/jcm14186585
APA StyleStopyra, W., & Grzybowski, A. (2025). Innovations in Intraocular Lens Power Calculation—A Review. Journal of Clinical Medicine, 14(18), 6585. https://doi.org/10.3390/jcm14186585






